Abstract
Cadmium (Cd)-induced growth inhibition is one of the primary factors limiting phytoremediation effect of Boehmeria nivea (L.) Gaud in contaminated soil. Sodium nitroprusside (SNP), a donor of nitric oxide (NO), has been evidenced to alleviate Cd toxicity in many plants. However, as an important mechanism of NO in orchestrating cellular functions, S-nitrosylation is still poorly understood in its relation with Cd tolerance of plants. In this study, higher exogenous NO levels were found to coincide with higher S-nitrosylation level expressed as content of S-nitrosothiols (SNO). The addition of low concentration (100 μM) SNP increased the SNO content, and it simultaneously induced an alleviating effect against Cd toxicity by enhancing the activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR) and reduced the accumulation of H2O2 as compared with Cd alone. Application of S-nitrosoglutathione reductase (GSNOR) inhibitors dodecanoic acid (DA) in 100 μM SNP group brought in an extra elevation in S-nitrosylation level and further reinforced the effect of SNP. While the additions of 400 μM SNP and 400 μM SNP + 50 μM DA further elevated the S-nitrosylation level, it markedly weakened the alleviating effect against Cd toxicity as compared with the addition of 100 μM SNP. This phenomenon could be owing to excess consumption of glutathione (GSH) to form SNO under high S-nitrosylation level. Therefore, the present study indicates that S-nitrosylation is involved in the ameliorating effect of SNP against Cd toxicity. This involvement exhibited a concentration-dependent property.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium, as a nonredox heavy metal element, is considered to be a potential toxicant in the environment. Various anthropogenic activities such as mineralization, wastewater irrigation, and widespread use of phosphate fertilizers contribute to its accumulation in soil, which results in a series of environmental problem and may negatively affect human health due to its high mobility from soil to plant and further to the food chain (Vig et al. 2003). In plants, Cd is taken up rapidly by roots and causes toxicity symptom including growth inhibition, leaf chlorosis and leaf roll, tissue necrosis, and even death by disrobing metabolisms like photosynthesis, antioxidant system, water statue, etc. (Sanità di Toppi and Gabbrielli 1999). At the cellular level, Cd is found to produce oxidative stress, visibly contributing to the toxicity of reactive oxygen species (ROS), viz. hydrogen peroxide (H2O2), superoxide (O −2 ), and hydroxyl radical (OH·). ROS are highly reactive toxic molecules and can disrupt membrane integrity and permeability and induce lipid peroxidation and ion leakage. Unlike other heavy metals, Cd does not participate in the generation of ROS (Haber-Weiss reaction and Fenton reaction) but can favor the overproduction of ROS indirectly. The mechanism includes reducing activity of antioxidative enzyme, lowering the content of antioxidant (Srivastava et al. 2004) and disrupting the electron transport chain (Qadir et al. 2004). Additionally, it has also been reported that Cd strongly binds to oxygen, nitrogen, and sulfur atoms (Nieboer and Richardson 1980). This binding affinity is related to free enthalpy of the formation of the product of metal and ligand. Due to this feature, Cd can inactivate enzymes by binding itself to cysteine residues.
Nitric oxide (NO), as a multifunctional signaling molecule, has been recognized as playing a crucial role in various plant physiological processes, e.g., germination, leaf expansion, lateral root development, flowering, stoma closure, cell death, and defense against biotic and abiotic stresses (Xiong et al. 2010). Since the past decade, various articles have reported the effects of exogenous NO on alleviating heavy metal toxicity. Intensive researches have been done on the physiological function of NO in ameliorating the oxidative stress induced by cadmium via broad but different effects (Singh et al. 2008). Squadrito and Pryor (1995) considered that NO might function as an antioxidant, by directly scavenging the ROS, such as converting superoxide radicals (O −2 ) to peroxynitrite (\( {\mathrm{ONOO}}^{-} \)) which is not so destructive for plant cell as O −2 (Delledonne et al. 2001). It also has been evidenced that NO stimulated the biosynthetic pathway of glutathione (GSH) in plant cells with an enhanced tolerance against oxidative stress (Xiong et al. 2010).
Due to its chemistry and diffusible properties, NO and its derivatives actively interact with molecules like thiol and amino acids (Alvarez and Radi 2003). Among them, S-nitrosylation of cysteine thiol of proteins to form an S-nitrosothiol (SNO) is regarded as an important redox-based post-translational modification (Foyer and Noctor 2005; Sandalio et al. 2001). Recent evidence (Barroso et al. 2006) suggests that many proteins are S-nitrosylated in plants under physiological or stress conditions, leading to the first insights into S-nitrosylation-dependent regulation of protein function. Lately, total S-nitrosothiol (SNO) levels were measured in Arabidopsis plants that were altered in S-nitrosoglutathione reductase (GSNOR) activity (Feechan et al. 2005b). It is established that GSNOR was an important enzyme that is involved in regulation of cellular S-nitrosylation (Liu et al. 2001). GSNOR is a member of the alcohol dehydrogenase family (ADH) and regulates the levels of S-nitrosothiols (SNOs) through catabolism of S-nitrosoglutathione (GSNO). GSNO may function both as an intracellular NO reservoir and as a vehicle for NO throughout the cell. GSNOR shows high specificity toward GSNO and serves as a biologically stable nitric oxide adduct (Jensen et al. 1998; Liu et al. 2001). In this manner, GSNOR controls trans-nitrosation equilibrium between GSNO and protein S-nitrosothiols (RSNO). Hence, S-nitrosylation and GSNOR play critical roles in the function of exogenous NO in the overall metabolism (Feechan et al. 2005).
Additionally, the GSNOR catalyzes the NADH-dependent reduction of GSNO to oxidized l-glutathione (GSSG) and NH3. The forming GSSG is then reduced to GSH from the NADPH-dependent reaction catalyzed by glutathione reductase (GR). GSH is an important antioxidant and is the most abundant low molecular weight thiol in plant cells (Noctor et al. 1998). Previous research has observed that GSH was converted to S-nitrosoglutathione (GSNO) via S-nitrosylation in plants under both biotic and abiotic stresses (Chaki et al. 2009). Given that GSH plays an important role in detoxifying heavy metal, it is necessary to understand the role of S-nitrosylation in plant tolerance against Cd toxicity. However, to our knowledge, there are some controversies regarding the endogenous NO-induced protein nitration and its role in ameliorating oxidative injury under different stresses (Chaki et al. 2009; Corpas et al. 2008). The knowledge of the connection between exogenous NO-induced S-nitrosylation and antioxidant system under cadmium stress is quite limited.
Boehmeria nivea (L.) Gaud (ramie) is an important fiber crop used for textile. Previous researches demonstrated that ramie is a promising species for Cd phytostabilization due to its large biomass and fast growth rate (Wang et al. 2008). While according to previous data, visible growth inhibition and diseases were found in ramie under high concentration Cd stress, which partly limits the application of ramie for phytostabilization. Hence, it is of great necessity to find a proper way to solve this problem.
In this work, using ramie plants as a model system and employing biochemical approaches, the presence and level of S-nitrosylation in leaves were studied while assessing SNO content. Cellular NO content and GSNOR activity were determined in order to investigate how sodium nitroprusside (SNP) impacted cellular S-nitrosylation level. GSNOR inhibitor dodecylic acid (DA) and NO scavenger carboxy-PTIO (c-PTIO) were applied in this study to get contrasting data. Additionally, the impact of S-nitrosylation on the activities of three key antioxidative enzymes [ascorbate peroxidase (APX), GR, and superoxide dismutase (SOD)] and contents of important antioxidants (GSH and AsA) have been analyzed. Finally, the growth indicators (length and biomass) of plants under different concentration of SNP were determined to explore the concentration-dependent effect of NO on alleviating Cd toxicity.
Materials and methods
Plant materials and growth conditions
One-month-old B. nivea (L.) Gaud (ramie) seedlings provided by the Institute of Bast Fiber Crops, Chinese Academy of Agricultural Sciences, were grown hydroponically in plastic pots (volume 3 l) containing 25 % Hoagland nutrient solution with aeration (three plants per pot). The nutrient solution was renewed every 2 days in 2 weeks. Then, the plants were transferred to fresh media. The treatments were arranged in a randomized block design with six replicates, totaling 14 containers. The Hoagland solution was adjusted to pH 6.5, and environmental chamber conditions were controlled at 25:10 light/dark, 14 h photoperiod, light intensity PAR 300 μM m−2 s−1, and 60 ± 5 % relative humidity. After 10 days grown in above conditions, seedlings were transferred to the designed nutrient solution for 72 h (supplement design is shown in Table 1) and then the plants were collected. The roots and shoots were separated and washed with 5 mM CaCl2 first and then repeatedly washed with deionized distilled water. Growth parameters (shoot length, biomass) were measured at the end of the experiment.
NO and SNO contents
For determining cellular levels of NO and SNO, samples (0.2 g) were cut into pieces. NO content was determined using a commercial reagent kit (Jiancheng Biotech Inc., Nanjing, China) following specifications. Cellular levels of SNO were detected as described by Feechan et al. (2005) with revises. Pieces were ground using a mortar and pestle in a solution containing 0.1 M TRIS–HCl (pH 7.5) and 1 mM PMSF at 4 °C. The homogenates were centrifuged at 10,000g at 4 °C for 10 min, and the supernatants were used for the assays. The supernatants were then filtered through a MicroBioSpin-6 column (Bio-Rad). SNO levels in a fraction filtered through a 5-kDa cutoff ultrafiltration membrane (low-mass SNO) were measured by photolysis chemiluminescence and normalized for protein content as detailed.
Determination of lipid peroxidation and H2O2 content
The malondialdehyde (MDA) content of leaves was determined using the thiobarbituric acid method (Chaoui et al. 1997) with little revises. Plant tissue (0.2 g) was homogenized with 10 ml 10 % (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 10,000g for 10 min. Two milliliters of the aliquot of the supernatant and 2 ml of 10 % TCA containing 0.5 % thiobarbituric acid (TBA) were added. The mixture was incubated at 100 °C for 10 min and then cooled quickly in an ice bath. The contents were centrifuged at 10,000g for 15 min, and the absorbance of the supernatant was measured at 532 nm and corrected for nonspecific absorbance at 600 nm. The concentration of MDA was calculated using 155 mM−1 cm−1 as extinction coefficient.
Fresh samples (0.2 g) were homogenized in 2 ml ice-cold acetone. Titanium reagent (2 % TiCl2 in conc. HCl) was added to a known volume of extract supernatant to give a Ti (IV) concentration of 2 %. The Ti–H2O2 complex, together with unreacted Ti, was then precipitated by adding 0.2 ml 17 M ammonia solution for each 1 ml of extract. The precipitate was washed five times with ice acetone and re-suspended, drained, and dissolved in 1 M H2SO4 (3 ml). The absorbance of the solution was measured at 410 nm against blanks, which had been similarly prepared but without plant tissue (Patterson et al. 1984).
Enzyme analysis
Leaves extract for determination of GSNOR activity was followed by Barroso et al. (2006) with revise. All operations were performed at 0–4 °C. Leaves were ground using a mortar and pestle in a solution containing 0.1 M TRIS–HCl (pH 7.5), 2 mM DTT, 0.1 mM EDTA, 0.2 % (v/v) Triton-X-100, and 10 % (v/v) glycerol. Homogenates were centrifuged at 27,000g for 25 min. To determine GSNOR activity, the leaf extracts were incubated in an assay mixture containing 20 mM TRIS–HCl (pH 8.0), 0.2 mM NADH, and 0.5 mM EDTA, and the reaction was started by adding GSNO to the mixture at a final concentration of 400 μM. The activity was expressed as nanomole NADH consumed per minute per milligram protein (ε340 = 6.22 mM−1 cm−1). Protein concentration was assayed according to Bradford (1976) using bovine serum albumin as standard.
APX and GR were extracted based on the former report (Wang et al. 2008) with little revision. Frozen leaves (0.2 g fresh weight) were homogenized in ice-cold 50 mM phosphate buffer pH 7.0 containing 0.1 % (v/v) Triton X-100 and 1 % (w/v) polyvinylpyrrolidone (PVP). The plant material and isolation buffer were applied at proportions 1:4 (w/v). The homogenate was centrifuged at 15,000g for 20 min at 4 °C, and the supernatant was used for enzyme assays. For APX, homogenizing buffer was supplemented with 2 mM ascorbate. APX activity was determined by estimating the rate of ascorbate oxidation (Nakano and Asada 1987). The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM sodium ascorbate, 0.1 mM H2O2, and enzyme extract. A decrease in absorbance at 290 nm was measured at 25 °C for 3 min (E=2.8 mM−1 cm−1).
GR activity was assayed by estimating the rate of NADPH oxidation (Foyer and Halliwell 1976). The decrease in absorption at 340 nm (E = 6.2 mM−1 cm−1) due to NADPH oxidation was recorded over 2.5 min. The reaction mixture consisted of 50 mM phosphate buffer (pH 7.8), 0.15 mM NADPH, 0.5 mM GSSG, and enzyme extract. During 1 min at 25 °C, 1 U = 1 mmol substrate reacted.
The extraction procedure of SOD was carried out at 4 °C according to Wang et al. (2008a). Superoxide dismutase (SOD) activity was assayed by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium following the method of Stewart and Bewley (1980). The activities of SOD, APX, and GR were expressed as units per milligram protein.
Metabolite analysis
The methods to determine the contents of GSH and GSSG followed the method of Anderson (1984). The assay was based on sequential oxidation of GSH by DTNB to produce TNB and reduction of GSSG by NADPH in the presence of GR. Frozen leaves were homogenized in ice-cold 5 % (w/v) TCA and then centrifuged at 20,000g for 10 min at 4 °C. To determine GSSG content, 2-vinylpyridine was added to the extract. GSH content was obtained from the difference between the total glutathione and GSSG.
Statistical analysis
For each treatment including the control, there were three plants arranged in a randomized block design manner. Each analysis involved six replications. Data was presented as mean ± standard error (SE) and analyzed by one-way ANOVA followed by separation of treatment (including control) applying post hoc Tukey's test.
Results
S-nitrosylation and GSNOR
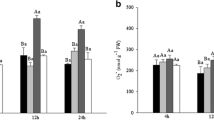
To check the possible changes which could affect S-nitrosylation pattern in ramie leaf during Cd stress, cellular NO content was first determined in ramie leaves. Results (Fig. 1a) revealed Cd-reduced NO accumulation in ramie leaves by 27.6 % compared to CK. With the treatment of 100 μM SNP and 400 μM SNP, the cellular NO content was elevated to 9.86 and 16.11 μM/gprot. The cellular S-nitrosylation level was measured as SNO content. As shown in Fig. 1b, the SNO content in ramie leaves was also reduced by Cd and was significantly elevated with supplement of 100 and 400 μM SNP, which was consistent with variation of cellular NO content. This indicated that exogenous NO (SNP) could induce cellular S-nitrosylation, and this effect seemed to be concentration-dependent. Additionally, it is well known that GSNO reductase (GSNOR) plays an important role in regulating cellular S-nitrosylation level. Downregulation of GSNOR activity was regarded to elevate the cellular S-nitrosylation level in humans, bacteria, and plants (Zheng et al. 2011). To detect whether GSNOR is involved in regulating SNP-induced S-nitrosylation, GSNOR activity was determined and expressed as NADH was consumed (Fig. 2). In this study, GSNOR activity was found to be reduced under 5 mg l−1 Cd exposure. The addition of 100 μM SNP elevated GSNOR activity by 29.1 % compared to Cd treatment only, and 400 μM SNP led to a more elevation (50.8 %) in GSNOR activity. The addition of 50 μM DA inhibited the GSNOR activity by 63.7 and 65.43 % in 100 and 400 μM SNP groups, respectively. Furthermore, the addition of 50 μM DA brought a visible increase in SNO content both in the treatment of 100 and 400 μM SNP groups. This indicated that GSNOR involved in regulating the S-nitrosylation was induced by exogenous nitric oxide. It is noteworthy that the addition of 100 μM c-PTIO (NO scavenger) in 100 μM SNP group not only significantly reduced the cellular NO content but also lowered the S-nitrosylation level.
Effects of different treatments on the a cellular NO content and b cellular SNO content in shoots of 14 days treatment ramie plants grown in nutrient solutions without or with 5 mg l−1 CdCl2. Data represent as mean ± standard error (SE) of six replicates. Bars with different letters indicate statistically significant differences at P < 0.05. Error bars show SE
Effects of different treatments on the activity of GSNOR in shoots of 14 days treatment ramie plants grown in nutrient solutions without or with 5 mg l−1 CdCl2. Data represent as mean ± standard error (SE) of six replicates. Bars with different letters indicate statistically significant differences at P < 0.05. Error bars show SE
Plant growth
As shown in Table 2, 5 mg l−1 Cd exposure significantly inhibited the ramie growth as compared to the control plants (p < 0.01). The plant shoot and biomass decreased to 40.1 and 49.3 %, respectively. Supplement with 100 μM SNP was found to alleviate Cd-induced growth inhibition. The plant height increased from 14.6 to 18.7 cm and the biomass increased from 11.9 to 19.1 g. Moreover, 50 μM dodecanoic acid (DA) further increased the plant height and biomass to 22.8 cm and 21.6 g, which was very close to CK (24.4 cm and 23.3 g). However, this effect was weakened when plants were treated with 400 μM SNP, the plant height was 16.4 cm, and biomass was 15.8 g respectively. The addition of DA in 400 μM SNP group further reduced this effect.
Oxidative stress and antioxidant system
Cd uptake by plants has been reported to induce a variety of physiological changes, such as membrane integrity (Smeets et al. 2005) and ROS accumulation (Sanità di Toppi and Gabbrielli 1999). The relationship between Cd toxicity and oxidative reactions in ramie was studied in terms of MDA and ROS contents. When plants were subject to environmental stress, oxidative damage resulted in membrane lipid peroxidation, which could be estimated by MDA content. An increase in MDA content is also assumed to be a common symptom of heavy metal stress. It could be clearly seen that MDA content markedly increased 67.8 % over control after Cd treatment (Fig. 5a). This increase was found reversed in 100 μM SNP treatment. The MDA content was reduced by 19.8 % in 100 μM SNP group and by 38.6 % in DA + 100 μM SNP group compared to Cd group. It indicated that DA + 100 μM SNP provided a more robust ability to alleviate the lipid peroxidation. However, the MDA contents in 400 μM SNP group and DA + 400 μM SNP group did not exhibit distinction statically from Cd group. H2O2 is an important ROS involved in many mechanisms in plants and is regarded to be responsible for Cd-induced lipid peroxidation and other dexterous effects (Dong et al. 2006). As is shown in Fig. 5b, the variations in H2O2 content were similar with MDA content. It is noteworthy that both 100 μM and 400 μM SNP treatment (with or without DA) alleviated oxidative stress for decreasing ROS content. However, addition of DA in high (400 μM) SNP concentration group further elevated MDA content compared with 400 μM SNP group, which exhibited an opposite effect to that in low (100 μM) SNP concentration group.
Antioxidant system has been evidenced to play a crucial role in scavenging ROS and maintaining the cellular homeostasis (Drążkiewicz et al. 2003). As shown in Fig. 4, Cd toxicity seriously impaired the antioxidant system. The activity of APX, GR, and SOD in leaves of plants was significantly decreased by 26.1, 35.3, and 32.6 %, respectively, with the treatment of Cd from control (p < 0.001). This effect was reversed with the addition of 100 μM SNP. However, 400 μM SNP group exhibited different results from 100 μM SNP group. High concentration of SNP (400 μM) did not significantly impact GR and SOD activity, but lowered APX activity. Supplement with 50 μM DA in 100 μM SNP group further elevated the activities of those enzymes as compared to 100 μM SNP group, while in the group treated with 400 μM SNP + 50 μM DA, little distinction was observed from 400 μM SNP group. The general trend of GSH and GSSG contents was similar to the enzyme in Fig. 4. The difference is that GSSG content was decreased both in 100 μM SNP and 400 μM groups with the addition of 50 μM DA.
Discussion
In the past decade, experimental designs using NO donors revealed that exogenously applied NO can provide protection, e.g., against heavy metal toxicity, due to its ability to directly scavenge ROS or to activate antioxidant enzymes (Laspina et al. 2005; Xiong et al. 2010). With the addition of exogenous NO (SNP), the antioxidant system was significantly improved (Fig. 3 and Fig. 4). The experiment data were found in various plants such as tomato (Gratão et al. 2008), rice (Panda et al. 2011), and sunflower (Laspina et al. 2005). Recent study revealed that NO-induced S-nitrosylation could be involved in plant tolerance against various stresses. Bai et al. (2011) found in Antiaris toxicaria seeds that NO reinforces APX and CAT activities by improving S-nitrosylation level of antioxidant proteins, thus reducing the Cd-induced carbonylation degree. Ortega-Galisteo et al. (2012) identified six peroxisomal proteins as putative targets of S-nitrosylation which were associated with photorespiration, β-oxidation, and reactive oxygen species detoxification. In our study, Cd induced a decline in S-nitrosylation level (presented as SNO contend) in 1-month-old B. nivea (L.) Gaud seedlings (Fig. 1b). This decline might be the result from an inhibited production of endogenous NO in Cd-treated plant (Fig. 1a). Luna et al. (1994) found that the accumulation of S-nitrosothiols correlated with cellular NO levels. Our data (Fig. 1) in the group treated with Cd + 100 uM SNP and Cd + 400 μM confirmed their findings and demonstrated that intracellular S-nitrosylation level existed a positive correlation with exogenous NO content.
Effects of different treatments on a GSH content and b GSSG content in shoots of 14 days treatment ramie plants grown in nutrient solutions without or with 5 mg l−1 CdCl2. Data represent as mean ± standard error (SE) of six replicates. Bars with different letters indicate statistically significant differences at P < 0.05. Error bars show SE
Effects of different treatments on the activity of a APX, b GR, and c SOD in shoots of 14 days treatment ramie plants grown in nutrient solutions without or with 5 mg l−1 CdCl2. Data represent as mean ± standard error (SE) of six replicates. Bars with different letters indicate statistically significant differences at P < 0.05. Error bars show SE
Both sodium nitrate and NO are the decomposition products of SNP. It is well documented that both sodium nitrite and NO can cause cellular S-nitrosylation in plant. To investigate which product of SNP caused the variation of cellular S-nitrosylation, we used c-PTIO as a specific NO scavenger. The results indicated the addition of 100 μM c-PTIO in 100 μM SNP-scavenged cellular NO in ramie leaves. Simultaneously, the increase in SNO content was significantly reversed. Therefore, given that c-PTIO has little effect on sodium nitrate content, we can infer that exogenous NO (SNP) elevated cellular S-nitrosylation level most likely due to its contribution to increased cellular NO content in ramie leaves, instead of other products of SNP.
S-Nitrosoglutathione reductase (GSNOR) is the major enzyme that catalyzes S-nitrosoglutathione (GSNO) metabolism and controls intracellular levels of protein S-nitrosylation. Previous study found a higher than normal SNO level in Arabidopsis thaliana which lacks GSNOR gene (Feechan et al. 2005). We added 50 μM dodecanoic acid (DA), a specific inhibitor of GSNOR (Sanghani et al. 2009), to Cd + 100/400 μM SNP group and observed a significant increase in SNO level (Fig. 1). This result indicated that GSNOR is involved in the regulation of exogenous NO-induced S-nitrosylation level in ramie leaves. However, the knowledge about the relation between abiotic and biotic stresses and GSNOR activity regulation is still contradicted. A very recent article (Kubienová et al. 2014) has observed opposite variations in GSNOR activity in different types of stress conditions. However, in the experiment of pea plants, Barroso et al. (2006) found that GSNOR was inhibited by the addition of 50 μM CdCl2 because of the lowered GSNO content. In this study, the increase of GSNOR activities in 100 and 400 μM SNP groups might be a result from the elevated SNO level for the same reason (Fig. 2). This indicated that the self-regulation of GSNOR activity is probably based on its substrate (GSNO) content.
It is known that in S-nitrosylation, the addition of a NO moiety to a specific cysteine residue in glutathione or proteins to form S-nitrosothiols (SNO) or S-nitrosylated proteins emerged as a principal mechanism by which NO orchestrates cellular functions in Arabidopsis (Zheng et al. 2011). To investigate whether NO regulates antioxidant system via S-nitrosylation or not, DA was added in 100 and 400 μM SNP groups to observe the alternations of antioxidant system (Figs. 3, 4, and 5).
Effects of different treatments on the a MDA content and b H2O2 content in shoots of 14 days treatment ramie plants grown in nutrient solutions without or with 5 mg l−1 CdCl2. Data represent as mean ± standard error (SE) of six replicates. Bars with different letters indicate statistically significant differences at P < 0.05. Error bars show SE
GSH was initially studied in this experiment because it could build a bridge to study the connection between the antioxidant system and S-nitrosylation. In the recent years, many articles have reported the effect of exogenous nitric oxide on GSH production (Arasimowicz-Jelonek et al. 2011; Xu et al. 2010). However, how NO regulates GSH content in plant is still complex and contradictory. A conceivable explanation suggests that NO, as a signal molecular, is directly involved in the GSH synthesis-related gene expression and upregulates the production of GSH in plants (Innocenti et al. 2007). In our experiment (Fig. 3), the addition of DA was found to increase GSH content and improved antioxidant system efficiency under low level of S-nitrosylation (100 μM SNP group). When S-nitrosylation reached to a relative high level (100 μM SNP group), the addition of DA produced an opposite result. A former study indicated that exogenous nitric oxide also exhibited similar concentration-dependent effect on antioxidant system (Wang et al. 2012). This finding could be explained as the dual role of S-nitrosylation in GSH production. On the one hand, high level of S-nitrosylation means that excess GSH has been consumed by NO to form S-nitrosoglutathione (GSNO) via S-nitrosylation reaction. Additionally, the addition of DA reduced GSSG content both in 100 and 400 μM SNP groups. This finding is probably due to the variation in GSNOR activity. It is well established that GSNOR catalyzed an irreversible NADH-dependent reduction of GSNO to a mixture of products including GSSG, NH3, hydroxylamine, and glutathione sulfinic acid (Liu et al. 2001). Reduced GSNOR activity could inhibit the transformation of GSSG to GSH and then affect regeneration of GSH. However, on the other hand, the forming GSNO, as an important S-nitrosylation production, is thought to function as a mobile reservoir of NO bioactivity and could probably provide a consistent trigger in GSH production. According to our results, whether the effect of S-nitrosylation on GSH production is negative or positive might probably depend on cellular S-nitrosylation level.
It has been evidenced that the accumulation of H2O2 and other ROS could induce cell lipid peroxidation. In this study, supplement with 100 and 400 μM SNP promoted the GSH production compared with Cd group. Elevation in GSH content could strengthen antioxidant system, such as improving efficiency of ascorbate-glutathione cycle (Drążkiewicz et al. 2003) and promoting the biosynthesis of phytochelatins (PCs), a peptide that can sequester Cd2+ by forming heavy metal-binding complexes (Kuźniak and Skłodowska 2001). Similar elevations (Fig. 4) were found in APX, SOD, and GR, which were probably owing to interactions or mutual adjustments among antioxidants and antioxidative enzymes. Elevation in enzyme activity and antioxidant content indicated a more powerful ability of plant to scavenging ROS. The reasons were presented as follow: (i) superoxide anion (O −2 ) and hydrogen peroxide (H2O2) are two main ROS that are involved in Cd-induced oxidative stress. SOD converts O −2 into H2O2 which is then scavenged by enzymes, e.g., APX. (ii) APX is regarded as the most important peroxidase in H2O2 detoxification (Noctor and Foyer 1998). APX can provide a powerful capacity to directly scavenge H2O2 through ascorbate–glutathione cycle (Jiménez et al. 2002). (iii) Elevation in GR activity helps to regenerate the substrate (AsA) of the reaction catalyzed by APX and then enhance efficiency of this reaction. Those enzymes and antioxidants can constitute or represent a relatively integrated chain of defense against ROS. These promotions in antioxidants and antioxidative enzyme contribute to ROS scavenging capacity of B. nivea (L.) Gaud seedling and then ameliorate lipid peroxidation (Fig. 5) and other Cd-induced toxicity. Of course, ROS system in plant is very complicated, and further study is still needed on the relationship between S-nitrosylation and the whole ROS system.
Besides, there exists a possibility that exogenous nitric oxide induced S-nitrosylation and reversed Cd-induced enzyme protein carbonylation in leaves. Bai et al. (2011) suggest that this modification was conductive to preserve activity of antioxidant enzyme in A. toxicaria seeds under desiccation stress. Both protein carbonylation and S-nitrosylation are important mechanisms used to regulate protein activities at the post-translational level (Grennan 2007). Excess protein carbonylation causes an irreversible oxidative process, and it would be expected to contribute to inhibitions or impairments of multiple enzymes, thus affecting cellular functions ranging from protein synthesis, energy production, and cytoskeleton dynamics to signal transduction (Sohal et al. 2002). This would open new avenues in the research of the physiological function of NO and S-nitrosylation in higher plants. However, further investigation still needs to be conducted to support this hypothesis.
In summary, the presence of S-nitrosylation and its concentration-dependent effect on ramie plant antioxidant system was demonstrated. The ameliorating effects of exogenous NO on Cd-induced toxicity may result from its function to supplement intracellular NO and S-nitrosylation level that was lowered by Cd. Treatment with 100 μM SNP leads to a best alleviation of growth inhibition and least MDA content. However, this effect was weakened or reversed when SNP concentration reached 400 μM. The presence of S-nitrosoglutathione reductase was involved in regulating the functional effect. When GSNOR activity was inhibited, the increased SNO led to a reduced GSH content. Endogenous NO or the intracellular SNO level is usually affected under many biotic and abiotic stresses. The present study builds a potential bridge for studying connecting oxidative stress and NO or NO-derived molecules that mediated post-translational modification. Additionally, the results of this experiment provide potential illumination on how exogenous nitric oxide ameliorates Cd-induced B. nivea (L.) growth inhibition and verified its role of repairing heavy metal-contaminated areas. However, the complete elucidation and the detailed signaling mechanism of NO need further investigation.
Abbreviations
- SNP:
-
Sodium nitroprusside
- ROS:
-
Reactive oxygen species
- SNO:
-
S-Nitrosothiol
- GSNOR:
-
S-Nitrosoglutathione reductase
- GSH:
-
Glutathione
- APX:
-
Ascorbate peroxidase
- GR:
-
Glutathione reductase
- O2 − :
-
Superoxide anion radical
- MDA:
-
Malondialdehyde
References
Alvarez B, Radi R (2003) Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25:295–311
Anderson ME (1984) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:548–555
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Gwóźdź EA (2011) The message of nitric oxide in cadmium challenged plants. Plant Sci 181:612–620
Bai X, Yang L, Tian M, Chen J, Shi J, Yang Y, Hu X (2011) Nitric oxide enhances desiccation tolerance of recalcitrant Antiaris toxicaria seeds via protein S-nitrosylation and carbonylation. PLoS One 6:e20714
Barroso JB, Corpas FJ, Carreras A, Rodríguez-Serrano M, Esteban FJ, Fernández-Ocaña A, Chaki M, Romero-Puertas MC, Valderrama R, Sandalio LM (2006) Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J Exp Bot 57:1785–1793
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chaki M, Fernández-Ocaña AM, Valderrama R, Carreras A, Esteban FJ, Luque F, Gómez-Rodríguez MV, Begara-Morales JC, Corpas FJ, Barroso JB (2009) Involvement of reactive nitrogen and oxygen species (RNS and ROS) in sunflower–mildew interaction. Plant Cell Physiol 50:265–279
Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Corpas FJ, Chaki M, Fernández-Ocaña A, Valderrama R, Palma JM, Carreras A, Begara-Morales JC, Airaki M, del Río LA, Barroso JB (2008) Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol 49:1711–1722
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci 98:13454–13459
Dong J, Wu F, Zhang G (2006) Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 64:1659–1666
Drążkiewicz M, Skórzyńska-Polit E, Krupa Z (2003) Response of the ascorbate–glutathione cycle to excess copper in Arabidopsis thaliana (L.). Plant Sci 164:195–202
Feechan A, Kwon E, Yun B-W, Wang Y, Pallas JA, Loake GJ (2005) A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci 102:8054–8059
Foyer C, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071
Gratão P, Monteiro C, Antunes A, Peres L, Azevedo R (2008) Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium‐induced stress. Ann Appl Biol 153:321–333
Grennan AK (2007) Protein S-nitrosylation: potential targets and roles in signal transduction. Plant Physiol 144:1237–1239
Innocenti G, Pucciariello C, Le Gleuher M, Hopkins J, Stefano M, Delledonne M, Puppo A, Baudouin E, Frendo P (2007) Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta 225:1597–1602
Jensen D, Belka G, Du Bois G (1998) S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J 331:659–668
Jiménez A, Gómez JM, Navarro E, Sevilla F (2002) Changes in the antioxidative systems in mitochondria during ripening of pepper fruits. Plant Physiol Biochem 40:515–520
Kubienová L, Tichá T, Jahnová J, Luhová L, Mieslerová B, Petřivalský M (2014) Effect of abiotic stress stimuli on S-nitrosoglutathione reductase in plants. Planta 239:139–146
Kuźniak E, Skłodowska M (2001) Ascorbate, glutathione and related enzymes in chloroplasts of tomato leaves infected by Botrytis cinerea. Plant Sci 160:723–731
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS (2001) A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410:490–494
Luna CM, González CA, Trippi VS (1994) Oxidative damage caused by an excess of copper in oat leaves. Plant Cell Physiol 35:11–15
Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol 28:131–140
Nieboer E, Richardson DHS (1980) The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environmental Pollution Series B, Chemical and Physical 1:3–26
Noctor G, Foyer CH (1998) ASCORBATE AND GLUTATHIONE: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Noctor G, Arisi A-CM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49:623–647
Ortega-Galisteo AP, Rodríguez-Serrano M, Pazmiño DM, Gupta DK, Sandalio LM, Romero-Puertas MC (2012) S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: changes under abiotic stress. J Exp Bot 63:2089–2103
Panda P, Nath S, Chanu TT, Sharma GD, Panda SK (2011) Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.). Acta Physiol Plant 33:1737–1747
Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium(IV). Anal Biochem 139:487–492
Qadir S, Qureshi MI, Javed S, Abdin MZ (2004) Genotypic variation in phytoremediation potential of Brassica juncea cultivars exposed to Cd stress. Plant Sci 167:1171–1181
Sandalio LM, Dalurzo HC, Gomez M, Romero‐Puertas MC, Del Rio LA (2001) Cadmium‐induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Sanghani PC, Davis WI, Fears SL, Green S-L, Zhai L, Tang Y, Martin E, Bryan NS, Sanghani SP (2009) Kinetic and cellular characterization of novel inhibitors of S-nitrosoglutathione reductase. J Biol Chem 284:24354–24362
Sanità di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot 63:158–167
Smeets K, Cuypers A, Lambrechts A, Semane B, Hoet P, Van Laere A, Vangronsveld J (2005) Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol Biochem 43:437–444
Sohal RS, Mockett RJ, Orr WC (2002) Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med 33:575–586
Squadrito GL, Pryor WA (1995) The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem Biol Interact 96:203–206
Srivastava S, Tripathi RD, Dwivedi UN (2004) Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa—an angiospermic parasite. J Plant Physiol 161:665–674
Stewart RRC, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–248
Vig K, Megharaj M, Sethunathan N, Naidu R (2003) Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res 8:121–135
Wang X, Liu Y, Zeng G, Chai L, Song X, Min Z, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62:389–395
Wang Q, Liang X, Dong Y, Xu L, Zhang X, Hou J, Fan Z (2012) Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul 69:11–20
Xiong J, Fu G, Tao L, Zhu C (2010) Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys 497:13–20
Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330
Zheng W, Liu Y, Pan S, Yuan W, Dai Y, Wei J (2011) Involvements of S-nitrosylation and denitrosylation in the production of polyphenols by Inonotus obliquus. Appl Microbiol Biotechnol 90:1763–1772
Acknowledgments
The authors would like to thank the financial support from the National Natural Science Foundation of China (Grant No. 41271332), the Natural Science Foundation of Hunan Province, China (Grant No. 11JJ2031), and the Science and Technology Planning Project of Hunan Province, China (Grant No. 2012SK2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wang, D., Liu, Y., Tan, X. et al. Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea (L.) Gaud under cadmium stress. Environ Sci Pollut Res 22, 3489–3497 (2015). https://doi.org/10.1007/s11356-014-3581-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3581-5