Abstract
The large estuary that the River Po forms at its confluence into the Adriatic Sea comprises a multitude of transitional environments, including coastal lagoons. This complex system receives the nutrients transported by the River Po but also its load of chemical contaminants, which may pose a substantial (eco)toxicological risk. Despite the high ecological and economic importance of these vulnerable environments, there is a substantial lack of information on this risk. In light of the recent amendments of the European Water Framework Directive (2013/39/EU), the present study investigated the sediment contamination of six coastal lagoons of the Po delta and its effects on Manila clams (Ruditapes philippinarum), exposed in situ for 3 months. Sediment contamination and clam bioaccumulation of a wide range of chemicals, i.e. trace metals (Cd, Cr, Ni, Hg, Pb, As), polybrominated diphenyl ethers (PBDEs), alkylphenols (APs), organochlorine compounds (PCBs, DDTs), polycyclic aromatic hydrocarbons (PAHs) and organotins (TPhT, TBT), suggested a southward increase related to the riverine transports. Where the River Po influence was more direct, the concentrations of contaminants were higher, with nonylphenol and BDE-209 exceeding sediment quality guidelines. Biometric indicators suggested the influence of contamination on organism health; an inverse relationship between PBDEs in sediments and clam condition index has been found, as well as different biota-sediment accumulation factors (BSAFs) in the lagoons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many contaminants discharged to riverine environments are easily sorbed to sediment particles and are thereby transported for long distances along watercourses, often alternating deposition and resuspension processes but remaining bioavailable to exert biological effects (Mearns et al. 2013; Jahnke et al. 2014). In fact, many studies have demonstrated that contaminated sediments can directly threaten benthic species, but many other organisms can be indirectly exposed to sediment chemicals via trophic transfer (Chapman et al. 2013). In highly anthropised environments, it is difficult to assess whether the multitude of chemicals generally present may pose a risk to the aquatic community or to human consumption. As may be perceived, this problem is common in transitional and coastal environments, which, often located at the closure of drainage basins, receive both nutrients and contaminants from the multiple sources of entire basins. If nutrients can support a higher productivity, the loads of contaminants expose the communities of these important environments to potential toxic effects. The nature of coastal lagoons and estuarine areas typically facilitates deposition processes, increasing the risk posed by contaminated sediments. At the same time, in many regions worldwide, the higher productivity of these environments has substantially favoured aquaculture activities with important economic benefits; thus, the protection of these vulnerable ecosystems and the control of human risks due to seafood consumption should be of utmost importance (EC 2013; Newton et al. 2013).
The Po River is the most important Italian watercourse, and its wide drainage basin contributes to more than 35 % of Italian agricultural, livestock and industrial production. These human activities originate important organic loads, estimated in 114 × 106 inhabitant equivalents (AdbPo 2006). The Po River also transports inorganic nutrients, trace metals and a multitude of compounds, making it the primary source of land-derived nutrients to the Northern Adriatic Sea and one of the main sources of contaminants to Adriatic environments (Giani et al. 2012). Its delta has been traditionally exploited for different types of aquacultures, and its suitability for mollusc farming recently led to the development of flourishing commercial activities (Abbiati et al. 2010). In particular, the clam Ruditapes philippinarum, called the Manila clam, which was first introduced in Italy in the 1980s for marine farming, was soon shown to be more tolerant and faster growing than the native species, Ruditapes decussatus, allowing much higher production yields (Turolla 2008). As a result, through intensive farming, the Manila clam rapidly colonised several coastal areas of the Adriatic Sea and the Po River delta, making Italy the second-highest producer in the world after China (FAO 2014).
Bivalves are widely used as sentinel species because of their limited biotransformation activities and high tolerance to xenobiotics, in addition to their economic importance. For example, being distributed in many intertidal areas around the world, the Manila clam has been used to investigate environmental quality (Sacchi et al. 2013; Dedeh et al. 2014). Previous studies have demonstrated that several priority and endocrine-active compounds that originate from the tributaries of the River Po are transported with particulate material along its course, up to the transitional and coastal environments of the river delta (Viganò et al. 2015; Casatta et al. 2015). Originally designed for a specific action, pesticides, flame retardants, plasticisers or surfactants were found to have endocrine-disrupting activity and side effects on organisms, either mimicking or blocking hormones and thus disrupting normal physiological functions. Recently, it was shown that relatively few of the multitude of chemicals reaching the Po delta were potentially of (eco)toxicological concern; for example, among the many flame retardants reaching these environments, only polybrominated diphenyl ethers (PBDEs) were identified as concerning (Casatta et al. 2015). In this study, we provide further evidence of these recent results by focusing on chemicals that were previously identified as being of particular interest: alkylphenols among surfactants; PBDEs among flame retardants; DDT, polychlorinated biphenyls and polycyclic aromatic hydrocarbons among legacy pollutants; and organotin compounds. In parallel, we analysed trace metal contamination, paying particular attention to Ni and Cr, given their abundance of geochemical origins in the Po River basin (Amorosi and Sammartino 2007). Importantly, most of the considered chemicals are listed as priority substances in the recent amendments of the European Water Framework Directive (WFD; EC 2013). In addition to analysing sediment contamination or clam bioaccumulation, we also focused on bivalve responses to the combination of habitat and contaminant factors that likely occur in the delta system. Furthermore, in consideration of the endocrine-disrupting properties of the aforementioned chemicals, we also investigated whether specific gonadal effects could be induced by the exposure to the lagoons of the delta. To achieve all of these objectives, groups of young R. philippinarum were exposed in field for 3 months to bed sediments of the major Po delta lagoons. The present results are discussed to assess whether some of these contaminants may pose a risk to aquatic organisms or to human consumption, contributing to the prioritisation of environmental problems in estuarine lagoons and thus to the applicability of the WFD.
Material and methods
Area of study
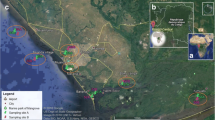
The exposure sites were located in six lagoons of the Po River delta (Fig. 1). The lagoons were chosen to represent potentially different transitional ecosystems. Table 1 reports the major geographical and morphological features of these lagoons. Notably, 3 of them—i.e. Marinetta, Canarin and Sacca di Goro—receive direct freshwater inflows from the Po River. A brief description of each lagoon is given below.
Following a north–south direction, the first lagoon investigated was Caleri. The hydrodynamics of this large and shallow area primarily depends on the water exchange with the sea through a narrow mouth, which is located close to our exposure site. This easternmost part is designated for clam aquaculture. Marinetta is connected to the sea by a narrow mouth and directly receives freshwater through a deltaic branch of the Po River (Po di Levante). Only a small area, in the westernmost part of the lagoon, is designated for clam farmers (Vincenzi et al. 2014).
Barbamarco is the smallest and shallowest lagoon examined in this study. It is connected to the Adriatic Sea through two main openings, and no direct freshwater inputs are present. According to coastal currents, freshwater input indirectly comes from the distributaries of the Po di Tramontana or the Po di Maistra via the sea mouths. The opening of the only other waterway located in the southern part of the lagoon is strictly regulated and discontinuous (Spillman et al. 2009).
Canarin currently presents only a small mouth to the sea in its northern part. It directly receives freshwater through minor distributaries originating from the primary branch of the Po River, the Po di Pila, which accounts for more than the 50 % of freshwater discharge and sediment load of the Italian river (Nelson 1970). Water circulation in the entire southern area of the lagoon is very limited, whereas in the northern part, it is more effective, due to the strong and direct freshwater input. Canarin is subjected to eutrophication, with extensive growth of algae and frequent anoxic periods (ARPAV 2004).
Sacca di Scardovari is the largest embayment of the area and is located between the Po di Tolle and Po di Gnocca distributaries. As a result of the main coastal currents, it receives Po waters indirectly, through the wide mouth that connects the lagoon to the Adriatic Sea. The southern area hosts intensive bivalve cultures (ARPAV 2004).
The last and southernmost exposure site is located in Sacca di Goro, one of the most important Italian rearing sites for R. philippinarum. It is separated from the Adriatic Sea by a narrow sandy barrier that regulates seawater exchanges and has several freshwater drainage canals, with the main input coming from the Po di Volano (Sacchi et al. 2013). The outermost area of the lagoon is part of a regional “area of biological protection”, which was recognised as being suitable for the spontaneous reproduction and growth of clams and other species of commercial interest.
Clam exposure and experimental design
In each lagoon, an area of 5 m × 5 m was selected, and juvenile clams of R. philippinarum from a common batch (mean length, 29.4 ± 5.7 mm; mean weight, 6.8 ± 1 g) were seeded on 12 and 13 March 2014 at the density of 3 kg/m2, according to farming practises. We exposed a common pool of clams, avoiding autochthonous organisms, to exclude the effects of life history adaptation to specific conditions (Paul-Pont et al. 2010). Moreover, although the conditions were much more variable than a laboratory study, this experimental design appeared to be the most faithful to real environmental conditions, and considering its length of time, it was the best way to highlight significant alterations for the populations inhabiting the lagoons. After 3 months of exposure (mid-June), approximately 100 clams were sampled at each of the six sites and brought on ice to the laboratory, where they were kept at −80 °C until the next measurements and analyses. Clams were properly rinsed with milliQ water, blotted on filter paper and characterised for shell length and wet weight. After lyophilisation, clams were pooled and homogenised. The dry weight was determined on three pooled samples for each lagoon and the lipid content was determined in a subsample of the Soxhlet extracts. Chemical analyses were then conducted on a pooled and homogenised sample of dried clam tissues that was obtained for each exposure site. The Clam Condition Index (CI) was calculated as the ratio between the dry weight of soft tissues (mg) and the shell length (mm; Cataldo et al. 2001). CI is a physiologic parameter generally used to provide information about the state of the nourishment of bivalves. High CI values are related to the presence of organic constituents that support growth, whereas low values reflect periods of stress, involving utilisations of reserves (Beninger and Lucas 1984). From the same exposure sites, and contemporaneously to clam sampling, the upper layer of bed sediments (0–10 cm) was collected using a small Ponar grab sampler. Composite sediment samples were refrigerated during transport and stored at −80 °C within a few hours of sampling, until freeze-drying and analysis. The sediment samples were sieved to determine the particle-size composition and to separate the fine fraction (<63 μm), on which chemical analyses were performed. Total organic carbon (OC) and nitrogen (N) were also measured.
Chemical analysis
Organochlorine compound, polybrominated diphenyl ethers (PBDEs) and polycyclic aromatic hydrocarbons (PAH) analysis
Chemical analyses of organochlorine compounds and PBDE in sediments and clams were described in previous studies (Poma et al. 2014a; Poma et al. 2014b; Casatta et al. 2015). A detailed description is provided in the Online resources. Briefly stated, dried samples (0.5–1 g) of clams and of the fine fraction of sediments were spiked with 50 μL of an internal standard solution that contained decabromobiphenyl (BB-209, 1000 μg/L) and the labelled compounds [13C12] PCB-101, [13C12]PCB-153, [13C12]p,p′-DDT, and [13C12]BDE-28, [13C12]BDE-47, [13C12]BDE-99, [13C12]BDE-153, [13C12]BDE-154, [13C12]BDE-183 (250 μg/L), purchased from Wellington Laboratories, Inc., Canada. Unless otherwise indicated, all other analytical standards were purchased from Sigma-Aldrich, Germany. Regarding the analysis of PCBs, PBDEs and DDTs, the samples were extracted using a hot Soxhlet apparatus (Büchi, Switzerland) using an n-hexane/acetone mixture (3:1, v/v). The clean-up procedure was performed using a multi-layer column (1.5 × 20 cm) packed (bottom to top) with 1.5 g of acidified silica gel 30 % with sulphuric acid (Sigma-Aldrich, Germany) and 1.5 g of Florisil® (100–200 mesh, Sigma-Aldrich, Germany). The column was pre-washed with 15 mL of n-hexane/dichloromethane (1:1 v/v), and the elution was performed by collecting 40 mL of the same solvent. One milliliter of toluene was added to the extract, concentrated by Turbovap on a gentle nitrogen stream, and then reconstituted to 100 μL using toluene. Gas chromatography (GC) analysis for organochlorine compounds and PBDEs was performed using a Thermo Electron TraceGC 2000 coupled with a PolarisQ Ion Trap (ThermoElectron—Austin, TX) mass spectrometer and equipped with a PTV injector and an AS 3000 auto-sampler. The system was managed by ThermoFinnigan Xcalibur software version 1.4.1. Detection and quantification was done in selected ion monitoring (SIM) mode.
PAHs were extracted from sediments and clams using an ultrasonic bath (Martinez et al. 2004). Before extraction, dried samples of clams (0.2 g) and a fine fraction of sediment (1 g) were spiked with an internal standard solution that contained 50 ng of each labelled compound (acenaphthene-d10, phenanthrene-d10, chrysene-d12 e perylene-d12) purchased from Sigma-Aldrich. Samples were extracted three times using dichloromethane (20 mL) at 40 °C and 59 kHz for 20 min; the extracts were jointed, evaporated to 1 mL under a nitrogen stream and cleaned up using a column packed with a mixture of silica gel and neutral alumina (2:1 v/v). First, the columns were washed with dichloromethane (20 mL), and then, compounds were eluted by collecting 20 mL of dichloromethane. Finally, 1 mL of toluene was added, and the extract was evaporated to 200 μL under a gentle nitrogen stream. A gas chromatography–mass spectrometry (GC-MS) analysis of PAHs was performed with a Focus GC (Thermo Fisher, USA) equipped with a TriPlus autosampler. GC was coupled to a DSQ single quadrupole mass spectrometer (Thermo Fisher, USA). A capillary column (SUPELCO SLB-5 MS 30 m × 0.25 mm I.D., 0.25 μm film thickness) was used in the following conditions: carrier gas, helium (1.3 mL/min); injector temperature, 270 °C (split mode, 10:1); initial oven temperature was 90 °C (held for 1.5 min) increased to 270 °C at 20 °C/min and maintained in isothermal condition for 15 min; transfer line at 270 °C. Detection and quantification was done in SIM mode.
Alkylphenols (AP) analysis
Determinations of AP were performed in accordance with the CEN/TC CSS99040 method with a modification of the chromatographic analysis, as previously reported (Casatta et al. 2015). The extraction and clean-up were performed using pressurised liquid extraction (PLE) with an ASE-200 instrument (Thermo Fisher Scientific). Briefly, 0.67 g of freeze-dried sample was mixed with neutral alumina and copper at a ratio of 1:3:3. The extraction was conducted using an acetone/n-hexane mixture (1:1 v/v, three cycles, 60 °C). The extract was evaporated to 0.5 mL and analysed by ultra-performance liquid chromatography tandem mass-spectrometry (UPLC/MS-MS; Waters Acquity interfaced to an AB-Sciex API-5000).
Organotins analysis
The extraction of organotins was performed using PLE with an ASE-200 instrument (Thermo Fisher Scientific). The determined compounds were triphenyl-tin, Sn(C6H5)3 + and tributyl-tin, Sn(C4H9)3 +. One gram of freeze-dried sample was mixed with neutral alumina and copper at a ratio of 1:5:5. The mixture was placed in 66 mL extraction cells between two layers of diatomaceous earth. Extraction was performed with methanol (three cycles, 110 °C). The volume of the organic extract was reduced to 1 mL. The determination was performed by liquid chromatography tandem mass-spectrometry (LC/MS-MS) using a water/methanol gradient. Each solvent contained 5 mM of ammonium formate and 0.1 % formic acid. The gradient analysis started at 90/10 and was changed to 10/90 in 10 min. Finally, an isocratic step of 5 min was applied. The procedure was validated using a certified reference material of freshwater sediment (Quevauviller and Ariese 2001).
Trace metal analysis
Total mercury concentrations were determined with thermal decomposition, amalgamation and atomic absorption spectrometry in accordance with US-EPA method 7473 (US-EPA 1998) using an automated Hg Mercury Analyzer (AMA254, FKV, Bergamo, Italy). After freeze-drying and homogenising, each sample was analysed in triplicate (0.04 mg for clam tissue and 0.1 g for sediments), and precision was generally better than 5 %. Accuracy was checked using certified reference materials BCR 278 Mussel tissue from the Bureau Communautaire de Référence for organisms and GBW07305 from the National Standard Centre of China for sediments. For both materials, the accuracy was within ±10 % of certified values. As, Ni, Cr, Pb and Cd concentrations were measured using graphite furnace atomic absorption spectrometry (GF-AAS) with a Perkin Elmer AAnalyst 600 with Zeeman correction, equipped with a graphite atomisation system, after the microwave-assisted digestion (Preekem EU Excel 2000) of dried samples (0.1 g) with 5 mL of concentrated HNO3 acid (VWR, Suprapur). Procedural blanks were regularly run to check for ambient or reagent contamination.
Quality assurance and quality control
Quality control (QC) steps were taken to monitor and ensure the precision and accuracy of the obtained experimental results. QC practices included the analysis of quality control samples within each set of samples. They included calibration standards, certified reference materials (where available), spiked samples, duplicate sample analysis and blanks. Additional QC measures included the analysis of blind duplicate samples. Quality assurance (QA) activities included the review of analytical methods, the evaluation of non-conformances and the review of quality data. The international standards followed were ISO 17025 and ISO 9001. The limit of detection (LOD) and the limit of quantification (LOQ) were evaluated for all investigated organic pollutants. Specifically, LODs were evaluated as the concentration, allowing for a signal threefold higher than the noise to be obtained. LOQs were evaluated as three times the LOD and are reported in Table S1 (Online resources).
Regarding biological samples, the method performance for PCBs, PBDEs, DDTs and PAHs was checked by analysing the NIST SRM2974a. Sediment quality assurance was conducted by analysing candidate reference material BROC-2 provided by the RIVO (The Netherlands Institute for Fisheries Research). For PCBs and PBDEs, all measured values (n = 4) were within the range of the expected concentration (±30 %), with relative standard deviations (RSD) below 20 %. Internal standard recoveries were calculated for each sample, and the analysis was repeated if the value was below 30 %. A procedural blank was analysed every eight samples to check for laboratory contaminations. The results of the procedural blanks were always lower than the determined LODs for organochlorine compounds and PBDEs. The analytical methods for AP were validated by spiking a known amount (between 0.5 and 2 ng/g) of the pollutants onto sediments and then running the analytical protocol using the standard addition method for calibration. The recoveries ranged between 65 and 112 %: the PLE procedure that was employed was effective in both extracting the organic pollutants and avoiding the use of a post-extraction column for the removal of polar contaminants. The matrix effect was evaluated for all pollutants. A labelled internal standard, i.e. 13C6-OP2EO, was added to the extracted samples. Linear regressions were obtained over a concentration range of 3 orders of magnitude for all organic pollutants investigated. For each trace metal and each sample, an analysis was run in duplicate, and the precision was generally better than 5 %. The accuracy of recovery was between 75 and 92 %, as estimated using reference materials BCR 278 for organisms and GBW07305 for sediments.
Histological analysis
Simultaneously to the collection of samples for chemical analysis, 15 individuals per exposure site were freshly dissected, and their gonads were immediately fixed in 10 % neutral buffered formalin for 24 h. The tissues were subsequently dehydrated in a series of increasing concentrations of ethanol solutions (70–80–90–100 %) and then embedded in paraffin wax (Diapath, melting point 56–58 °C). Serial sections were cut at 5 μm using the rotary microtome (Leica RM 2145) and then treated with hematoxylin-eosin staining solution. A microscopic examination under the optic microscope (×4–×40) aimed to determine the stages of gonadal development. Clam gonad development was classified in accordance with Lubet (1959).
Hazard quotient (HQ)
The evaluation of hazard from sediment chemistry followed the calculations proposed by Piva et al. (2011). The HQ is initially based on the calculation for each pollutant of a ratio to reference (RTR), i.e. the ratio between the concentrations measured and those indicated by various sediment quality guidelines. In this study, the considered guidelines were Italian legislation D.M. 260/2010, the Region III BTAG Marine Sediment Screening Benchmarks (US-EPA 2006) and the guidelines provided by European and Canadian authorities for nonylphenol and PBDE, respectively (EC 2002; Environment Canada 2013). The RTR is weighted (RTRw) to account for the typology of a contaminant, i.e. whether it is a “priority” or “priority and hazardous” pollutant, according to the WFD (EC 2013). In the calculation of the specific hazard quotient, an average RTRw is obtained for all parameters with RTR < 1 (i.e. values below the SQG), whereas for those with RTR ≥ 1, the RTRw are individually added into the summation. With this calculation, the HQ increases according to both the number and the magnitude of the exceeding parameters. The resulting values of HQ are then assigned to one out of five classes of chemical hazards: Absent, Slight, Moderate, Major and Severe (Piva et al. 2011).
Data analysis
Statistical analyses of the clam length and weight measurements were performed using GraphPad Prism v.6 (La Jolla, CA, USA). The normality and homogeneity of data were verified using Shapiro-Wilk’s test and Levene’s test, respectively. The differences among the six groups were evaluated using one-way ANOVA, followed by Tukey’s post hoc test. The criterion for significance was set at p < 0.05. The Pearson correlation coefficients (r) were calculated using Statistica 7 (StatSoft, Inc., 2004). The data are reported as the mean ± standard deviation (SD).
Results and discussion
Sediments and clams
The results of the main physical and chemical parameters of the six exposure areas of the Po River delta and the particle-size composition of their sediments are shown in Table 1. The temperature, dissolved oxygen and pH did not show relevant variations among the lagoons, likely due to the results of variable combinations of water circulation and depth, freshwater and seawater inputs. The values measured for these three parameters should have not deviated from the tolerance limits defined for optimal the clams’ growth (Vincenzi et al. 2007), as reported in Table 2. On the contrary, the other environmental parameters, i.e. OC, C/N and particularly salinity, suggest that Caleri, Barbamarco and Scardovari can be can be distinguished from the other three lagoons. In fact, the higher salinity values confirm that they do not receive direct freshwater inputs and are primarily characterised by their relationship with the marine coastal environment. Scardovari receives no direct freshwater contributions, but its wide sea mouth is exposed to the indirect effects of the Po di Tolle and the other northern branches of the Po River (Fig. 1). In fact, the lower OC and C/N values of this wide lagoon are shown to be more similar to the other three environments receiving direct contributions from the Po River, i.e. Marinetta, Canarin and Goro. Although wide variations may exist, C/N ratio can be a useful indicator of the origin of the organic matter (Yu et al. 2010). Accordingly, the higher values of Caleri and Barbamarco (Table 1) suggest a higher contribution of fresh algal organic material, benthic marine macrophytes or, more in general, of marine particulate organic matter (Faganeli et al. 1988). The lower values observed in the other lagoons might also be due to algal production, but a denser bacterial community, typically characterised by lower C/N ratios, is likely more important and supported (directly or not) by the River Po (Faganeli et al. 1988; Yu et al. 2010).
Table 2 reports the main morphological parameters determined in clams after 3 months of exposure. Significant differences among clams from the six lagoons were observed: clams exposed in Caleri and Barbamarco, the two lagoons less influenced by freshwater, grew significantly more than those exposed in all other lagoons. Caleri, in particular, hosted the largest specimens (p < 0.05), with an average growth rate of approximately 1.3 mm/month. By contrast, clams exposed in the Lagoon of Canarin grew very slowly. Their average increase in shell length was only 0.23 mm/month, and they had the lowest wet and dry weight soft tissues (p < 0.05; Table 2). The lipid contents were only partially consistent with the growth trend. The highest content was found in the exposed group in Caleri, the lagoon substantially providing the best growth conditions, according to all indicators, but a high lipid content was also found in Canarin clams, the site apparently offering the worst conditions. The ecotoxicological interpretation of lipid contents has often been controversial; compared with previous studies’ observations on fish or other taxa, the amount of lipid reserves may show unexpected trends, not necessarily related to organism health. Higher lipid reserves were observed, for example, in fish that lived in polluted environments and had several histological damages. The investment in lipid reserve, rather than in growth or reproduction, seems to be a complex process that depends on several factors, such as food quality and quantity, species tolerance or contaminants (Marshall Adams 1999; Kerambrun et al. 2014). In this respect, the clam condition index (CI) has potentially a higher informative content, and it shows the following rank order: Caleri > Barbamarco/Goro/Marinetta > Scardovari > Canarin (Table 2). This finding confirms that the best environmental conditions were in the northernmost lagoon, Caleri, whereas the worst were in Canarin. These data further reflect the mentioned distinction between the marine lagoons and those exposed to Po River waters, and they confirm that many factors interact to determine the final results.
The histological analyses of clam gonads, necessarily conducted on a subsample of each exposure group, did not reveal any significant alterations (Table S9). All individuals reached similar gonadal maturation, with only a minor percentage of individuals in a post-spawning phase. Importantly, we obtained a homogeneous stadium of development; we did not, however, note differences in the stage of reproductive maturity among the clams exposed in the six lagoons after 3 months of exposure (Fig. S1, Online resources).
Alkylphenols and flame retardants
Alkylphenols belong to the group of non-ionic surfactants and are widely used for many applications. They have been considered for many years as endocrine disrupters, leading to several official restrictions (David et al. 2009). Nevertheless, they are important components of the sediment contamination of Po delta environments. They represent approximately 50 % of the overall amount of organic chemicals detected in the six lagoons (Fig. 2a). In this class of contaminants, there is a marked prevalence of nonylphenol (NP) and its mono-ethoxylate (NP1EO), generally accounting for 90 % of total AP contamination (Table 3). Similar results have previously been reported for Po River sediments, which, transported to the estuary, are evidently responsible for the present results (Viganò et al. 2015). Accordingly, a lower NP and NP1EO contamination is present in Caleri and Barbamarco, the two lagoons that do not receive the waters of the Po River (Table 3). Marinetta, Canarin, Scardovari and Goro show NP concentrations in the range of 57.3–202.3 ng/g dw. It is important to note that these values exceed the predicted no effect concentration for sediments (PNECsediment) of 39 ng/g provided by European authorities (EC 2002). NP may thus threaten the health of benthic organisms living in areas exposed to the Po River. Nevertheless, the present AP concentrations are in the low range of most values reported in the literature (David et al. 2009 and references therein). Consistent with sediment contamination, NP and its ethoxylates (primarily NP1EO) bioaccumulated in clam tissues at concentrations higher than all other chemicals in this study (Table 3). Although nonylphenols and octylphenols are listed as hazardous substances in the WFD, no environmental quality standard (EQS) is given for biota for secondary poisoning or human health protection.
The contamination profile for organic pollutants (a) and metals (b) determined in whole sediment samples from the six lagoons considered in this study. AP, alkylphenols; PAH, polycyclic aromatic hydrocarbons; PBDE, polybrominated diphenyl ethers; PCB, polychlorinated biphenyls; DDT, p,p′-DDT and its metabolites and isomers; TBT, tributyl-tin
Regarding PBDEs, there is increasing scientific evidence of the endocrine-disrupting properties of several congeners and of their induction of neurobehavioral and reproductive disorders and thyroid hormone-level alterations in both humans and wildlife (Yogui and Sericano 2009). In this study, the decabrominated congener BDE-209 accounted for more than 95 % of the contamination in all sediment samples, followed by BDE-47 and BDE-99 (Table 3). These results confirm the prevailing uses of deca-BDE technical formulations previously documented for the Po River basin and provide further evidence that the BDE-209 load can be transported to its deltaic region and, thereby, to the Adriatic Sea (Viganò et al. 2011; Viganò et al. 2015; Casatta et al. 2015). Similarly to AP, the lowest PBDE concentrations were in fact observed in Caleri and Barbamarco, which receive no inputs from the Po River. The concentrations of PBDEs in this study are in line with those reported for sediments from other estuarine and coastal areas (Ramu et al. 2010; Stewart et al. 2014). The Canadian authorities set Sediment Quality Guidelines for the protection of aquatic life, intended to protect benthic and pelagic organisms, from PBDE contamination (Environment Canada 2013). In particular, the threshold concentration of BDE-209 in sediment was set to 19 ng/g dw. According to this value, the sediments from the Lagoon of Canarin were 1.5 times higher than the guideline (28.6 ng/g dw, normalised to 1 % OC). It is interesting to observe that the concentration in the fine material (<63 μm) of the three other lagoons influenced by the Po transport, in turn, exceeded this guideline (40.7, 47.3 and 25.1 ng/g dw normalised to 1 % OC for Marinetta, Scardovari and Goro, respectively). These results deserve more attention because several sites of the Po delta and coastal environments could be more favourable for the sedimentation of fine particles, thus exposing these areas to a higher risk. In this context, it is worth mentioning the inverse relationship found between PBDEs in lagoons’ fine materials and the CI of exposed clams (R 2, 0.76; p = 0.02). Although this result does not imply a primary causative role of these flame retardants, it increases concern regarding their presence and potential effects in the whole River Po environment. Regarding the other congeners, only BDE-47 and BDE-99 were detectable in sediment samples, at concentrations far below existing thresholds (Environment Canada 2013).
BDE-209 bioaccumulated in clams exposed to Scardovari and Goro, whereas it was not detected (<3 ng/g dw) in clams from Marinetta or, unexpectedly, from Canarin, which contained the highest sediment contents of this congener. The latter result has no clear explanation, but it was confirmed by further analyses performed on wild specimens collected from the same lagoon (data not shown). Although this result suggests that exposed clams were equilibrated with lagoon sources of PBDE, the main cause of this apparent lack of accumulation remains unclear. Previous studies have hypothesised that bivalves can biotransform PBDEs (Wang et al. 2009; La Guardia et al. 2012; Poma et al. 2014b; Tian et al. 2015). Although this potential cannot be ruled out, clams exposed to Canarin also showed a lower biota-sediment accumulation factors for PCB than those exposed to Scardovari and Goro, which had a lower PCB in their sediments (Table 4; see below). Only a few minor congeners were detectable in clam tissues, at concentrations (from 0.008 to 0.012 ng/g ww) in the range of the limit provided by the WFD for biota (0.033 ng/g ww for the sum of six PBDE congeners, if re-calculated for bivalves, as detailed in previous studies; EC 2013; Casatta et al. 2015).
Legacy pollutants
Concerning legacy pollutants, the concentration of DDT isomers and 14 PCB congeners was analysed (Table 3). The widespread contamination of the highly persistent DDTs is a common result in areas directly or indirectly exposed to the effects of agriculture activities or to solid waste disposal, which can contribute to soil contamination (Mrema et al. 2013). The chronic toxicological endpoints of this pesticide are well known and include growth, reproductive and behavioural responses (US-EPA 2000; Mrema et al. 2013). Almost all DDT isomers were found in lagoon sediments, with a prevalence of p,p′-DDE. The spatial trend of ∑DDT suggests a role of the Po River in contaminating four lagoons, with Caleri and Barbamarco again showing lower concentrations. In this context of diffuse contamination, it is interesting to observe that with the exception of Caleri, the parental insecticide p,p′-DDT was found in all lagoons, although at a low concentration range. This finding seems to confirm recent studies that showed an important source of poorly transformed DDT residues within the River Po basin, precisely in the area of Lake Maggiore, where previous industrial activities were located (Guzzella et al. 1998; Bettinetti et al. 2012; Viganò et al. 2015). Accordingly, the p,p′-DDE/p,p′-DDT ratio calculated for our samples showed values of approximately 3 or higher, indicating aged inputs (Hitch and Day 1992). The present results are below the maximum concentrations set by the Italian legislation for sediments of transitional coastal systems (1, 0.8 and 1.8 ng/g for DDTs, DDDs and DDEs, respectively; D.M. 260/2010; although other areas with higher sedimentation rates could easily exceed these limits). Partly in agreement with sediment contaminations, the pattern of DDTs’ bioaccumulation in clams seems to confirm the importance of River Po transport, although the minimum values observed in Canarin and the relatively high values of Caleri, both unexpected, further propose the complexity of the deltaic system and factor interactions. As discussed below, the relative importance of suspension feeding and the likely differences in chemical bioavailability can be deemed relevant factors (Sfriso et al. 2014).
The PCB contamination was measured in sediment samples, and their congener profiles reflect what was previously reported for the Po River, whose contamination was generally characterised by the prevalence of PCB-101, PCB-138, PCB-149, PCB-153 and PCB-180 (Viganò et al. 2015). This profile is the result of the technical mixtures that were more widely used in Northern Italy, i.e. Aroclor 1254 and 1260 (Viganò et al. 2007). No significant differences in the congener distributions were observed in the sediments of the six lagoons, identifying the common origin of this widespread contamination. Moreover, the direct or indirect contribution of the Po River seemed to be evident from a north–south increasing trend of contamination. The prevailing marine currents, moving the same direction, likely contribute to this pattern (Table 3; Falcieri et al. 2014) and that is the reason why the lagoons located south of the main branch of the Po di Pila may be exposed to additional loads of chemicals. As shown in Table 3, clams’ bioaccumulation of PCBs provided further evidence of these exposure conditions in the Po delta. Adverse effects of PCBs on biota and on humans were widely studied and comprise reproductive deficiencies, immune- and neuro-toxicity, carcinogenicity and endocrine effects (Erickson 2001). Compared to the literature, present sediment concentrations of ΣPCB are lower than those reported, for example, in the Venice Lagoon or in other transitional environments (Van Ael et al. 2012; Moschino et al. 2012). Moreover, no sediment sample exceeded the threshold of 8 ng/g dw set by the Italian legislation for the protection of marine and human health (D.M. 260/2010). Nevertheless, the present PCB levels are close to this value or other threshold values (e.g. Lopes et al. 2012), so it is easy to predict that less favourable grain-size compositions would generate limit exceedances and thus present a risk to the environments of the Po delta. Thus, although PCB use was banned in the 1970s, these chemicals are worthy of attention in the Po River deltaic region. Clam congener profiles substantially reflected sediment contamination, confirming the low capacity of clams to metabolise PCBs and their bioavailability to the aquatic community of the deltaic region. Comparable levels of contamination were reported for some fish species from the closing section of the Po River (Viganò et al. 2015) and for wild clams in the Sacca di Goro (Casatta et al. 2015).
PAHs and organotins
PAHs are ubiquitous environmental pollutants. Because they are human carcinogens and mutagens and toxic to all living organisms, both the European Community and the US Environmental Protection Agency have listed them as priority pollutants (Tobiszewski and Namieśnik 2012). PAH concentrations in sediments are shown in Table 3. The PAH levels were above the LOD for all chemicals analysed, with the exception of acenaphthylene. In all sediments, the PAH contamination was due to fluoranthene, pyrene, benzo[k]fluoranthene, perylene and also to phenanthene. Diagnostic ratios are often used to distinguish the influence of the multiple possible sources of PAHs. The application of these ratios indicated a main pyrolytic origin in all six exposure sites (Wang et al. 2008; Tobiszewski and Namieśnik 2012). The distribution patterns of the single PAHs are very similar across lagoons, and the role of the River Po as main determinant of delta contamination seems to be less evident. Other local sources of PAH likely superimpose, and the widespread use of fishing and recreational motor boats is the most probable pyrolytic sources. In any case, present sediment values are far below the SQG for transitional water bodies, indicated by the Italian legislation (D.M. 260/2010), and below the concentrations reported in other transitional environments (Van Ael et al. 2012; Moschino et al. 2012; Solé et al. 2013). The bioaccumulation profiles seem to be more heterogeneous and suggestive of different bioavailabilities, particularly higher in Scardovari and Goro but also in Barbamarco (Table 3). The causal factors are likely different, and at least for Scardovari and Goro, boat traffic and the widespread bed sediment reworking due to benthic organisms and clam culture activities can be hypothesised (Eggleton and Thomas 2004).
The WFD lists polyaromatic hydrocarbons among priority hazardous substances (EC 2013), but limits in biota are set only for fluoranthene and benzo[a]pyrene. The concentrations in clam tissues, as reported in Table 3, are far below both the European thresholds and the Environmental Assessment Concentrations defined by OSPAR for shellfish for the protection of marine biota (OSPAR 2009).
Finally, concerning organotin compounds, the WFD has recognised severe adverse effects on aquatic ecosystems (EFSA 2004). In this study, triphenyltin (TPhT) was undetectable in sediments and clams from the six lagoons, whereas tributyltin (TBT) was detected only at low levels. The highest concentration found in our sediment samples (0.64 ng/g dw in Canarin) is far below the SQG of 5 ng/g for TBT in transitional ecosystems set in the Italian Decree n. 260/2010 and even lower than TBT concentrations previously reported for Sacca di Goro (ARPA-EMR 2013). Moreover, the concentrations of TBT bioaccumulated in our clam tissues are at least 2 orders of magnitude below the tolerable daily intake (TDI) value of 0.25 μg/kgbw adopted for antifouling agents (EFSA 2004). Consequently, organotin contamination should not pose a threat for biota or human health.
Trace metals
In consideration of the known adverse effects of metals on biota at all levels of organisation, from cellular to the physiological and biochemical levels (De Paiva et al. 2015), we also measured the concentrations of trace metals in lagoon sediments (Table 3). Similar spatial trends were observed for all metals in the six sites, with increasing concentrations from the northernmost site, the Lagoon of Caleri, to the southern Sacca di Goro. As observed for some organic contaminants, the Lagoon of Canarin showed the highest whole sediment levels for all trace metals (Fig. 2b, Table 3). For some trace metals, e.g. Pb, Cd or Hg, the fine materials of northern lagoons are the most contaminated. Therefore, these data suggest that the sediment quality of the lagoons results from the interaction of the Po River transport, with its prevailing north–south direction, and the local properties of each lagoon, particularly their hydrodynamics. The present concentrations are below the SQGs for metals in transitional water bodies indicated by the Italian legislation (D.M. 260/2010) and below the guidelines set for the protection of both human and biota (US-EPA 2006; OSPAR 2009). The only exception is Ni, whose concentration (26.9 and 21 μg/g dw in Canarin and in Sacca di Goro, respectively) slightly exceeded the US-EPA benchmark for marine sediments of 15.9 μg/g dw (US-EPA 2006). It is known that the contamination of Ni but also of Cr in the Po River is mainly related to the geological composition of some areas of its drainage basins, with documented effects on sediment chemistry (Amorosi and Sammartino 2007). The present concentrations remain, however, below the threshold of 30 and 50 μg/g dw for Ni and Cr, respectively, as indicated in the Italian legislation (D.M. 260/2010); they are of low concern for biota, at least in terms of their exposure to sediments.
Concerning metal bioaccumulation, Ni, Cd and Cr concentrations in clam tissues reflected the Po River footprint described for sediments: the lowest concentration was found in clams exposed in the Lagoon of Caleri, with an increasing trend moving to the south of the deltaic area. Hg and As, on the contrary, did not parallel sediment contamination; the two showed comparable concentrations in clams from all six lagoons (Table 3). Although Cd and Ni are listed in the WFD as priority pollutants, no EQS is given for biota. A threshold is instead provided for Hg (0.02 μg/g ww; EC 2013), but it is higher than all present results, which range from 0.008 to 0.014 μg/g ww.
Hazard quotient, biota-sediment accumulation factors (BSAF) and potential for trophic transfer
To provide a synthetic evaluation of the contamination profile of each lagoon, all data on sediment chemistry were elaborated according to a previously described Weight Of Evidence model, which produces hazard quotient estimates (Piva et al. 2011). These HQs do not necessarily reflect the real ecosystem situation because many other pollutants can be present in the environment, causing interactive or synergistic effects on biota. However, as a general evaluation of the chemical status, the HQs determined in the present study indicated that Caleri and Barbamarco appeared to be the least affected (HQs of 0.1 and 0.2, respectively), and their chemical hazard was summarised as Absent. The contamination in Marinetta, Goro and Scardovari were found to present Slight or Moderate hazards (HQ values in the range of 2.1–5.6). Conversely, Canarin exhibited the highest chemical hazard, classified as Major due to its HQ of 11.4, toward the less restrictive normative limits. This finding strengthens the importance of the fact that two priority and hazardous pollutants, i.e., BDE-209 and NP, exceeded the existing SQGs. This profile primarily reflects the influence of the Po River’s contribution to the delta area, with a major hazard recorded in the lagoons that are directly exposed to the Po River.
However, sediment chemistry and the mentioned estimates do not necessarily describe in its entirety real environmental conditions. Differences in bioavailability and the presence of other chemicals, not included among the listed chemicals, may have unexpected critical roles. The use of BSAFs, which integrate bioaccumulation results and thus direct information on organisms’ exposure, may provide additional insights to evaluate the environmental conditions of the six lagoons. The Manila clam burrows in surficial sediments and feeds on suspended particles collected above the sediment surface. The clam is thereby exposed to contaminants via multiple exposure pathways, i.e. through direct contact with sediment/interstitial water and the ingestion of particulate matter, which, in turn, is variably composed of, e.g. detritus, phytoplankton or benthic microalgae, depending on tidal hydrodynamics, land-use characteristics within catchments or simply the availability of food items (Dang et al. 2009; Komorita et al. 2014).
The BSAF of the main chemicals examined in this study are reported in Table 4. As expected from the low trophic level of the Manila clam, most BSAF were in the range 0.01–0.8, and all were below 1.7, i.e. the value proposed as a theoretical threshold for simple partitioning processes of non-ionic organic compounds (Ozkoc et al. 2007). Nevertheless, some differences in chemicals’ uptake among the lagoons seemed to be present. Hydrophobic organic compounds associate primarily with organic matter in sediments. However, it is recognised that not only the amount but also the nature and composition of organic matter can greatly modify sorption processes and thus partitioning between sediment particles and the dissolved phase (Karickhoff et al. 1979). Regarding particle composition, a fraction residual of the incomplete combustion of fossil fuels and biomasses, generally termed “black carbon”, has received considerable attention by environmental scientists. It is abundant in aquatic environments and has been demonstrated to greatly reduce chemicals’ bioavailability (Moermond et al. 2004; Koelmans et al. 2006). As particulate matter can control bioavailability, the amount and composition of dissolved organic carbon (DOC) can further reduce chemicals’ bioavailability in the dissolved phase, in either the interstitial or overlying water column (Haitzer et al. 1998; Akkanen et al. 2012). The chemicals in the dissolved phase are generally considered the most readily bioavailable; however, the ingestion of particle-bound fraction also contributes to bioaccumulation, although with a different relative importance, depending primarily on chemical hydrophobicity but also on diet composition (Mackay and Fraser 2000; Wang and Zhang 2013; Khairy et al. 2014). In such a potential complexity of interactions, it is interesting to observe the results of Fig. 3. It shows the BSAF of APs and PCBs determined in Manila clams from the six lagoons. The lagoons influenced by the Po River have a lower BSAF. However, whereas the differences in bioavailability are clear for the APs, regarding PCBs, the clams of Scardovari and particularly Goro show an increasing BSAF. APs are moderate/low hydrophobic chemicals for which the uptake from water is expected to be more important than for PCBs. The Po River transports colloidal and DOC, so the lower BSAF of APs may be explained by a higher content of dissolved organic carbon and colloids capable of reducing the dissolved fraction of APs in interstitial and overlying water (Pettine et al. 1998; Boldrin et al. 2005). In the case of PCBs, colloids, DOC and black carbon, which are likely transported by the river, are expected to reduce bioavailability. However, in this case, the ingestion of particle-bound PCBs is expected to be more important than for APs. Scardovari and particularly Goro are characterised by intensive clam culture activities; therefore, sediment reworking and resuspension are surely more important than in Canarin, where this anthropic pressure is substantially absent, or in Marinetta. Previous studies have demonstrated that sediment resuspension increases chemical exposure and bioaccumulation (e.g. Josefsson et al. 2010; Roberts 2012). Similar trends seemed to be evident for PAHs, DDTs and some trace metals, although statistical significance was not reached.
As mentioned, a large part of the Po basin is known to contain high natural concentrations of Cr and Ni, constituting a background level against which anthropogenic influence must be assessed (Amorosi and Sammartino 2007). In this study, although we could describe the discrete concentrations of Cr and, particularly, of Ni in sediments, we calculated the lowest BSAF values among all trace metals considered (Table S10). This finding suggests that these potentially toxic components are not easily bioavailable to biota in these conditions. There is a limited bioaccumulation capacity in clam soft tissues; these chemicals thus do not constitute a threat for the environment.
The present data for the different pollutants in clam tissues do not suggest a threat to wildlife communities. The values we found in clams after chronic exposure are much lower than any critical toxicity thresholds calculated for the protection of higher trophic organisms (Lazorchak et al. 2003; ODEQ 2007; EC 2008; Environment Canada 2013). In the evaluation of the possible risks for human health, it should be considered that our study describes the results of a chronic test. As stated above, the concentrations measured in clam tissues at the end of the exposure period might not properly reflect the concentration of chemicals that could be bioaccumulated by clams reared in these lagoons for much longer periods. However, in the Po delta area, clams are widely reared for human consumption. To provide a tentative evaluation of the risks following bivalve ingestion, we calculated the potential contribution of the pollutants accumulated in clams in a diet. Considering a daily seafood consumption of 30 g/person for people living along Adriatic Sea coasts (Boscolo et al. 2007), and deriving the dietary intake, the present results for alkylphenols indicate a negligible risk for humans, i.e. more than 2 orders of magnitude lower than the TDI (Nielsen et al. 2000; Ademollo et al. 2008). The present concentrations are also below any limit set for human carcinogenic risk due to shellfish consumption for DDT (US-EPA 2000) and the limits set by European Regulation for the six ICES PCBs and for Cd (1259/2011/EU and 1881/2006/EU, respectively). In addition, when considering the thresholds meant for human consumption, it should be taken into account that clams undergo a depuration process before commercialisation. This procedure tends to lower, even if not always significantly, the chemical concentrations in clam tissues (Yakan et al. 2013; Anacleto et al. 2015). Consequently, reported concentrations in clams should be of even lesser concern for human consumption.
Conclusions
This study has examined the sediment contamination levels in six main lagoons of the Po River delta, and it has discussed the effects on clams (R. philippinarum) exposed for 3 months to the lagoons’ different environments. Despite the great complexity of the Po delta, the wide dataset on sediment properties and chemical contamination provided evidence of the important role of the Po River in influencing the quality of some of the lagoons, particularly those with lower interactions with seawater. The geographical distribution of the main river branches and the relative proportions of freshwater and seawater inputs of each lagoon result in a north–south environmental trend. The northernmost lagoon, Caleri, showed the lowest sediment contamination and the best clam growth performance, whereas more southern lagoons, particularly Canarin, showed higher levels of chemicals and worse clam growth. Despite these differences, the histological examination of gonadal tissues did not show alterations among the exposure groups. As demonstrated, the present results confirm that sediment contaminations through the Po River transports may be of concern, and NP and BDE-209 in particular may present a threat to aquatic organisms. Concentrations of PCBs were very close a concerning level, and together with DDTs, they might easily become a problem in areas characterised by higher sedimentation rates. Likely because of low bioavailability, trace metals seemed to be of low concern individually. As observed in other studies, sediment contamination was not always paralleled by bioaccumulation in clam tissues. Since clams are exposed both via (interstitial) water and suspended particles that they ingest, different bioavailabilities as well as natural and anthropogenic disturbances of lagoon sediments may partly explain the bioaccumulation results. No potential negative implications for human health were evidenced in clams exposed for 3 months to the six lagoons.
References
Abbiati M, Mistri M, Bartoli M et al (2010) Trade-off between conservation and exploitation of the transitional water ecosystems of the northern Adriatic Sea. Chem Ecol 26:105–119. doi:10.1080/02757541003693193
AdbPo (2006) Caratteristiche del Bacino del Fiume Po e primo esame dell’impatto ambientale delle attività umane sulle risorse idriche - Aprile 2006 [Italian] 643
Ademollo N, Ferrara F, Delise M et al (2008) Nonylphenol and octylphenol in human breast milk. Environ Int 34:984–987. doi:10.1016/j.envint.2008.03.001
Akkanen J, Tuikka A, Kukkonen JVK (2012) On the borderline of dissolved and particulate organic matter: partitioning and bioavailability of polycyclic aromatic hydrocarbons. Ecotoxicol Environ Saf 78:91–98. doi:10.1016/j.ecoenv.2011.11.010
Amorosi A, Sammartino I (2007) Influence of sediment provenance on background values of potentially toxic metals from near-surface sediments of Po coastal plain (Italy). Int J Earth Sci 96:389–396. doi:10.1007/s00531-006-0104-8
Anacleto P, Maulvault AL, Nunes ML et al (2015) Effects of depuration on metal levels and health status of bivalve molluscs. Food Control 47:493–501. doi:10.1016/j.foodcont.2014.07.055
ARPA-EMR (2013) Report sullo stato delle acque superficiali triennio 2010–2012 [Italian]. 176
ARPAV (2004) Le lagune del Delta del Po: ecosistemi fragili. Rilevamento in continuo della qualità delle acque [Italian]. 33
Beninger PG, Lucas A (1984) Seasonal variations in condition, reproductive activity, and gross biochemical composition of two species of adult clam reared in a common habitat: Tapes decussatus L. (Jeffreys) and Tapes philippinarum (Adams & Reeve). J Exp Mar Bio Ecol 79:19–37. doi:10.1016/0022-0981(84)90028-5
Bettinetti R, Quadroni S, Manca M et al (2012) Seasonal fluctuations of DDTs and PCBs in zooplankton and fish of Lake Maggiore (Northern Italy). Chemosphere 88:344–351. doi:10.1016/j.chemosphere.2012.03.009
Boldrin A, Langone L, Miserocchi S et al (2005) Po River plume on the Adriatic continental shelf: dispersion and sedimentation of dissolved and suspended matter during different river discharge rates. Mar Geol 222–223:135–158. doi:10.1016/j.margeo.2005.06.010
Boscolo R, Cacciatore F, Berto D, Giani M (2007) Polychlorinated biphenyls in clams Tapes philippinarum cultured in the Venice Lagoon (Italy): contamination levels and dietary exposure assessment. Food Chem Toxicol 45:1065–1075
Casatta N, Mascolo G, Roscioli C, Viganò L (2015) Tracing endocrine disrupting chemicals in a coastal lagoon (Sacca di Goro, Italy): sediment contamination and bioaccumulation in Manila clams. Sci Total Environ 511C:214–222. doi:10.1016/j.scitotenv.2014.12.051
Cataldo DH, Boltovskoy D, Stripeikis J, Pose M (2001) Condition index and growth rates of field caged Corbicula fluminea (Bivalvia) as biomarkers of pollution gradients in the Paraná river delta (Argentina). Aquat Ecosyst Health Manag 4:187–201. doi:10.1080/14634980127712
Chapman PM, Wang F, Caeiro SS (2013) Assessing and managing sediment contamination in transitional waters. Environ Int 55:71–91. doi:10.1016/j.envint.2013.02.009
Dang VH, Nguyen TH, Lee G-S et al (2009) In vitro exposure to xenoestrogens induces growth hormone transcription and release via estrogen receptor-dependent pathways in rat pituitary GH3 cells. Steroids 74:707–714. doi:10.1016/j.steroids.2009.03.002
David A, Fenet H, Gomez E (2009) Alkylphenols in marine environments: distribution monitoring strategies and detection considerations. Mar Pollut Bull 58:953–960
De Paiva MD, da Costa Marques MR, Baptista DF, Buss DF (2015) Metal bioavailability and toxicity in freshwaters. Environ Chem Lett 13:69–87. doi:10.1007/s10311-015-0491-9
Dedeh A, Ciutat A, Tran D, Bourdineaud J-P (2014) DNA alterations triggered by environmentally relevant polymetallic concentrations in marine clams Ruditapes philippinarum and polychaete worms Hediste diversicolor. Arch Environ Contam Toxicol 67:651–658. doi:10.1007/s00244-014-0059-x
EC (2002) 4-Nonylphenol (branched) and nonylphenol. CAS Nos: 84852-15-3 and 25154-52-3. EINECS Nos: 284-325-5 and 246-672-0. Summ Risk Assess Report 1–32
EC (2008) European Union Risk Assessment Report on Nickel 1715
EC (2013) Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013, amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Official J Eur Communities L 226:24, 8.2013
EFSA (2004) Opinion of the Scientific Panel on Contaminants in the food chain on a request from the Commission to assess the health risks to consumers associated with exposure to organotins in foodstuffs. EFSA J 102:1–119
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 30:973–980. doi:10.1016/j.envint.2004.03.001
Environment Canada (2013) Canadian Environmental Protection Act, 1999 -Federal Environmental Quality Guidelines Polybrominated Diphenyl Ethers (PBDEs), 25
Erickson M (2001) PCB properties, uses, occurrence and regulatory history. In: The University Press of Kentucky (ed) PCBs Recent Adv Environ Toxicol Heal Eff pp xi–xxx
Faganeli J, Malej A, Pezdic J, Malacic V (1988) C : N : P ratios and stable C isotopie ratios as indicators of sources of organic matter in the Gulf of Trieste (Northern Adriatic). Oceanol Acta 11:377–382
Falcieri F, Benetazzo A, Sclavo M et al (2014) Po River plume pattern variability investigated from model data. Cont Shelf Res 87:84–95. doi:10.1016/j.csr.2013.11.001
FAO (2014) The State of World Fisheries and Aquaculture 2014 243
Giani M, Djakovac T, Degobbis D et al (2012) Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuar Coast Shelf Sci 115:1–13. doi:10.1016/j.ecss.2012.08.023
Guzzella L, Patrolecco L, Langone L, Guilizzoni P (1998) DDT and other organochlorine compounds in the Lake Maggiore sediments: a recent point source of contamination. Fresenius Environ Bull 7:79–89
Haitzer M, Höss S, Traunspurger W, Steinberg C (1998) Effects of dissolved organic matter (DOM) on the bioconcentration of organic chemicals in aquatic organisms—a review. Chemosphere 37:1335–1362
Hitch R, Day H (1992) Unusual persistence of DDT in some Western USA soils. Bull Environ Contam Toxicol. doi:10.1007/BF00194381
Jahnke A, MacLeod M, Wickström H, Mayer P (2014) Equilibrium sampling to determine the thermodynamic potential for bioaccumulation of persistent organic pollutants from sediment. Environ Sci Technol 48:11352–11359. doi:10.1021/es503336w
Josefsson S, Leonardsson K, Gunnarsson JS, Wiberg K (2010) Bioturbation-driven release of buried PCBs and PBDEs from different depths in contaminated sediments. Environ Sci Technol 44:7456–7464. doi:10.1021/es100615g
Karickhoff S, Brown D, Scott T (1979) Sorption of hydrophobic pollutants on natural sediments. Water Res 13:241–248. doi:10.1016/0043-1354(79)90201-X
Kerambrun E, Henry F, Rabhi K, Amara R (2014) Effects of chemical stress and food limitation on the energy reserves and growth of turbot, Scophthalmus maximus. Environ Sci Pollut Res Int 21:13488–13495. doi:10.1007/s11356-014-3281-1
Khairy HM, Faragallah HM, Hussein NR and Dorgham MM (2014) Environmental characteristics and nutritional level of chronically eutrophic bay on Alexandria Sea coast, Egypt. Indian J Geo Mar Sci 43
Koelmans AA, Jonker MTO, Cornelissen G et al (2006) Black carbon: the reverse of its dark side. Chemosphere 63:365–377. doi:10.1016/j.chemosphere.2005.08.034
Komorita T, Kajihara R, Tsutsumi H et al (2014) Food sources for Ruditapes philippinarum in a coastal lagoon determined by mass balance and stable isotope approaches. PLoS One 9, e86732. doi:10.1371/journal.pone.0086732
La Guardia MJ, Hale RC, Harvey E et al (2012) In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the bivalve (Corbicula fluminea) and gastropod (Elimia proxima). Environ Sci Technol 46:5798–5805. doi:10.1021/es3004238
Lazorchak JM, McCormick FH, Henry TR, Herlihy AT (2003) Contamination of fish in streams of the Mid-Atlantic Region: an approach to regional indicator selection and wildlife assessment. Environ Toxicol Chem 22:545–553. doi:10.1002/etc.5620220312
Lopes C, Persat H, Babut M (2012) Transfer of PCBs from bottom sediment to freshwater river fish: a food-web modelling approach in the Rhône River (France) in support of sediment management. Ecotoxicol Environ Saf 81:17–26. doi:10.1016/j.ecoenv.2012.04.007
Lubet P (1959) Recherches sur le cycle sexuel et l’émission des gamètes chez les mytilides et les pectinides (Mollusques bivalves). Rev Des Trav l’Institut Des Pêches Marit 23:387–548
Mackay D, Fraser A (2000) Kenneth Mellanby Review Award. Bioaccumulation of persistent organic chemicals: mechanisms and models. Environ Pollut 110:375–391
Marshall Adams S (1999) Ecological role of lipids in the health and success of fish populations. In: Arts MT, Wainman BC (eds) Lipids in freshwater ecosystems. Springer, New York, NY, pp 132–160
Martinez E, Gros M, Lacorte S, Barceló D (2004) Simplified procedures for the analysis of polycyclic aromatic hydrocarbons in water, sediments and mussels. J Chromatogr A 1047:181–188. doi:10.1016/j.chroma.2004.07.003
Mearns AJ, Reish DJ, Oshida PS et al (2013) Effects of pollution on marine organisms. Water Environ Res 85:1828–1933. doi:10.2175/106143013X13698672322949
Moermond CTA, Roozen FCJM, Zwolsman JJG, Koelmans AA (2004) Uptake of sediment-bound bioavailable polychlorobiphenyls by benthivorous carp (Cyprinus carpio). Environ Sci Technol 38:4503–4509
Moschino V, Delaney E, Da Ros L (2012) Assessing the significance of Ruditapes philippinarum as a sentinel for sediment pollution: bioaccumulation and biomarker responses. Environ Pollut 171:52–60. doi:10.1016/j.envpol.2012.07.024
Mrema EJ, Rubino FM, Brambilla G et al (2013) Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 307:74–88
Nelson BW (1970) Hydrography, sediment dispersal, and recent historical development of the Po River Delta, Italy. In: Morgan J (ed) Deltaic sediment. Mod Anc Spec Publ SEPM, pp 152–184
Newton A, Icely J, Cristina S et al (2013) An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar Coast Shelf Sci 140:95–121
Nielsen E, Østergaard G, Thorup I et al (2000) Toxicological evaluation and limit values for nonylphenol, nonylphenol ethoxylates, tricresyl, phosphates and benzoic acid. Environ Proj Copenaghen DK Danish Environ Prot Agency N 512:43
ODEQ (2007) Guidance for assessing bioaccumulative chemicals of concern in sediment. Or Dep Environ Qual Environ Cleanup Program 89
OSPAR (2009) Background Document on CEMP Assessment Criteria for QSR 2010. Monit Assess Ser 24
Ozkoc HB, Bakan G, Ariman S (2007) Distribution and bioaccumulation of organochlorine pesticides along the Black Sea coast. Environ Geochem Health 29:59–68. doi:10.1007/s10653-006-9064-y
Paul-Pont I, de Montaudouin X, Gonzalez P et al (2010) How life history contributes to stress response in the Manila clam Ruditapes philippinarum. Environ Sci Pollut Res Int 17:987–998. doi:10.1007/s11356-009-0283-5
Pettine M, Patrolecco L, Camusso M, Crescenzio S (1998) Transport of carbon and nitrogen to the Northern Adriatic Sea by the Po River. Estuar Coast Shelf Sci 46:127–142. doi:10.1006/ecss.1997.0303
Piva F, Ciaprini F, Onorati F et al (2011) Assessing sediment hazard through a weight of evidence approach with bioindicator organisms: a practical model to elaborate data from sediment chemistry, bioavailability, biomarkers and ecotoxicological bioassays. Chemosphere 83:475–485. doi:10.1016/j.chemosphere.2010.12.064
Poma G, Binelli A, Volta P et al (2014a) Evaluation of spatial distribution and accumulation of novel brominated flame retardants, HBCD and PBDEs in an Italian subalpine lake using zebra mussel (Dreissena polymorpha). Environ Sci Pollut Res Int 21:9655–9664. doi:10.1007/s11356-014-2826-7
Poma G, Roscioli C, Guzzella L (2014b) PBDE, HBCD and novel brominated flame retardant contamination in sediments from Lake Maggiore (Northern Italy). Environ Monit Assess 186:7683–7692. doi:10.1007/s10661-014-3959-3
Quevauviller P, Ariese F (2001) New sediment reference material for the quality control of butyltin and phenyltin analysis. TrAC Trends Anal Chem 20:207–218. doi:10.1016/S0165-9936(01)00059-0
Ramu K, Isobe T, Takahashi S et al (2010) Spatial distribution of polybrominated diphenyl ethers and hexabromocyclododecanes in sediments from coastal waters of Korea. Chemosphere 79:713–719. doi:10.1016/j.chemosphere.2010.02.048
Roberts DA (2012) Causes and ecological effects of resuspended contaminated sediments (RCS) in marine environments. Environ Int 40:230–243. doi:10.1016/j.envint.2011.11.013
Sacchi A, Mouneyrac C, Bolognesi C et al (2013) Biomonitoring study of an estuarine coastal ecosystem, the Sacca di Goro lagoon, using Ruditapes philippinarum (Mollusca: Bivalvia). Environ Pollut 177:82–89. doi:10.1016/j.envpol.2013.01.042
Sfriso A, Facca C, Raccanelli S (2014) PCDD/F and dioxin-like PCB bioaccumulation by Manila clam from polluted areas of Venice lagoon (Italy). Environ Pollut 184:290–297
Solé M, Manzanera M, Bartolomé A et al (2013) Persistent organic pollutants (POPs) in sediments from fishing grounds in the NW Mediterranean: ecotoxicological implications for the benthic fish Solea sp. Mar Pollut Bull 67:158–165. doi:10.1016/j.marpolbul.2012.11.018
Spillman CM, Hamilton DP, Imberger J (2009) Management strategies to optimise sustainable clam (Tapes philippinarum) harvests in Barbamarco Lagoon, Italy. Estuar Coast Shelf Sci 81:267–278. doi:10.1016/j.ecss.2008.11.003
Stewart M, Olsen G, Hickey CW et al (2014) A survey of emerging contaminants in the estuarine receiving environment around Auckland, New Zealand. Sci Total Environ 468–469:202–210. doi:10.1016/j.scitotenv.2013.08.039
Tian S, Zhang Y, Song C et al (2015) Bioaccumulation and biotransformation of polybrominated diphenyl ethers in the marine bivalve (Scapharca subcrenata): influence of titanium dioxide nanoparticles. Mar Pollut Bull 90:48–53. doi:10.1016/j.marpolbul.2014.11.031
Tobiszewski M, Namieśnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119. doi:10.1016/j.envpol.2011.10.025
Turolla E (2008) The breeding of Manila clams in Po Delta. Graf, Adriat
US-EPA (1998) Test methods for evaluating solid waste, physical/chemical methods; SW-846, US Government Printing Office, Washington, DC.
US-EPA (2000) Guidance for assessing chemical contaminant data for use in fish advisories, volume 2: Risk assessment and fish consumption limits, 3rd edition. U S Environ Prot Agency Wash DC 823:00–008
US-EPA (2006) Region III BTAG Marine Sediment Screeneng Benchmarks 6
Van Ael E, Covaci A, Blust R, Bervoets L (2012) Persistent organic pollutants in the Scheldt estuary: environmental distribution and bioaccumulation. Environ Int 48:17–27
Viganò L, Farkas A, Guzzella L et al (2007) The accumulation levels of PAHs, PCBs and DDTs are related in an inverse way to the size of a benthic amphipod (Echinogammarus stammeri Karaman) in the River Po. Sci Total Environ 373:131–145. doi:10.1016/j.scitotenv.2006.11.006
Viganò L, Roscioli C, Guzzella L (2011) Decabromodiphenyl ether (BDE-209) enters the food web of the River Po and is metabolically debrominated in resident cyprinid fishes. Sci Total Environ 409:4966–4972. doi:10.1016/j.scitotenv.2011.07.062
Viganò L, Mascolo G, Roscioli C (2015) Emerging and priority contaminants with endocrine active potentials in sediments and fish from the River Po (Italy). Environ Sci Pollut Res Int. doi:10.1007/s11356-015-4388-8
Vincenzi S, Caramori G, Rossi R, De Leo GA (2007) A comparative analysis of three habitat suitability models for commercial yield estimation of Tapes philippinarum in a North Adriatic coastal lagoon (Sacca di Goro, Italy). Mar Pollut Bull 55:579–590. doi:10.1016/j.marpolbul.2007.09.016
Vincenzi S, De Leo GA, Munari C, Mistri M (2014) Rapid estimation of potential yield for data-poor Tapes philippinarum fisheries in North Adriatic coastal lagoons. Hydrobiologia 724:267–277. doi:10.1007/s10750-013-1742-z
Wang Z-Y, Zhang H-Y (2013) Rational drug repositioning by medical genetics. Nat Biotechnol 31:1080–1082. doi:10.1038/nbt.2758
Wang Z, Yang C, Brown C, et al. (2008) A case study: distinguishing pyrogenic hydrocarbons from petrogenic hydrocarbons. Int Oil Spill Conf
Wang Z, Ma X, Lin Z et al (2009) Congener specific distributions of polybrominated diphenyl ethers (PBDEs) in sediment and mussel (Mytilus edhongulis) of the Bo Sea, China. Chemosphere 74:896–901. doi:10.1016/j.chemosphere.2008.10.064
Yakan SD, Henkelmann B, Schramm K-W, Okay OS (2013) Bioaccumulation-depuration kinetics and effects of phenanthrene on Mediterranean mussel (Mytilus galloprovincialis). J Environ Sci Health A Tox Hazard Subst Environ Eng 48:1037–1046. doi:10.1080/10934529.2013.773799
Yogui GT, Sericano JL (2009) Polybrominated diphenyl ether flame retardants in the U.S. marine environment: a review. Environ Int 35:655–666. doi:10.1016/j.envint.2008.11.001
Yu F, Zong Y, Lloyd JM et al (2010) Bulk organic g13C and C/N as indicators for sediment sources in the Pearl River delta and estuary, southern China. Estuar Coast Shelf Sci 87:618–630. doi:10.1016/j.ecss.2010.02.018
Acknowledgments
This research was founded by the Ritmare Project, activity SP3_WP2_AZ3_UO03, “Esame del rischio ecotossicologico dovuto a inquinanti tradizionali ed emergenti in organismi residenti e degradazione di inquinanti organici nei sedimenti contaminati”. The authors are also grateful for the kind and valuable help of E. Turolla from the Istituto Delta di Ecologia Applicata, Ferrara, of V. Locaputo from the Water Research Institute, Bari, and of D. Mastroianni, Water Research Institute, Roma.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 7196 kb)
Rights and permissions
About this article
Cite this article
Casatta, N., Stefani, F., Pozzoni, F. et al. Endocrine-disrupting chemicals in coastal lagoons of the Po River delta: sediment contamination, bioaccumulation and effects on Manila clams. Environ Sci Pollut Res 23, 10477–10493 (2016). https://doi.org/10.1007/s11356-015-5656-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5656-3