Abstract
The low bioavailability of Pb and low number of Pb-tolerant plant species represent an important limitation for Pb phytoextraction. It was recently suggested that halophyte plant species may be a promising material for this purpose, especially in polluted salt areas while Pb mobility may be improved by synthetic chelating agents. This study aims to evaluate Pb extraction by the halophyte Sesuvium portulacastrum in relation to the impact of EDTA application. Seedling were cultivated during 60 days on Pb artificially contaminated soil (200, 400, and 800 ppm Pb) in the presence or in the absence of EDTA (3 g kg−1 soil). Results showed that upon to 400 ppm, Pb had no impact on plant growth. However, exogenous Pb induce a decrease in shoot K+ while it increased shoot Mg2+ and had no impact on shoot Ca2+ concentrations. Lead concentration in the shoots increased with increasing external Pb doses reaching 1,390 ppm in the presence of 800 ppm lead in soil. EDTA addition had no effect on plant growth but strongly increased Pb accumulation in the shoot which increased from 1,390 ppm in the absence of EDTA to 3,772 ppm in EDTA-amended plants exposed to 800 ppm exogenous Pb. Both Pb absorption and translocation from roots to shoots were significantly enhanced by EDTA application, leading to an increase in the total amounts of extracted Pb per plant. These data suggest that S. portulacastrum is very promising species for decontamination of Pb2+-contaminated soil and that its phytoextraction potential was significantly enhanced by addition of EDTA to the polluted soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Discharge and disposal of waste products containing heavy metals have resulted in the contamination of soils and water with these pollutants (Min et al. 2003). Since heavy metals are toxic to biological systems and not biodegradable, they constitute a major environmental concern and need to be removed from the ecosystem. Lead (Pb) is one of the most toxic anthropogenic pollutants which have been released to the environment since the industrial revolution and it progressively accumulated in different terrestrial and aquatic ecosystems (Barrutia et al. 2010). Anthropogenic activities such as mining, smelting, burning of fossil fuels, dumping of municipal sewage sludge, and the manufacture of pesticides and fertilizers are the primary sources of lead dispersal (Chehregani et al. 2009). This element presents high potential risks to human health, but the clean-up of Pb-contaminated soil is one of the most difficult tasks for environmental engineering. Current Pb removal techniques from soils include essentially soil washing with chemicals, but cannot be widely used because they are ex situ-expensive approaches and produce large amounts of secondary wastes.
In recent years, several biological methods were established for reclamation of affected soils, including phytoremediation consisting in the use of plants for pollutant removal from contaminated sites. It appears as a cost effective and environmentally friendly alternative (Salla et al. 2011; Vettori et al. 2012). Researchers have shown that some plants have the ability to uptake high amounts of toxic heavy metals and thus may contribute to clean up contaminated water and soils (Yadav 2010; Ghaderian and Ghotbi Ravandi 2012). However, this process depends upon the availability of the metal in the media and the plant capacity to absorb these contaminants (Abou Auda et al. 2011). Lead presents a limited solubility in soil, and is generally not available for plant uptake due to complexation with organic matter; sorption on oxides and clays; or precipitation as carbonates, hydroxides, and phosphates (Cecchi et al. 2008; Vega et al. 2010). Lead contamination is frequently limited to the uppermost horizons of soil profiles (Shahid et al. 2012). Only a very minor fraction of the total soil Pb is in the soil solution, so the Pb concentration in plant shoots is relative low (Shahid et al. 2011). Hence, the two major limitations to Pb phytoremediation consist in its low bioavailability in soil and poor translocation from the roots to the shoots.
In order to enhance both the availability of Pb in soil and translocation from the roots to the shoots, synthetic chelating agents such as EDTA, diethylenetrinitrilopentaacetic acid (DTPA), nitrilotriacetic acid (NTA), pyridine-2,6-dicarboxylic acid (PDA), or ethylenediamine disuccinate (EDDS) have been recommended (De la Rosa et al. 2004; Ehsan et al. 2007; Andra et al. 2009; Lambrechts et al. 2011). Among the chelating agents used to extract metals from soils, EDTA is regarded as the most effective in solubilizing soil-bound Pb (Nascimento et al. 2006; Yukselen and Gokyay 2006). It has therefore been extensively used in soil decontamination technologies (Lasat et al. 2000). Moreover, beside dissolving Pb adsorbed to soil particles (Wu et al. 2010), EDTA has been shown to increase Pb movement to roots via mass flow or diffusion, enhance metal uptake, and trigger root to shoot translocation of heavy metals (Nascimento et al. 2006). The addition of EDTA into the soil thus significantly enhances lead translocation from the roots to the shoots and therefore increases lead accumulation in the harvestable above-ground parts in several plant species (Liu et al. 2008). Nevertheless, several studies have shown that the species used for assisted-phytoextraction of lead by this chelator are glycophytes such as maize, Brassica napus (Komárek et al. 2007; Zaier et al. 2010a). However, those plants are salt-sensitive glycophytes plant species and are therefore unable to survive in salt areas. Halophyte plant species are naturally able to cope with high salinity levels and are promising candidates for soil desalination by producing higher biomass concomitant to elevated salt concentration in harvestable parts (Debez et al. 2010). Recent studies also suggested that halophytes may be useful for phytoremediation of heavy metal-contaminated salty soils (Ghnaya et al. 2005, 2007). Yet, the use of this chelator on halophyte plants is not documented. The objectives of this research were to determine the tolerance and accumulation ability of the halophyte specie, Sesuvium portulacastrum, to Pb2+ and to explore EDTA usefulness for enhancing the phytoremediation of Pb-contaminated soil.
Material and methods
Soil sampling and characterization
The soil used in this study was collected from upper horizon (0–20 cm depth) from Borj-Cedria region (30 km north of Tunis). Samples were air dried at room temperature, followed by a 2-mm sieving. The clayey sandy soil was characterized by low level of organic matter and electric conductivity about 1 mmhos cm−1. The main characteristics of the soil are listed in Table 1. The pH was measured potentiometrically in 1 M KCl after 24 h in the water/soil ratio of 5/1 (v/w). Electrical conductivity (EC) and the percentage of organic carbon were determined with the method of Kalra and Maynard (1991). The total nitrogen (Nt) was determined by the Kjeldahl’s method. Available phosphorus was determined according to Egner et al. (1960). K+ and Na+ contents were determined in the same homogenate by flame spectrometry (Corning Photometer).
Plant material and pot experiment
S. portulacastrum L. (Aizoaceae), a dicotyledonous halophyte commonly known as sea purslane, was propagated by cutting. Three-centimeter long-stem segments with one node and two opposite leaves were taken from mother plants, cultivated in greenhouse on a mixture of sandy soil and loam, and irrigated with tap water. Cuttings were sterilizes for 5 min in saturated calcium hypochlorite solution, thoroughly rinsed with distilled water, and placed for 7 days in an aerated nutrient solution diluted 10 times, supplemented with Fe-EDTA and micronutrients according to Arnon and Hoagland (1940). Rhizogenesis took place after 1 week. The plants were then transferred and precultured for 1 week in a modified Hoagland nutrient solution containing 5 mM Ca(NO3)2, 5 mM KNO3, 1 mM KH2PO4, 50 lM H3BO3, 1 mM MgSO4, 4.5 μM MnCl2, 3.8 μM ZnSO4, 0.3 μM CuSO4, 0.1 mM (NH4)6Mo7O24, and 10 μM FeEDTA. Then the plants were transplanted and cultured in the selected soil contaminated artificially with different Pb concentrations of 0, 200, 400, and 800 ppm (added as lead nitrate).
Amendment treatments
Air-dried soil was placed in each plastic tanks (30 kg DW per tank). EDTA was added in a single application to the tanks at 3 g kg−1 soil. The tanks containing soil were divided into four experimental groups: (1) control with no amendment (CK), (2) plants were cultivated with EDTA, (3) plants were cultivated with different Pb concentrations (0, 200, 400, and 800 ppm, respectively), and (4) plants were cultivated with different Pb concentrations (0, 200, 400, and 800 ppm, respectively) + EDTA. EDTA was added to the irrigation solutions as ethylenediaminetetraacetic acid disodium salt in a single application to the soil surface at 3 g kg−1 soil.
Experiment was carried out under natural light and ambient temperature in order as to keep all plants under conditions as similar as possible to field conditions. The experimental design was completely randomized with eight replicates for each treatment. Plants were harvested 60 days after plant transplantation.
Plant harvest and analysis
Two harvests were performed, at the beginning of treatment and 60 days later. Shoots were harvested, successively rinsed three times with cold water, and blotted between two layers of filter paper. Roots were carefully removed from the substrate, dipped in a cold solution of HCl (0.01 M) during 5 min to eliminate heavy metals adsorbed at the root surface (Aldrich et al. 2003), then washed three times with cold distilled water and blotted dry with filter paper. The fresh weight (FW) was measured immediately, and the dry weight (DW) was estimated after 48 h of desiccation in an oven at 60 °C.
Cations concentration
Dried samples (c.a. 100 mg) were ground to a fine powder using a porcelain mortar and a pestle and digested in 4/1 (v/v) HNO3/HClO4 (20 ml) mixture at 100 °C. After total evaporation, 30 ml of 0.5 % HNO3 were added and Pb2+and Ca2+ concentrations were determined by atomic absorption spectrometry (Spectra AA 220 FS). Potassium concentration was determined by flame spectrometry (Corning Photometer).
Analysis of results
Relative growth rate
The relative growth rate (RGR) based on whole plant dry weight production, was calculated according to Hunt (1990) as RGR = (Ln (W 2) − Ln (W1))/(t 2 − t 1), where W is the dry matter at the beginning (W 1) and the end (W 2) of the 60-day treatment period, and (t 2 − t 1) is the duration of this period.
Bioconcentration factor
The Pb2+ uptake and accumulation were depicted by a bioconcentration factor (BCF), which provides an index of the plant’s ability to accumulate Pb2+ with respect to the concentration of this pollutant in the soil (Zayed et al. 1998). It is calculated as follows:BCF = Pb2+ concentration in dry shoots at the harvest/initial concentration of Pb2+ in soil.
Translocation factor
The translocation factor (TF) depicts the ability of the species to translocate the metal from roots to shoots (Ghnaya et al. 2007).
TF = Pb2+ in dry shoots (μg g−1)/Pb2+ in dry roots (μg g−1).
Statistical analysis
Analyses of variance (ANOVA) with orthogonal contrasts and mean comparison procedures were used to detect differences between treatments. Mean separation procedures were conducted using the multiple range tests with Fisher’s least significant difference (LSD; p < 0.05).
Results
Effects of treatments on plant growth
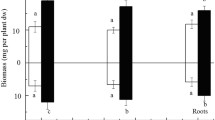
S. portulacastrum plant morphology was not significantly modified in the presence of Pb2+ as compared to control and the plant did not exhibit any toxicity symptom due to the presence of metal, even at the highest concentration (800 ppm). The measurement of plant dry weight in the absence of EDTA showed that, up to 400 ppm, lead did not significantly affect plant biomass production in S. portulacastrum (Fig. 1). The depressive effect of Pb2+ on whole-plant biomass production became significant at the highest concentration (800 ppm) only. In addition, the recorded reduction never exceeded 25.4 % as compared with control plants (Fig. 1).
Variation of plant biomass production in Sesuvium portulacastrum exposed for 60 days to various Pb(NO3)2 concentrations with and without addition of EDTA. Data are the means of eight replicates and vertical bars are standard errors. Values marked with the same letter are not significantly different at p = 0.05
With respect to the possible effect of EDTA on plant growth, results showed that the addition of EDTA (3 g/kg soil) 50 days after S. portulacastrum transplantation slightly, but significantly improved biomass production of plant cultivated at the highest Pb concentration (Fig. 1). Additionally, plant morphology was not affected by EDTA addition in the soil.
Lead accumulation
Low Pb2+ concentrations (not exceeding 5 ppm) were detected in control plants which could be due to the presence of impurities of salts used for irrigation solution or to the presence of pre-existing trace of Pb in non-amended soil (Fig. 2). Roots and shoots Pb2+ concentrations increased progressively with increasing Pb2+ supply to the soil. The shoot Pb2+ concentrations were higher than the root Pb2+ concentrations (1,390 and 1,208 ppm in shoots and roots respectively at 800 ppm Pb2+). The addition of EDTA significantly increased shoot Pb2+ concentrations comparatively to non-amended soil. For example, a threefold increased was noticed at the highest external dose (800 ppm Pb2+). Root Pb2+ accumulation was also enhanced by EDTA addition, although to a lower extent than in the shoots.
Changes in Pb2+ shoot and root concentrations (ppm) of S. portulacastrum exposed for 60 days to treated by various Pb(NO3)2 concentrations with and without addition of EDTA. Data are the means of eight replicates and vertical bars are standard errors. Bars marked with same letter are not significantly different at p = 0.05
The phytoextraction potential of plants could be estimated by the total amount of metal accumulated in the shoots, which represents the product of shoot biomass by metal concentration. This parameter was clearly enhanced by EDTA supply (Fig. 3). Indeed, at 800 ppm Pb2+, the total amount of lead accumulated in the shoots was 1,196 μg plant−1 in the absence of EDTA. The supply of EDTA increased this accumulation up to 2655 μg plant−1. In the same way, both the TF and BCF, used to evaluate the capacity of plants to absorb and to transport metal from the roots to the shoots, was significantly increased by the addition of EDTA (Table 2).
Changes in lead amounts (μg plant−1) in shoots of S. portulacastrum exposed for 60 days to various Pb(NO3)2 concentrations with and without addition of EDTA. For each treatment, this parameter is the product of Pb shoot concentrations (μg g−1 DW) by shoot DW (g plant−1). Data are the means of eight replicates and vertical bars are standard errors. Bars marked with same letter are not significantly different at p = 0.05
Ca2+, K+, and Mg2+ nutrition
The presence of Pb2+ in the soil led to reduction in the shoot K+ concentrations (Fig. 4). Such a decrease was proportional to external Pb2+ concentration. The strongest reduction was recorded in plants exposed to Pb2+ in combination with EDTA. At 800 ppm Pb2+, shoot K+ was reduced to 29 % compared to control, but in the presence of EDTA this reduction reached 48 %.
Changes in potassium concentration (mmol g−1 DW) in shoots of S. portulacastrum exposed for 60 days to various Pb(NO3)2 concentrations with and without addition of EDTA. Data are the means of eight replicates and vertical bars are standard errors. Bars marked with same letter are not significantly different at p = 0.05
The presence of Pb2+ alone did not significantly modify the shoot Ca2+ concentration (Fig. 5). In fact, slight reduction in Ca2+ shoots concentration was detected in plants subjected to lead in combination with EDTA. This decrease does not exceed 8 % in plant subjected to 800 ppm + EDTA as compared to control.
Changes in calcium concentration (mmol g−1 DW) in shoots of S. portulacastrum exposed for 60 days to Pb(NO3)2 concentrations with and without addition of EDTA. Data are the means of eight replicates and vertical bars are standard errors. Bars marked with same letter are not significantly different at p = 0.05
The shoot Mg2+ concentration was higher in plants treated with Pb2+ alone or in combination with EDTA than in untreated ones (Fig. 6). This effect becomes significant with higher doses of Pb2+.
Changes in magnesium concentration (mmol g−1 DW) in shoots of S. portulacastrum exposed for 60 days to various Pb(NO3)2 concentrations with and without addition of EDTA. Data are the means of eight replicates and vertical bars are standard errors. Bars marked with same letter are not significantly different at p = 0.05
Discussion
One of the main limits of phytoextraction is the low solubility and availability of heavy metal for root uptake. This is specially the case for lead which exhibits the lowest solubility among all heavy metals at classical soil pH. Application of EDTA has been proposed to increase Pb mobility in soil solution. Another problem concerns the identification of suitable plant species for Pb phytoextraction. In several cases, Pb contamination concerns saline depressions which represent site of industrial and urban rejections. The application of phytoextraction in saline soils, where conventional hyperaccumulators glycophytes species such Noccaea caerulescens, Brassica juncea, or Arabidopsis halleri cannot be used because these species lack mechanisms of NaCl tolerance. Identification of halophytes (salt-tolerant plants) accumulator species constitute the major prerequest for further rehabilitation of metals polluted saline soils. In this context, it has recently been suggested that salt-tolerant species may be more adapted to cope with heavy metals stresses than glycophytic plants (De la Rosa et al. 2004; Ghnaya et al. 2005). S. portulacastrum is one of the fast-growing halophyte specie which could be used for this purpose and was therefore tested for its potential to accumulate Pb2+ (Zaier et al. 2010b).
In the present study, we tested the Pb tolerance and accumulation ability by the halophyte species S. portulacastrum. We also assess EDTA efficiency for improvement of Pb2+ by this species. Our results indicated that S. portulacastrum plants do not exhibited any toxicity symptom such as chlorosis, necrosis, or significant growth reduction even when treated with 800 ppm Pb2+. This confirms its strong tolerance to Pb already showed under hydroponically conditions (Zaier et al. 2010b). To evaluate the impact of Pb on the growth activity during the treatment period, we measured the RGR, which is a recommended parameter to evaluate the specific effect of the constraints on the growth activity during treatment independently of the initial size of seedlings, especially for this plant multiplied by cutting (Ghnaya et al. 2005). The variation of the RGR values with the concentration of Pb accumulated in the shoots showed that the presence of Pb treatment alone or in combination with EDTA induced only a slight decrease in the growth rate (Fig. 7) despite a large shoot Pb2+ accumulation, especially in plants amended with EDTA. This, once again, confirms the high Pb tolerance in S. portulacastrum. With respect to the possible impact of EDTA on the plant activity, the same figure (Fig. 7) showed that the addition of this chelators had no impact on growth in the absence of Pb and alleviated the depressive effect of the metal when plants were subjected to different doses of Pb.
Relationship between the variations of the RGR values and Pb2+ concentration in the shoots of S. portulacastrum with and without addition of EDTA. RGR measures the quantity of biomass deposited by 1 g of biomass per unit of time. It was estimated as ∆ ln (∆ W)/∆t, where ∆W is the dry weight, ln stands for natural logarithm, and ∆ represents the difference between final and initial value (Hunt 1990)
In sensitive plants, lead and other toxic heavy metals interfere with essentials nutrients uptake and translocation, and adversely affect the acquisition of macro- and micronutrients in plants, thus leading to nutrients deficiencies (Sharma and Dubey 2005; Brunet et al. 2008). Potassium is an essential nutrient for plants growth and its absorption may be restricted by Pb2+ (Larbi et al. 2002). In our experimental conditions, S. portulacastrum showed a reduction in the shoot K+ concentration (Fig. 4), while Ca2+ concentration remained unaffected (Fig. 5) and Mg concentration clearly increased (Fig. 6). This result is consistent with several previous studies (Sharma and Dubey 2005; Zaier et al. 2010b). Since Pb2+ has no chemical similarity with K+, this suggest that it exerts an indirect effect on K+ uptake, probably by complexing ATP and reducing energy availability (Asp et al. 1994). In fact, K+ absorption is controlled by ATPases that hydrolyze ATP to provide energy for the transport of K+ in the interior of the cell root. It is undeniable that K+ is an important element for plant growth; however, opposite to glycophytes, halophytes are characterized by the ability to replace K+ by Na+ for specific functions, such as vacuolar osmotic equilibrium (Mühling and Läuchli 2002). So S. portulacastrum is able to express normal growth although the reduced K-shoot concentrations. With respect to the Pb effect on Ca and Mg nutrition, many previous studies showed reduction in calcium contents in the tissues of lead-exposed plants such as rye, maize, B. juncea, tomato, and mustard (Antosiewicz 2005; Zaier et al. 2010b). This effect was attributed to direct toxic effect of Pb through the inhibition of Ca2+ transporters at the root level (Wojas et al. 2007; Antosiewicz and Hennig 2004) and/or indirect effect by replacement of Ca2+ ions with Pb2+ ions due to the high affinity of the latter for Ca2+ binding sites on biological structures (White and Broadley 2003). In fact, these authors demonstrated that, due to the reduced specificity of some Ca transporters to Ca2+, theses transporters can mediate the absorption of homologues metallic cations as Pb2+ across root cellular membranes (White and Broadley 2003; Antosiewicz and Hennig 2004; Wojas et al. 2007). In our work, Fig. 5 showed that this species maintained Ca contents in the shoots in the presence of Pb alone. This could be explained by the possible capability of this plant to conserve the function of Ca transporters under Pb contamination. Mg shoot concentrations were enhanced in the presence of Pb in S. portulacastrum (Fig. 6). The same results were found by Kibria et al. (2009) in both Amaranthus gangeticus and Amaranthus oleracea and Zaier et al. (2010b) in B. juncea which is in favor of synergy between Pb2+ and Mg2+ for absorption and translocation.
In the presence of Pb2+ alone or in combination with EDTA, S. portulacastrum accumulated higher concentrations of Pb2+ in the shoots, reaching 1,389.8 and 3,771.3 ppm, respectively at 800 and 800 ppm combined with EDTA (Fig. 2). Lead hyperaccumulation is a rare phenomenon in Plant Kingdom (Baker et al. 1994). Only two species until now were reported to accumulate high lead concentration in their shoots—Noccaea rotundifolium (8,200 ppm) from a lead/zinc mining area of Cave del Predil, northern Italy (Reeves and Brooks 1983) and some populations of N. caerulescens (2,740 ppm) colonizing a lead mine district in Pennines, England (Shimwell and Laurie 1972). Considering the present study, without addition of EDTA and owing to its high potential of Pb concentration in the shoots (>1,000 ppm) without suffering from toxicity, S. portulacastrum could be classified as “Pb-accumulator” specie. Other argument supporting the capability of this halophyte plant species for Pb extraction is the high values of translocation factor. Hence, our results indicate that the concentrations of Pb2+ in the shoots (1,389.9 ppm) exceed those in the roots (1,208 ppm) at external lead dose of 800 ppm demonstrating an extraordinary capability of this specie to transport Pb in the xylem vessel. This experiment also showed that S. portulacastrum translocates Pb more efficiently when cultivated on 800 ppm Pb-polluted soils than when subjected to hydroponic medium containing 800 ppm Pb as we demonstrated in our previous work (Zaier et al. 2010b). In Aneurolepidium chinense, Gnaphalium polycaulon, and Medicogo sativa cultivated under 400 ppm, the shoot Pb concentration were 73.15, 82.61, and 389.61 ppm, respectively, but it was up to 864 ppm in S. portulacastrum which demonstrates the superiority of the halophyte species in this process.
On the other hand, considering the low bioavailability of Pb in soil, we suggested that increased mobility of Pb by synthetic chelators could enhance the potential of metal accumulation in the shoot. Such an “assisted-phytoextraction” was indeed reported to significantly enhance Pb translocation from roots to shoots by lowering ending of this element with cell walls (Barrutia et al. 2010; Saifullah et al. 2009; Ben Rejeb et al. 2013). Nevertheless, the applied concentration of the chelating agent must be appropriate and not toxic for plant growth and microbial biomass. In fact, some studies suggested that the addition of this synthetic chelator may have a significantly adverse effect on plant growth (Lai and Chen 2005; Quartacci et al. 2006; Ben Rejeb et al. 2013). In our study, 3 g kg−1 soil of EDTA did not significantly affect S. portulacastrum growth in the absence and in presence of different Pb concentrations in the soils. This suggests that this dose of EDTA has no deleterious impact on S. portulacastrum. Used EDTA concentration significantly enhanced shoots Pb concentrations in S. portulacastrum. Thus, beside its impact on Pb bioavailability for root absorption, EDTA seems to also promote Pb translocation from roots to shoots, as clearly demonstrated by the recorded increase in the TF values (Table 2). Compared to plants subjected to Pb alone, the Pb concentrations in shoots of S. portulacastrum increased from 460.47 to 881.53 ppm at 200 ppm of external Pb and from 1,389.84 to 3,771.28 ppm at 800 Pb following EDTA supply. The total amounts of extracted Pb were higher in the presence of EDTA because this chelator enhanced shoots lead concentration without adverse effects on plant shoot biomass production. This result was consistent with others studies showing that the enhancement of heavy metals availability in soils by the addition of EDTA improves metal phytoextraction (Liphadzi and Kirkham 2006; Van Engelen et al. 2007). In fact, Lai and Chen (2005) found that, in a soil artificially polluted with several heavy metals, EDTA application induced the accumulation of significant amounts of Cd2+ and Pb2+ in shoots of Dianthus chinensis. The BCF is a common index used to estimate plant’s ability to pump heavy metals from the substrate and to compare species for phytoextraction potentials. Hence, in our study, the BCF values were significantly enhanced by the addition of EDTA (Table 2). Thus, EDTA ameliorates both Pb absorption and translocation efficiencies in this species. It has been hypothesized that improved translocation may be related to the presence of more stable Pb-EDTA complex (Andra et al. 2009).
Conclusion
The halophyte specie, S. portulacastrum, exhibited a strong tolerance and accumulation ability for lead when cultivated on Pb-contaminated soil. The Pb2+ concentrations in plants were significantly increased as a consequence of EDTA application. The used concentration of EDTA (3 g kg−1 soil) has no negative impact on plant growth. This chelator significantly promoted Pb translocation from roots to shoots. Thus, S. portulacastrum may be suitable for the remediation of Pb-contaminated saline soil and its putative interest for this purpose may be higher in a global strategy of assisted phytoextraction.
References
Abou Auda M, Abu Zinada I, El Shakh AE (2011) Accumulation of heavy metals in crop plants from Gaza Strip, Palestine and study of the physiological parameters of spinach plants. J Ass Arab Uni Bas App Sci 10:21–27
Aldrich MV, Gardea-Torresdey JL, Peralta-Videa JR, Parsons JG (2003) Uptake and reduction of Cr(VI) to Cr(III) by mesquite (Prospis spp.): chromate-plant interaction in hydroponics and solid media studied using XAS. Environ Sci Technol 37:1859–1864
Andra SS, Datta R, Sarkar D, Saminathan SKM, Mullens CP, Bach SBH (2009) Analysis of phytochelatin complexes in the lead tolerant vetiver grass [Vetiveria zizanioides (L.)] using liquid chromatography and mass spectrometry. Environ Pollut 157:2173–2183
Antosiewicz DM (2005) Study of calcium-dependent lead-tolerance on plants differing in their level of Ca-deficiency tolerance. Environ Pollut 134:23–34
Antosiewicz DM, Hennig J (2004) Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environ Pollut 129:237–245
Arnon DI, Hoagland DR (1940) Crop production in artificial solutions and in soils with special reference to factors affecting yields and absorption of inorganic nutrients. Soil Sci 50:463–484
Asp H, Gussarsson M, Adalsteinson S, Lenseń P (1994) Control of potassium influx in roots of birch (Betulapendula) seedlings exposed to cadmium. J Exp Bot 45:1823–1827
Baker AJM, Reeves RD, Hajar ASM (1994) Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae). New Phytol 127:61–68
Barrutia O, Garbisu C, Hernandez-Allica J, Garcia-Plazaola JI, Becerril JM (2010) Differences in EDTA-assisted metal phytoextraction between metallicolous and non-metallicolous accessions of Rumex acetosa L. Environ Pollut 158:1710–1715
Ben Rejeb K, Ghnaya T, Zaier H, Benzarti M, Baioui R, Ghabriche R, Wali M, Lutts S, Abdelly C (2013) Evaluation of the Cd2+ phytoextraction potential in the xerohalophyte Salsola kali L. and the impact of EDTA on this process. Ecol Eng 60:309–315
Brunet J, Repellin A, Varrault G, Terryn N, Zuily-Fodi Y (2008) Lead accumulation in the roots of grass pea (Lathyrus sativus L.): a novel plant for phytoremediation systems? CR Biogeosciences 331:859–864
Cecchi M, Dumat C, Alric A, Felix-Faure B, Pradere P, Guiresse M (2008) Multimetal contamination of a calcic cambisol by fallout from a lead-recycling plant. Geoderma 144:287–298
Chehregani A, Noori M, Yazdi HL (2009) Phytoremediation of heavy-metal-polluted soils: screening for new accumulator plants in Angouranmine (Iran) and evaluation of removability. Ecotoxicol Environ Safety 72:1349–1353
De la Rosa G, Jose RPV, Milka M, Jason GP, Irene CA, Jorge LGT (2004) Cadmium uptake and translocation in tumbleweed (Salsola kali), a potential Cd-hyperaccumulator desert plant species: ICP/OES and XAS studies. Chemosphere 55:1159–1168
Debez A, Huchzermeyer B, Abdelly C, Koyro HW (2010) Current challenges and future opportunities for a sustainable utilization of halophytes. In: M. Öztürk et al. (Eds.) Sabkha Ecosystems. Tasks for Vegetation Science pp: 59–77.
Egner H, Riehm H, Domingo WR (1960) Untersuchungen uber die chemische Bodenanalyse als Grundlage fur die Beurteilung des Nahrstoffzustandes der Boden, II. Chemische Extraktionsmethoden zur Phosphor-und Kalium bestimmung. Kungl Lantbrukshogsko Annal 26:45–46
Ehsan S, Prasher SO, Marshall W (2007) Simultaneous mobilization of heavy metals and polychlorinated biphenyl (PCB) compounds from soil with cyclodextrin and EDTA in admixture. Chemosphere 68:150–158
Ghaderian SM, Ghotbi Ravandi AA (2012) Accumulation of copper and other heavy metals by plants growing on Sarcheshmeh copper mining area, Iran. J Geochem Explor 123:25–32
Ghnaya T, Nouairi I, Slama I, Messedi D, Grignon C, Abdelly C, Ghorbel MH (2005) Cadmium effects on growth and mineral nutrition of two halophytes: Sesuvium portulacastrum and Mesembryanthemum crystallinum. J Plant Phys 162:1133–1140
Ghnaya T, Slama I, Messedi D, Grignon C, Abdelly C (2007) Cd-induced growth reduction in the halophyte Sesuvium portulacastrum is significantly improved by NaCl. J Plant Res 120:309–316
Hunt R (1990) Basic growth analysis. Plant growth analysis for beginners. UNWIN HYMA, London, p 112
Kalra YP, Maynard DG (1991) Methods for forest soil and plant analysis. Information report NOR-X-319. Northern Forestry Center, Forestry Canada, Northwest Region, p 116
Kibria MG, Islam M, Osman KT (2009) Effects of lead on growth and mineral nutrition of Amaranthus gangeticus L. and Amaranthus oleracea L. Soil & Environ 28:1–6
Komárek M, Tlustŏs P, Szákova J, Richner W, Brodbeck M, Sennhauser M (2007) The use of maize and poplar in chelant-enhanced phytoextraction of lead from contaminated agricultural soils. Chemosphere 67:640–651
Lai HY, Chen ZS (2005) The EDTA effect on phytoextraction of single and combined metals-contaminated soils using rainbow pink (Dianthus chinensis). Chemosphere 60:1062–1071
Lambrechts T, Gustot Q, Couder E, Houben D, Iserentant A, Lutts S (2011) Comparison of EDTA-enhanced phytoextraction and phytostabilisation strategies with Lolium perenne on a heavy metal contaminated soil. Chemosphere 85:1290–1298
Larbi A, Morales F, Abadía A, Gogorcena Y, Lucena JJ, Abadía J (2002) Effects of Cd and Pb in sugar beet plants grown in nutrient solution: induced Fe deficiency and growth inhibition. Funct Plant Biol 29:1453–1464
Lasat MM, Pence NS, Garvin DF, Ebbs SD, Kochian LV (2000) Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens. J Exp Bot 51:71–79
Liphadzi MS, Kirkham MB (2006) Availability and plant uptake of heavy metals in EDTA-assisted phytoremediation of soil and composted biosolids. S Afr J Bot 72:391–397
Liu D, Islam E, Li T, Yang X, Jin X, Mahmood Q (2008) Comparison of synthetic chelators and low molecular weight organic acids in enhancing phytoextraction of heavy metals by two ecotypes of Sedum alfredii Hance. J Hazard Mater 153:114–122
Min XB, XieX D, Chailly LY, Liang YJ, Li MI, Yong KE (2003) Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue. Trans Nonferrous Metals Soc China 23:208–218
Mühling KH, Läuchli A (2002) Effect of salt stress on growth and cation compartmentation in leaves of two plant species differing in salt tolerance. J Plant Physiol 159:137–146
Nascimento CWA, Amarasiriwardena D, Xing B (2006) Comparison of natural organic acids and synthetic chelates at enhancing phytoextraction of metals from a multi-metal contaminated soil. Environ Pollut 140:114–123
Quartacci MF, Argilla A, Baker AJM, Navari-Izzo F (2006) Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere 63:918–925
Reeves RD, Brooks RR (1983) Hyperaccumulation of lead and zinc by two metallophytes from mining areas of Central Europe. Environ Pollut 31:277–285
Saifullah E, Meers M, Qadir P, de Caritat FMG, Tack G, Du Laing MHZ (2009) EDTA-assisted Pb phytoextraction. Chemosphere 74:1279–1291
Salla V, Hardaway CH, Sneddon J (2011) Preliminary investigation of Spartina alterniflora for phytoextraction of selected heavy metals in soils from Southwest Louisiana. Microchem 97:207–212
Shahid M, Pinelli E, Pourrut B, Silvestre J, Dumat C (2011) Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicol Environ Saf 74:78–84
Shahid M, Pinelli E, Dumat C (2012) Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J Hazard Mater 220:1–2
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Shimwell DW, Laurie AE (1972) Lead and zinc contamination of vegetation in the southern Pennines. Environ Pollut 3:291–301
Van Engelen DL, Sharpe-Pedler RC, Moorhead KK (2007) Effect of chelating agents and solubility of cadmium complexes on uptake from soil by Brassica juncea. Chemosphere 68:401–408
Vega F, Andrade M, Covelo E (2010) Influence of soil properties on the sorption and retention of cadmium, copper and lead, separately and together, by 20 soil horizons: comparison of linear regression and tree regression analyses. J Hazard Mater 174:522–533
Vettori L, Missaglia A, Felici C, Russo A, Tamantini I, Carrozza GPC, Cinelli F, Toffanin A (2012) Erratum to “Bioremedation and phytoremedation: synergism in lead extraction from contaminated soils”. J Biotechnol 160:268 [J. Biotechnol. 150S (2010) S509–S510]
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Wojas S, Ruszczynska A, Bulska E, Wojciechowski M, Antosiewicz DM (2007) Ca2+-dependent plant response to Pb2+ is regulated by LCT1. Environ Pollut 147:584–592
Wu X, Jia Y, Zhu H, Wang H (2010) Bioaccumulation of cadmium bound to humic acid by the bivalve Meretrix meretirx Linnaeus from solute and particulate pathways. J Environ Sci (China) 22:198–203
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Yukselen MA, Gokyay O (2006) Leachability of metals from soil contaminated by mining activities. Environ Eng Sci 23:125–132
Zaier H, Ghnaya T, Ben Rejeb K, Lakhdar A, Rejeb S, Jemal F (2010a) Effects of EDTA on phytoextraction of heavy metals (Zn, Mn and Pb) from sludge-amended soil with Brassica napus. Biores Technol 101:3978–3983
Zaier H, Ghnaya T, Lakhdar A, Baioui R, Ghabriche R, Mnasri M, Sghair S, Lutts S, Abdelly C (2010b) Comparative study of Pb-phytoextraction potential in Sesuvium portulacastrum and Brassica juncea: tolerance and accumulation. J Hazard Mater 183:609–615
Zayed A, Gowthaman S, Terry N (1998) Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J Environ Qual 27:715–721
Acknowledgments
This work was conducted in the Laboratory of Extremophile Plants in the Biotechnology Center of Borj Cedria Tunisia and supported by the Tunisian Ministry of Higher Education and Scientific Research (LR10CBBC02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Both authors Hanen Zaier and Tahar Ghnaya equally contributed to this work.
Rights and permissions
About this article
Cite this article
Zaier, H., Ghnaya, T., Ghabriche, R. et al. EDTA-enhanced phytoremediation of lead-contaminated soil by the halophyte Sesuvium portulacastrum . Environ Sci Pollut Res 21, 7607–7615 (2014). https://doi.org/10.1007/s11356-014-2690-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2690-5