Abstract
Cadmium (Cd) is a detrimental metal in the environment and it is easily taken up by plants, thus entering the food chain and posing a severe threat to human health. Phytoremediation being low cost, highly stable, and environmentally friendly has been considered as a promising green technology for Cd remediation. The addition of exogenous substances to the culture media has been recognized as an efficient strategy to improve plant phytoremediation capability. Pot trials were conducted to investigate the combined effects of exogenous calcium (Ca) and spermidine (Spd) on Cd-induced toxicity in Boehmeria nivea (L.) Gaudich. (ramie). Results showed that the application of 5-mM exogenous Ca significantly alleviated Cd toxicity in ramie by reducing Cd accumulation, depressing H2O2 and malondialdehyde contents, increasing plants dry weights and chlorophyll concentrations, as well as altering the activities of total superoxide dismutase and guaiacol peroxidase. Furthermore, as a non-Cd hyperaccumulator plant, ramie hyperaccumulated Cd and suffered more severe toxic effects of Cd by the treatment of 1 mM Ca/Cd. The aggravated Cd toxicity could be compensated by the addition of exogenous Spd via the promotion of plant growth and the reduction of the oxidative stress. Overall, the combination effects of 1 mM Ca and Spd appeared to be more superior compared to other treatments in the plants under Cd stress with a higher Cd accumulation ability and the evaluated Cd stress tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a highly toxic heavy metal mainly derived from atmospheric deposition, industrial processes, and the over use of phosphate fertilizers (Fagerberg et al. 2015; Feng et al. 2010). It is easily taken up by plants, thus entering the food chain and posing a potential severe threat to human health (Ansari and Neha 2015; Hong and Yan 2015). Heavy metals, such as Cd, usually cannot be degraded, and most conventional chemical and physical remediation technologies are either easy to cause secondary contamination or too costly (Cassier-Chauvat and Chauvat 2015; Fan et al. 2008; Singh and Prasad 2014). Phytoremediation is thought to be a cost-effective, environmentally friendly, and aesthetically pleasing alternative in the remediation of metal-contaminated soil (Ghosh and Singh 2005; Koptsik 2014). However, the efficiency of phytoremediation is generally limited by the poorly metal bioavailability and the relatively lower plant growth rate (Chaudhry et al. 2002). Significant progresses in improving phytoremediation capability of the plants have been made in the last few years through comparative physiological, cytology, and genomic studies (Andreolli et al. 2013; Bhargava et al. 2012; Chen et al. 2014). The application of exogenous agents to the plant culture media has been recognized as an effective strategy to strengthen the phytoremediation efficiency of the contaminated soil (Farid et al. 2015; Zaier et al. 2014). Hyperaccumulator plants could take up extraordinarily high amounts of heavy metals from the environment. But, those plants usually are rare herbs and possess small biomass (Adki et al. 2014; Rascio and Navari-Izzo 2011). Researches have demonstrated that non-metal hyperaccumulator plants could increase their hyperaccumulation potential by the addition of exogenous substances such as ethylenediaminetetraacetic acid (EDTA) and some plant growth promoters (López et al. 2005; López et al. 2007).
Polyamines (PAs), phytohormone-like aliphatic nitrogenous compounds containing two or more amino, play crucial roles in plant growth, antisenescence, and the environmental stress tolerance (Cai et al. 2015; Tiburcio et al. 2014). The most common PAs found in plants are putrescine (Put), spermine (Spm), and spermidine (Spd) (Velikova et al. 2000). Among which, Spd not only acts as a direct stress-protecting compound in the plants but also plays as a stress-signaling regulator in the stress tolerance phenomena (Kasukabe et al. 2004). Some reports indicated that the application of exogenous Spd enhanced Cd tolerance in Hydrocharis dubia (Yang et al. 2013), winter rape seedlings (Markowska-Kozak 2005) and Salvinia natans (Xu et al. 2008). Calcium (Ca), an essential element for plants, is recognized as a central regulator for plant growth and development. Additionally, it plays an important role in the processes of plant intracellular signaling transduction, cell division, and plant photosynthesis (White and Broadley 2003). It has been reported that exogenous Ca application alleviated Cd toxicity in Lens culinaris Medic. Seedlings (Talukdar 2012), citrus plants (López-Climent et al. 2014), Pisum sativum L. seedlings (El-Beltagi and Mohamed 2013), and Matricaria chamomilla L. plants (Farzadfar et al. 2013).
Boehmeria nivea (L.) Gaudich. (ramie) is a widely distributed textile crop in China, Vietnam, and Laos. The previous studies proposed that ramie is a promising species for Cd remediation on account of its large biomass and fast growth rate (Wang et al. 2008; Xie et al. 2015). However, excess amount of Cd in the environment can interfere with numerous plant physiological processes such as plant growth and development, the photosynthesis, and some other metabolic processes. Parts of these harmful effects caused by Cd seem related to the oxidative stress and the membrane damages in the plants (Li et al. 2016; Tran and Popova 2013). Thus, the abilities to promote plant growth, enhance photosynthesis rate, and improve antioxidant capacity are closely linked with the increased tolerance of Cd in the plants. As mentioned above, the phytoremediation efficiency could be enhanced by the addition of exogenous substances. In our earlier studies, the effects of exogenous nitric oxide, selenium and silicon, citric and oxalic acids on Cd-induced toxicities in ramie were investigated (Li et al. 2014; Tang et al. 2015; Wang et al. 2015a). The coworkers found that the supplementation of exogenous substances could improve the tolerance of Cd in ramie by enhancing the antioxidative capacity and alleviating the other Cd-induced deleterious effects. However, little information is available on the mechanisms of metal-induced tolerance of ramie in the presence of Ca and Spd. Therefore, the aim of this study was to evaluate the combination effects of exogenous Ca and Spd on Cd-induced toxicity in ramie and to gather more information on the relevant mechanisms involved. Cd contents in different parts of ramie, plants dry weights (DW), chlorophylls contents and the activities of total superoxide dismutase (SOD) and guaiacol peroxidase (POD) in ramie leaves were determined. In addition, the concentrations of hydrogen peroxide (H2O2), malondialdehyde (MDA), and vitamin E were also recorded. Hydroponics experiments were chosen because we were interested in the effects of Ca and Spd on the metal accumulation and transportation, the plant growth, and the Cd-induced adverse effects to plants, separating them from the influences of the complex soil environments. In addition, hydroponic solution is a useful research tool because it can provide a more suitable condition to achieve reliable and quick identification of the plants concerning its potential for heavy metal tolerance (Sanita di Toppi and Gabbrielli 1999).
Materials and methods
Plant materials and growth conditions
Boehmeria nivea (L.) Gaudich (ramie) seedlings obtained from the Agriculture University of Hunan, China, were acclimatized to 1/16 strength Hoagland nutrient solution for 2 weeks. The dilution ratio (1/16) of the Hoagland solution was based on the preexperiment, in which we used 1/4, 1/8, 1/16, and 1/32 strength Hoagland nutrient solution for plant cultivation. Through the determination of biomass, chlorophylls, proline, and the total protein contents, we found that 1/16 strength Hoagland nutrient solution was the most suitable solution for ramie growth. To avoid nutrition deficiency, the solution was renewed every 3 days with aeration. Plants were grown in a growth chamber at 25/20 °C day/night temperatures, 14 h photoperiod (from 6:00 a.m. to 8:00 p.m.) and 60 ± 5 % relative humidity.
After 2 weeks of acclimation, ramie seedlings were subjected to the following experiment nutrient solution in a completely randomized design: (1) control (Ck), (2) 45 μM Cd (Ck + Cd), (3) 45 μM Cd + 0.1 mM Spd (Cd + Spd), (4) 45 μM Cd + 1 mM Ca (Cd + Ca1), (5) 45 μM Cd + 1 mM Ca + 0.1 mM Spd (Cd + Ca1 + Spd), (6) 45 μM Cd + 5 mM Ca (Cd + Ca5), (7) 45 μM Cd + 5 mM Ca + 0.1 mM Spd (Cd + Ca5 + Spd). Cd was supplied with CdCl2, and Ca was added with CaCl2 in the solution. Spd was sprayed on the leaves at 8:00 and 20:00 each day, while distilled water was used for non-Spd treatment samples. The selected Cd and Spd concentrations were based on preliminary experiment results and related literatures (Zhou et al. 2015). After 7 days of incubation, the plants were collected. The aboveground and underground parts of ramie were collected separately and dried at 80 °C for 24 h to record the dry weight (DW).
Chlorophyll analysis
The chlorophyll content was measured according to the method described by Lichtenthaler (1987). Morphologically similar leaves were selected and the midribs were removed. The photosynthetic pigments were extracted with ethanol of 95 % in the dark. The contents of chlorophylls were determined spectrophotometrically.
Determination of Cd content
Plant roots were soaked in 20-mM EDTA for 10 min to get rid of the adhered ions. Plant samples were rinsed with deionized water and dried at 70 °C for 48 h. Then, the dried samples were ground. Cd contents of leaves, stems, and roots in ramie were determined by an atomic absorption spectrometer (Analyst 300, Perkin Elmer, Germany) after digesting the grinded samples with HNO3–HClO4 (3:1). The translocation factor (TF) is defined as the ratio of Cd in the aboveground part to that in roots. It was calculated by the method of Kováčik (2013) with some modification:
Estimation of oxidative stress and antioxidant contents
H2O2 content was measured according to the method described by Velikova et al. (2000). Leaf tissue (0.2 g) was grinded with 3-mL 0.1 % (w/v) trichloroacetic acid (TCA) in an ice-bath and then centrifuged at 12,000 rpm for 15 min. Phosphate buffer (0.5 mL) (pH 7.0) and 1-mL KI (1 M) was added to the supernatant. H2O2 content was calculated using 0.28 μM−1 cm−1 as the extinction coefficient and the amount was expressed as μmol g−1 FW. The absorbance was read at 390 nm.
MDA content was measured according to the thiobarbituric acid (TBA) method described by Chaoui et al. (1997). Leaf tissue (0.2 g) was homogenized in 10-mL 10 % (w/v) TCA. The homogenate was centrifuged at 3000 rpm for 10 min. Then, 2-mL supernatant was mixed with 2 mL of 10 % TCA containing 0.5 % TBA. The mixture was heated at 95 °C for 30 min and then cooled rapidly in ice-bath. The content of MDA was determined at the wavelength of 532, 600, and 450 nm.
Vitamin E content and the activities of antioxidant enzymes (SOD, POD) were determined using commercial reagent kits purchased from Nanjing JianCheng Bioengineering Institute, China.
Vitamin E is a natural fat-soluble antioxidant, which plays a great part in scavenging the singlet oxygen and the superoxide anion free radical and limiting the extent of lipid peroxidation in plants. The estimation mechanism is based on the reduction of Fe3+ to Fe2+ in the presence of tocopherols. The content of vitamin E could be calculated via colorimetric assay for the colored complex produced by Fe2+ and bathophenanthroline.
For the extraction of enzymes, fresh leaves (0.2 g) were cut into pieces and then homogenized in 0.1-M phosphate buffer (pH 7.0). The homogenates were centrifuged for 10 min at 3500 rpm, and then the supernatant obtained was used for enzymes activities assays. All enzymatic extractions were carried out at 4 °C. The measured SOD was the total superoxide dismutase, which comprised of three isozymes, a CuZn-SOD, a Fe-SOD, and a Mn-SOD. The commercial kit used for SOD activity determination was based on the xanthine oxidase method, while the estimation mechanism of POD activity was measured according to the guaiacol oxidation in the presence of H2O2.
Statistical analysis
Data are presented as mean ± standard error (S.E.) of three replicates. Statistical analyses were performed by one-way analysis of variance (ANOVA). Graphical works were conducted by Origin 9.1. Duncan’s test was employed to determine the statistical significance at a probability level of P < 0.05.
Results and discussion
Effects of Ca and Spd on the plant growth
As seen in Fig. 1, plant growth was negatively affected by the application of Cd. The aboveground and underground DW of ramie in Cd treatment decreased by 24 and 28 % respectively as compared with the control groups. It is common that heavy metals affected negatively on plant growth since they could influence plant metabolism and cell divisions. The application of Cd and 1-mM Ca produced a synergistic effect on the plant growth inhibition. However, the aboveground and underground DW of ramie treated with 5-mM Ca/Cd increased by almost 14 and 7 % respectively, as compared to those plants treated with Cd alone. It has been demonstrated that Cd-induced growth inhibitions could be alleviated by the addition of exogenous Ca through effectively enhancing the mitotic index and reducing the rate of chromosomal aberration (Shi et al. 2014). Besides, the foliar spray with Spd appears to have little impact on plant growth under the treatment of Cd alone or in combination with 5-mM Ca. While, plants treated with 1-mM Ca/Spd/Cd produced significant increments on the aboveground DW compared to the ramie exposed to 1-mM Ca/Cd. It has been reported that Pas are involved in various biochemical and physiological processes related to plant growth and development (Walden et al. 1997), which could explain the prevention role of Spd in Cd-induced plant growth inhibition.
Effects of Cd, Spd, and Ca treatment on the aboveground (a) and underground (b) dry weight (DW) of Boehmeria nivea (L.) Gaudich. Data are means ± standard error. Different letters above the bars indicate significant difference (P < 0.05). Ck, Ck + Cd, Cd + Spd, Cd + Ca1, Cd + Ca1 + Spd, Cd + Ca5, and Cd + Ca5 + Spd correspond to control; 45 μM Cd, 45 μM Cd + 0.1 mM Spd, 45 μM Cd + 1 mM Ca, 45 μM Cd + 1 mM Ca + 0.1 mM Spd, 45 μM Cd + 5 mM Ca, and 45 μM Cd + 5 mM Ca + 0.1 mM Spd, respectively
Effects of Ca and Spd on Cd uptake and translocation
The effects of Ca and Spd on Cd uptake and translocation in ramie were shown in Fig. 2. Cd was mainly retained in the roots due to the protective reaction of the aerial part. Different concentrations of Ca showed varied changes of Cd contents in ramie. All concentrations of Ca application reduced the Cd content in roots. The 1 mM Ca/Cd treatment was notably effective for Cd uptake in the aboveground part, which increased the Cd concentration in steams by almost 270 % and in leaves by 314 % compared to those plants treated only with Cd. That probably is one of the reasons why plants treated with 1-mM Ca and Cd had much less DW than those plants treated only with Cd. The performance of Ca displayed obvious effect on Cd translocation. Exogenous Ca application improved the TF of Cd in ramie whether with 1-mM or 5-mM Ca, which suggested that Ca is beneficial to Cd translocation from roots to the aboveground parts. The increased TF of Cd is beneficial to Cd phytoremediation, since Cd retained in roots is easy to release back into the environment.
Effects of Cd, Spd, and Ca treatment on Cd concentrations of root (a), stem (b), leaf (c), and the translocation factor (d) in Boehmeria nivea (L.) Gaudich. Data are means ± standard error. Different letters (a, b, c, d, e, f) above the bars indicate significant difference (P < 0.05). Ck, Ck + Cd, Cd + Spd, Cd + Ca1, Cd + Ca1 + Spd, Cd + Ca5, and Cd + Ca5 + Spd correspond to control; 45 μM Cd, 45 μM Cd + 0.1 mM Spd, 45 μM Cd + 1 mM Ca, 45 μM Cd + 1 mM Ca + 0.1 mM Spd, 45 μM Cd + 5 mM Ca, and 45 μM Cd + 5 mM Ca + 0.1 mM Spd, respectively
There was little reducing on root Cd concentration in 1 mM Ca/Spd/Cd treatment as compared to 1 mM Ca/Cd treatment, which was probably due to the protection role of Spd against Cd toxicity. The effects of Spd on Cd uptake and translocation of ramie in other treatments, either with Cd or in combination with Ca, was negligible.
There is no special transport channel for non-essential element, such as Cd, within plants. Non-essential metal elements were transported into plants via transporters and channels for essential elements such as Ca and K (Clemens et al. 1998). The physicochemical similarities of Cd and Ca trigger the competition between these two ions for Ca-binding proteins and transporters (Rodríguez-Serrano et al. 2009). In this study, the promotion effects on Cd absorption and translocation were the most obvious in the presence of 1 mM Ca/Cd treatment. Low level of Ca induced hormesis in plants, which promoted the absorption of Ca by stimulating the activities of Ca transporters and channels in ramie. This, in turn, may also stimulate the Cd uptake and translocation in the plants. Similarly, Cho et al. (2012) demonstrated that low level of Ca treatment increased Cd contents and amplified Cd toxicity in rice seedlings. However, 5 mM of Ca was sufficient to counterbalance with the hormesis. Thus, the competition between these two ions caused a decline in the uptake of Cd in ramie roots and leaves. To better justify the relationship of these two elements, further studies on blocking and stimulating Ca channels in order to corroborate the way of Cd entrance into the plants should be conducted.
Suitable plants for phytoremediation purposes are called hyperaccumulators (López et al. 2007). So far, there are several criteria for the classification of Cd-hyperaccumulating species (Pollard et al. 2014). Ramie growth under natural conditions cannot be considered as a Cd-hyperaccumulator species. However, the treatment of 1-mM Ca/Cd satisfied at least two criteria and made ramie a Cd-hyperaccumulating plant: (i) a plant must accumulate 0.1 % of Cd in its aboveground tissue (Liu et al. 2015), and (ii) the Cd concentrations in the stems and leaves of plants need to be greater than the metal concentrations in roots (Lin et al. 2014). The results demonstrated that non-Cd-hyperaccumulator plants could hyperaccumulate Cd by treating with certain concentrations of Ca, which was beneficial to the improvement of phytoremediation efficiency.
The role of Ca and Spd on plant photosynthesis
Data of the total chlorophyll contents are shown in Table 1. Photosynthesis inhibition is a well-documented response of plants to the toxic metal ions (Deng et al. 2014; Ouyang et al. 2012; Zhang et al. 2015). In the present study, Cd significantly reduced the total chlorophyll contents in ramie leaves. The application of 5-mM Ca remitted the decline with a 15 % increase of the chlorophyll contents compared to that of the Cd treatment. It has been confirmed that appropriate concentrations of Ca played a virtual role in the stabilization of chlorophylls and in the maintenance of sufficient photochemical efficiency of photosystem II (PSII) in plants (Hochmal et al. 2015; Ramalho et al. 1995). Foliar spray with Spd increased chlorophyll contents by 16 % as compared to that of Cd treatment, by 27.7 % as compared to that of 1-mM Ca/Cd treatment, and by 4.4 % as compared to that of 5-mM Ca/Cd treatment, respectively. The results demonstrated that Spd could alleviate Cd-induced photosynthesis inhibition by increasing the contents of chlorophylls. Researches have proclaimed that Spd could regulate the structures and functions of the photosynthetic apparatus through its interaction with the thylakoid membrane (Yiu et al. 2009), which might be related to the increase of chlorophyll contents in plants. These results also indicated that the combination effects of Spd and 1-mM Ca was superior to other combined treatment in restoring Cd-induced chlorophyll contents decline.
Effects of Ca and Spd on plant oxidative damages
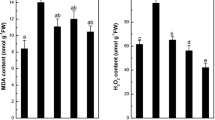
Plant oxidative damages could be quantified by determining the content of MDA and H2O2 (as shown in Fig. 3). MDA is a cytotoxic decomposition product of polyunsaturated fatty acids (PUFAs), which is typically used as a biochemical marker of membrane lipid peroxidation induced by heavy metals in plants (Chaoui et al. 1997). Cd application enhanced the MDA content of ramie. The addition of 5 mM Ca to cultivate solutions significantly relieved this upward trend caused by Cd. The decreased content of MDA may be resulted from the protecting role of Ca in the control of the stability and integrity of the membranes (Hirschi 2004). However, the treatment of 1-mM Ca/Cd even improved MDA content by approximately 25 % compared to that of the Cd treatment alone, which is probably owed to the increased uptake of Cd in this treatment. In addition, foliar spraying with Spd markedly decreased MDA content of the 1-mM Ca/Cd treatment group. The lower content of MDA in ramie with the application of Spd revealed that Spd can alleviate the membrane lipid peroxidation caused by Cd. It has been postulated that Spd interacted with negatively charged components of the membranes, thus regulating membrane permeability and resulting in the integrity and stabilization of the membranes, which could be responsible for the reduced MDA level caused by Spd. Besides, the effects of Spd on MDA content were smaller or not significant in other treatments, either with Cd or in combination with 5-mM Ca.
Effects of Cd, Spd, and Ca treatment on MDA (a) and H2O2 (b) concentrations in Boehmeria nivea (L.) Gaudich. Data are means ± standard error. Different letters above the bars indicate significant difference (P < 0.05). Ck, Ck + Cd, Cd + Spd, Cd + Ca1, Cd + Ca1 + Spd, Cd + Ca5, and Cd + Ca5 + Spd correspond to control; 45 μM Cd, 45 μM Cd + 0.1 mM Spd, 45 μM Cd + 1 mM Ca, 45 μM Cd + 1 mM Ca + 0.1 mM Spd, 45 μM Cd + 5 mM Ca, and 45 μM Cd + 5 mM Ca + 0.1 mM Spd, respectively
A growing body of researches indicated that there exists a correlation between the increase H2O2 content and the level of membrane lipid peroxidation in plants (Talukdar 2012). The present studies found that these two parameters (H2O2 and MDA) responded in a similar manner to Cd stress in ramie. H2O2 is a kind of reactive oxygen species (ROS), which could cause oxidative injury to proteins, DNA, and lipids (Apel and Hirt 2004). Figure 3 shows that the concentration of H2O2 in ramie exposed to Cd was significantly higher compared to that of the control plants. It confirmed that 45-μM Cd treatments induced oxidative stress to ramie. The addition of 5-mM Ca to the hydroponics medium containing Cd reduced H2O2 content by 29 %. The lower H2O2 level is likely related to the protection role of Ca against the oxidative stress produced by Cd. These are in consistent with some previous studies, which have also reported that Ca could negatively influence the H2O2 contents of plants under heavy metal stresses (Farzadfar et al. 2013; Talukdar 2012). However, H2O2 contents in leaves of ramie showed a remarkable enhancement when added with 1-mM Ca to Cd-contained culture media, but decreased when ramie was exposed to 1-mM Ca/Spd/Cd treatments. It has been documented that Spd can protect plants against oxidative stress through acting directly as free radical scavengers (Bors et al. 1989), which could be responsible for the declined H2O2 contents in ramie.
In normal conditions, ROS are continuously produced in plant as the by-products of aerobic metabolism processes, such as respiration and photosynthesis. The proper level of ROS act as signaling molecules controlling various plant physiological and biochemical processes related to the stomata behavior and pathogen defense (Kärkönen and Kuchitsu 2015; Lehmann et al. 2015). However, excess level of ROS react with a large variety of biomolecules and cause oxidative damages to proteins, DNA, and lipids, thus leading to tissue necrosis and programmed cell death in plants (Apel and Hirt 2004). The above results indicated that Cd induced oxidative damages in ramie through the upregulation of the H2O2 and MDA contents. With the purpose of protecting plants against oxidative damages and scavenging the overproduction of ROS, plants have developed a series of antioxidant mechanisms, which are involved in various enzyme and non-enzyme antioxidants (Mittler 2002).
In the present study, three representative antioxidants (vitamin E, SOD, and POD) were chosen to evaluate the function of Ca and Spd in the regulation of plant antioxidation upon Cd-stress (Table 1). The data showed that the vitamin E contents of ramie exposed to Cd were significantly reduced compared to control plants. The addition of 5-mM Ca significantly increased its contents by 16.88 %. Vitamin E is a group of lipid soluble chain-breaking antioxidants, which protects plants from oxidative stress by controlling peroxidation and eliminating or deactivating the free radicals (Caretto et al. 2002). The enhanced vitamin E level implied the antioxidative role of Ca in ramie. Whereas, when ramie seedlings were submitted to Cd, obvious decrease in the concentrations of vitamin E was observed with the addition of 1 mM Ca, while this decline was restored by the spraying with Spd. It has also been revealed that Spd played as a signaling regulator in stress signaling pathways by upregulating the expression of various stress-related genes in plants (Kasukabe et al. 2004), which may be related to the Spd-triggered changes of the antioxidant contents in ramie. Nevertheless, the addition of Spd to other treatments did not produce significant increment of vitamin E content compared to the plants treated with Cd alone and to the plants exposed to 5-mM Ca/Cd. As can be seen from Table 1, the activities of SOD and POD responded in a similar manner in all the treatments. Cd treatment increased SOD and POD activities by 95 and 49 %, respectively, compared to the control group. The application of 1-mM Ca amplified this increase. However, this increase could be counteracted by spraying Spd with almost 18 % reduction in SOD activities and 19 % reduction in POD activities. The addition of 5-mM Ca alone slowed down the Cd-induced upward trend of these two enzymes, while the combined application of Spd and 5-mM Ca appear to have little effect on these enzyme activities. SOD catalyzed superoxide radicals into H2O2, and subsequently, H2O2 can be degraded by POD (Mittler 2002). Plants survive from oxidative damages by modulating the activities of these enzymes. The enhanced SOD and POD activities indicated that Cd and 1 mM Ca/Cd treatment provoked the active oxygen removal system in plant. Numerous studies have documented that a certain concentration of Cd in plants contributed to the activation of enzyme activities of SOD and POD with the excessive accumulation of superoxide radical (O2 .−) and H2O2 (Hakimi et al. 2014; Hamilton et al. 2015; Wang et al. 2015b). The results also demonstrated that 1-mM Ca/Cd treatment caused more severe oxidative stress in ramie compared to the only Cd treatment with higher activities of SOD and POD. However, the 1-mM Ca/Spd/Cd and 5-mM Ca/Cd treatment resulted in a decline of SOD and POD activities in ramie when compared to the 1-mM Ca/Cd and the only Cd treatment group. This might be attributed to that the exogenous addition of Spd or 5-mM Ca strengthened the scavenging process of ROS and alleviated the oxidative stress incurred by Cd.
The foliar spraying with Spd only significantly mitigated Cd-induced oxidative damages under the treatment with 1-mM Ca and Cd. The protective effects of Spd seem to be triggered by certain concentrations of Ca, thus the only Spd and Cd treatment did not significantly mitigate Cd-induced oxidative stress. It was also probably due to the fact that the oxidative damages caused by Cd were already alleviated by the application of 5-mM Ca, and therefore the mitigation effects by exogenous Spd were not effective in the 5-mM Ca/Spd/Cd treatment.
Conclusions
Ramie is a potential candidate for Cd phytoremediation because it can accumulate high levels of Cd. On the other hand, Cd stress triggered several physiological responses, such as the stunted plant growth, reduced chlorophyll contents, increase MDA and H2O2 levels, and the modulated antioxidative enzyme activities in ramie. The application of 5-mM Ca alleviated Cd-induced toxicities by positively regulating the above responses. However, 1-mM Ca treatment increased the uptake and the transportation of Cd from ramie roots to the aboveground parts. Though it aggravated Cd toxicity, this toxicity could be mitigated by the foliar spraying with Spd. The results indicated that the combined application of 1-mM Ca and Spd could be considered as a feasible technique for the improvement of Cd accumulation in the aboveground parts of non-hyperaccumulator species. However, further studies are still required in order to gain insights into the exact mechanisms of the exogenous substance application on the enhancement of phytoremediation efficiency.
References
Adki V, Jadhav J, Bapat V (2014) At the cross roads of environmental pollutants and phytoremediation: a promising bio remedial approach. J Plant Biochem Biotechnol 23:125–140
Andreolli M, Lampis S, Poli M, Gullner G, Biró B, Vallini G (2013) Endophytic Burkholderia fungorum DBT1 can improve phytoremediation efficiency of polycyclic aromatic hydrocarbons. Chemosphere 92:688–694
Ansari MM, Neha KHA (2015) Effect of cadmium chloride exposure during the induction of collagen induced arthritis. Chem-Biol Interact 238:55–65
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manage 105:103–20
Bors W, Langebartels C, Michel C, Sandermann H Jr (1989) Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry 28:1589–1595
Cai G, Sobieszczuk-Nowicka E, Aloisi I, Fattorini L, Serafini-Fracassini D, Del Duca S (2015) Polyamines are common players in different facets of plant programmed cell death. Amino Acids 47:27–44
Caretto S, Paradiso A, D’Amico L, De Gara L (2002) Ascorbate and glutathione metabolism in two sunflower cell lines of differing α-tocopherol biosynthetic capability. Plant Physiol Bioch 40:509–513
Cassier-Chauvat C, Chauvat F (2015) Responses to oxidative and heavy metal stresses in Cyanobacteria: recent advances. Int J Mol Sci 16:871–886
Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Chaudhry Q, Schröder P, Werck-Reichhart D, Grajek W, Marecik R (2002) Prospects and limitations of phytoremediation for the removal of persistent pesticides in the environment. Environ Sci Pollut R 9:4–17
Chen B, Zhang Y, Rafiq MT, Khan KY, Pan F, Yang X, Feng Y (2014) Improvement of cadmium uptake and accumulation in Sedum alfredii by endophytic bacteria Sphingomonas SaMR12: effects on plant growth and root exudates. Chemosphere 117:367–373
Cho S-C, Chao Y-Y, Kao CH (2012) Calcium deficiency increases Cd toxicity and Ca is required for heat-shock induced Cd tolerance in rice seedlings. J Plant Physiol 169:892–898
Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI (1998) The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc Natl Acad Sci U S A 95:12043–12048
Deng G, Li M, Li H, Yin L, Li W (2014) Exposure to cadmium causes declines in growth and photosynthesis in the endangered aquatic fern (Ceratopteris pteridoides). Aquat Bot 112:23–32
El-Beltagi HS, Mohamed HI (2013) Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Not Bot Horti Agrobo 41:157–168
Fagerberg B, Barregard L, Sallsten G, Forsgard N, Östling G, Persson M, Borné Y, Engström G, Hedblad B (2015) Cadmium exposure and atherosclerotic carotid plaques—results from the Malmö diet and Cancer study. Environ Res 136:67–74
Fan T, Liu Y, Feng B, Zeng G, Yang C, Zhou M, Zhou H, Tan Z, Wang X (2008) Biosorption of cadmium(II), zinc(II) and lead(II) by Penicillium simplicissimum: Isotherms, kinetics and thermodynamics. J Hazard Mater 160:655–661
Farid M, Ali S, Ishaque W, Shakoor MB, Niazi NK, Bibi I, Dawood M, Gill RA, Abbas F (2015) Exogenous application of ethylenediamminetetraacetic acid enhanced phytoremediation of cadmium by Brassica napus L. Int J Environ Sci Te 12:3981–3992
Farzadfar S, Zarinkamar F, Modarres-Sanavy SAM, Hojati M (2013) Exogenously applied calcium alleviates cadmium toxicity in Matricaria chamomilla L. plants. Environ Sci Pollut R 20:1–10
Feng Y, Gong JL, Zeng GM, Niu QY, Zhang HY, Niu CG, Deng JH, Yan M (2010) Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem Eng J 162:487–494
Ghosh M, Singh S (2005) A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J Energy Environ 6:18
Hakimi L, Matinizadeh M, Shirvani A, Khalighi A (2014) Superoxide dismutase and peroxidase activities under lead and cadmium stresses in Berberis integerrima and Cercis siliquasrum. Adv Biores 5:20–23
Hamilton M, Esposito C, Malin M, R Cusumano L, Botton M (2015) Effects of copper and cadmium on development and superoxide dismutase levels in horseshoe crab (Limulus polyphemus) embryos. Springerplus 4:1–11
Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 136:2438–2442
Hochmal AK, Schulze S, Trompelt K, Hippler M (2015) Calcium-dependent regulation of photosynthesis. BBA-Bioenergetics 1847:993–1003
Hong Y, Yan S (2015) Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int J Mol Sci 16:1484–1494
Kärkönen A, Kuchitsu K (2015) Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112:22–32
Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Koptsik GN (2014) Problems and prospects concerning the phytoremediation of heavy metal polluted soils: a review. Eurasian Soil Sci 47:923–939
Kováčik J (2013) Hyperaccumulation of cadmium in Matricaria chamomilla: a never-ending story? Acta Physiol Plant 35:1721–1725
Lehmann S, Serrano M, L’Haridon F, Tjamos SE, Metraux JP (2015) Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 112:54–62
Li H, Liu Y, Zeng G, Zhou L, Wang X, Wang Y, Wang C, Hu X, Xu W (2014) Enhanced efficiency of cadmium removal by Boehmeria nivea (L.) Gaud. in the presence of exogenous citric and oxalic acids. J Environ Sci-China 26:2508–2516
Li X, Zhou Q, Sun X, Ren W (2016) Effects of cadmium on uptake and translocation of nutrient elements in different welsh onion (Allium fistulosum L.) cultivars. Food Chem 194:101–110
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Lin L, Ning B, Liao M, Ren Y, Wang Z, Liu Y, Cheng J, Luo L (2014) Youngia erythrocarpa, a newly discovered cadmium hyperaccumulator plant. Environ Monit Assess 187:1–7
Liu Z, Chen W, He X (2015) Influence of Cd2+ on growth and chlorophyll fluorescence in a hyperaccumulator: Lonicera japonica Thunb. J Plant Growth Regul 34:672–676
López ML, Peralta-Videa JR, Benitez T, Gardea-Torresdey JL (2005) Enhancement of lead uptake by alfalfa (Medicago sativa) using EDTA and a plant growth promoter. Chemosphere 61:595–598
López ML, Peralta-videa JR, Parsons JG, Benitez T, Gardea-Torresdey JL (2007) Gibberellic acid, kinetin, and the mixture indole—3-acetic acid—kinetin assisted with EDTA-induced lead hyperaccumulation in alfalfa plants. Environ Sci Technol 41:8165–8170
López‐Climent MF, Arbona V, Pérez‐Clemente RM, Zandalinas SI, Gómez‐Cadenas A (2014) Effect of cadmium and calcium treatments on phytochelatin and glutathione levels in citrus plants. Plant Biol 16:79–87
Markowska-Kozak E (2005) Spermidine as protector against cadmium-induced damages in winter rape seedlings. Acta Physiol Plant 17:67–68
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Ouyang H, Kong X, He W, Qin N, He Q, Wang Y, Wang R, Xu F (2012) Effects of five heavy metals at sub-lethal concentrations on the growth and photosynthesis of Chlorella vulgaris. Chinese Sci Bull 57:3363–3370
Pollard AJ, Reeves RD, Baker AJM (2014) Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci 217–218:8–17
Ramalho JC, Rebelo M, Santos ME, Antunes ML, Nunes MA (1995) Effects of calcium deficiency on Coffea arabica. Nutrient changes and correlation of calcium levels with some photosynthetic parameters. Plant Soil 172:87–96
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–81
Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, Luis A, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Shi HP, Zhu YF, Wang YL, Tsang PKE (2014) Effect of cadmium on cytogenetic toxicity in hairy roots of Wedelia trilobata L. and their alleviation by exogenous CaCl2. Environ Sci Pollut R 21:1436–1443
Singh A, Prasad SM (2014) Remediation of heavy metal contaminated ecosystem: an overview on technology advancement. Int J Environ Sci Te 12:353–366
Talukdar D (2012) Exogenous calcium alleviates the impact of cadmium-induced oxidative stress in Lens culinaris Medic. seedlings through modulation of antioxidant enzyme activities. J Crop Sci Biotechnol 15:325–334
Tang H, Liu Y, Gong X, Zeng G, Zheng B, Wang D, Sun Z, Zhou L, Zeng X (2015) Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ Sci Pollut R 22:9999–10008
Tiburcio A, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta 240:1–18
Tran TA, Popova LP (2013) Functions and toxicity of cadmium in plants: recent advances and future prospects. Turk J Bot 37:1–13
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Walden R, Alexandra C, Tiburcio AF (1997) Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol 113:1009–1013
Wang X, Liu Y, Zeng G, Chai L, Song X, Min Z, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62:389–395
Wang D, Liu Y, Tan X, Liu H, Zeng G, Hu X, Jian H, Gu Y (2015a) Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea (L.) Gaud under cadmium stress. Environ Sci Pollut R 22:3489–3497
Wang J, Zhang H, Zhang T, Zhang R, Liu R, Chen Y (2015b) Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int J Biol Macromol 77:59–67
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot-London 92:487–511
Xie J, Liu Y, Zeng G, Liu H, Zheng B, Tang H, Xu W, Sun Z, Tan X, Nie J, Jiang Z, Gan C, Wang S (2015) The effects of P. aeruginosa ATCC 9027 and NTA on phytoextraction of Cd by ramie (Boehmeria nivea (L.) Gaud). RSC Adv 5:67509–67517
Xu Q, Shi G, Wang H, Yang H, Zhao J, Xu Y (2008) Roles of exogenous spermidine in improving Salvinia natans tolerance towards cadmium stress. Ying Yong Sheng Tai Xue Bao 19:2521–2526 (in Chinese)
Yang H, Shi G, Li W, Wu W (2013) Exogenous spermidine enhances Hydrocharis dubia cadmium tolerance. Russ J Plant Physl 60:770–775
Yiu JC, Liu CW, Fang DYT, Lai YS (2009) Waterlogging tolerance of Welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiol Bioch 47:710–716
Zaier H, Ghnaya T, Ghabriche R, Chmingui W, Lakhdar A, Lutts S, Abdelly C (2014) EDTA-enhanced phytoremediation of lead-contaminated soil by the halophyte Sesuvium portulacastrum. Environ Sci Pollut R 21:7607–7615
Zhang Y, Xu S, Yang S, Chen Y (2015) Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma 252:911–924
Zhou L, Liu Y, Hu X, Zeng G, Wang Y, Hu X, Zhou Y, Tan X, Jiang L, Zeng X (2015) Time-dependent antioxidative responses of ramie (Boehmeria nivea (L.) Gaudich) to moderate cadmium stress and its up-regulation mechanism by spermidine antioxidant. RSC Adv 5:76141–76149
Acknowledgments
This work was supported by the Program for the National Natural Science Foundation of China (41271332, 51278176, 51408206, 51579098, and 51521006), the Fundamental Research Funds for the Central Universities, the Program for New Century Excellent Talents in University (NCET-13-0186), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13R17), and the Scientific Research Fund of Hunan Provincial Education Department (No. 521293050).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Gong, X., Liu, Y., Huang, D. et al. Effects of exogenous calcium and spermidine on cadmium stress moderation and metal accumulation in Boehmeria nivea (L.) Gaudich. Environ Sci Pollut Res 23, 8699–8708 (2016). https://doi.org/10.1007/s11356-016-6122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6122-6