Abstract
Heavy metal contamination is a globally recognized environmental issue, threatening human life very seriously. Increasing population and high demand for food resulted in release of various contaminants into environment that finally contaminate the food chain. Edible plants are the major source of diet, and their contamination with toxic metals may result in catastrophic health hazards. Heavy metals affect the human health directly and/or indirectly; one of the indirect effects is the change in plant nutritional values. Previously, a number of review papers have been published on different aspects of heavy metal contamination. However, no related information is available about the effects of heavy metals on the nutritional status of food plants. This review paper is focused upon heavy metal sources, accumulation, transfer, health risk, and effects on protein, amino acids, carbohydrates, fats, and vitamins in plants. The literature about heavy metals in food plants shows that both leafy and nonleafy vegetables are good accumulators of heavy metals. In nonleafy vegetables, the bioaccumulation pattern was leaf > root ≈ stem > tuber. Heavy metals have strong influence on nutritional values; therefore, plants grown on metal-contaminated soil were nutrient deficient and consumption of such vegetables may lead to nutritional deficiency in the population particularly living in developing countries which are already facing the malnutrition problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals have high density and mostly toxic in nature for human, plants, and animals regardless of their concentrations (LWTAP 2004) and have high atomic density five times greater than water or more than 4 g/cm3 (Hawkes 1997) or more than 5 g/cm3 (Saxena and Shekhawat 2013; Weast 1984). The nonessential metals are part of earth crust that enters the upper soil horizon and food chain through biogeochemical cycles (Tinsley 1979). Metals and metalloids such as cadmium (Cd), lead (Pb), mercury (Hg), and zinc (Zn) are called heavy metals because of their high densities (Oves et al. 2012), while arsenic (As) is included in this list because of similar properties (Chen et al. 1999). Essential and nonessential trace elements, when exceed the threshold limits, can cause different physiological, morphological, and genetical anomalies including reduced growth, mutagenic effects, and increased mortality (Khan et al. 2010a; Li et al. 2010; Luo et al. 2011).
Food crops are one of the important parts of our diet, and they may contain a number of essential and toxic metals (Yang et al. 2011; Waqas et al. 2015) depending on growing media characteristics. Vegetables are the major source of human exposure to heavy metal and contribute about 90 % of the total metal intake, while the rest 10 % intake occurs through dermal contacts and inhalation of contaminated dust (Martorell et al. 2011; Kim et al. 2009; Ferré-Huguet et al. 2008; Khan et al. 2014). Food safety is a burning issue regarding human health in the recent decades because of the high demand for food. This scenario leads to stimulate researchers and scientists to work on health risk associated with consumption of heavy metals, pesticides, and toxin-contaminated food (D’Mello 2003).
Essential and nonessential elements are regularly added to our food chain through excessive use of agrochemicals, municipal wastewater, industrial effluents, and raw sewage for irrigation (Tongesayi et al. 2013). The Agency for Toxic Substances and Diseases Registry (ATSDR 2011) has classified heavy metals and metalloids such as As, Pb, and Cd found in the environment as 1, 2, and 7 on the basis of toxicity.
Some elements like Cu, Cr, Fe, Mn, and Zn are essential for animals and human beings because they play an important role in different metabolic functions, enzymatic activities, sites for receptors, hormonal function, and protein transport at specific concentrations (Apostoli 2002; Antoine et al. 2012). Other elements like As, Cd, and Pb are nonessential and have no beneficial role in plants, animals, and humans (Chang 2000) and have no nutritional function, as they are highly toxic (Goldbold and Huttermann 1985; Nies 1999). It is necessary to characterize the sources and contents of heavy metals in soil in order to establish quality standards and to determine the threats to human health and food safety (Sun et al. 2013). Environmental pollution with heavy metals is unrelenting, covert, and permanent in nature (Wang et al. 2001). Biological organisms are incapable to degrade metals because of their nonbiodegradable nature and long half-life, and they persist in their body parts and environment leading to health hazards (Amaral and Rodrigues 2005; Nabulo et al. 2011). Heavy metals have the capability to move from contaminated soil and water and bioaccumulate in vegetables causing health risks (Stasinos and Zabetakis 2013; Rattan et al. 2005; Khan et al. 2015).
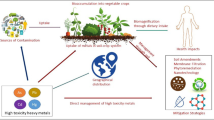
Soil characteristics play an important role in terms of food production, and the contamination of this vital resource with heavy metals and their ultimate uptake and bioaccumulation in food crops posses major environmental and health problems (Fig. 1), particularly in developing countries (Lin et al. 2007). Soil type and plant genotype and their interaction have significant effect on heavy metal concentrations (Ding et al. 2013). Mineral fertilizers have higher heavy metal concentrations as compared to organic manure; therefore, the application of mineral fertilizers results in soil heavy metal pollution (Hu et al. 2013).
So far, a number of experiments (pots and field) have been conducted to study the effect of soil parameters and other elements on mobility and bioavailability of heavy metals from soil to crop system (Li et al. 2007; Hart et al. 2006; Khan et al. 2013a, b). The mobility and bioavailability of heavy metals in contaminated soil is affected by a number of biological processes and physiochemical properties like soil pH, organic matter (OM) (Ahmad and Goni 2010; Ernst 1996; Alloway 1995), cation exchange capacity (CEC), soil texture, and soil microbiota. Soil pH has significant effects on availability and accumulation of heavy metals in the edible parts of plants (Hu et al. 2013; Wang et al. 2013a). Similarly, CEC and OM have negative impact on the mobility and bioavailability of heavy metals like Pb (Arshad et al. 2008; Ding et al. 2013; McLaughlin et al. 2011; Khan et al. 2015). Cd toxicity to the soil environment is well known; it is a toxic heavy metal and causes toxicity even at low concentration. The bioaccumulation rate of Cd is higher in the field crops as compared to other elements (Moustakas et al. 2001; Arao and Ae 2003).
For the improvement of nutritional composition of soil, different agricultural strategies like biofortification and proper use of fertilizers have been suggested (Graham et al. 2006; Bonierbale et al. 2007).
In recent years, most of the studies have focused on As contamination of food chain (Gilbert-Diamond et al. 2011; Huang et al. 2012; Kim et al. 2009) along with other heavy metals and metalloids that were found above the recommended limits (Tufuor et al. 2011). Food crop irrigated with industrial effluents and wastewater is the major source of soil and crop contamination with heavy metals and metalloids (Lee et al. 2008; Tiwari et al. 2011; Sipter et al. 2008).
The associated health hazards of toxic metal depend on concentrations of these metals in specific media and exposure time. Long time and chronic exposure can cause health hazards even at low concentrations of toxic metal (Mahalakshmi et al. 2012). Among the abiotic stresses to plants, heavy metal toxicity is one of the major stresses and the toxicity is based on physiochemical properties of heavy metals (Saxena and Shekhawat 2013; Zhuang et al. 2009).
The aims of this paper are to summarize the literature about the heavy metals as a major environmental issue and critically discuss the information about heavy metal (As, Cd, Cr, Cu, Ni, and Pb) contamination in soil and the grown food plants, metal bioaccumulation, soil-to-plant transfer, nutritional effects, and health risks.
Heavy metals in soil
Soil contamination with heavy metals is a serious global environmental problem (Facchinelli et al. 2001; Solgi et al. 2012; Wang et al. 2001) posing risks to human, animals, microbes, and plants and contaminating surface and groundwater. Heavy metals and other pollutants enter to the soil ecosystem through natural processes and anthropogenic activities. Trace element concentrations in soil environment are mainly dependent upon the geology of the area (Carral et al. 1995), while anthropogenic activities like solid waste disposal, wastewater irrigation, sludge application, automobile exhaust, mining and smelting processes, urbanization, agricultural activities, and industrialization also contribute heavy metals into the soil environment (Facchinelli et al. 2001; Lim et al. 2008; Montagne et al. 2007; Wei and Yang 2010; Chen et al. 1999; Tsai et al. 2001; Shi et al. 2005). Similarly, the physiochemical characteristics of soil have substantial effects on heavy metal concentration and its availability to plants (Fig. 1). Heavy metal toxicity is mainly associated with metal speciation in soil (Allen et al. 1980; Liu et al. 1996). The heavy metal concentrations in agriculture soil were in range of 0.1–40, 0.01–0.7, 5–3000, 2–100, 10, and 2–200 mg kg−1 for As, Cd, Cr, Cu, Ni, and Pb, respectively (O’Neill 1995; Allaway 1968), while the heavy metal concentrations in garden soil were in range of 3.06–15.89, 0.27–2.86, 14.08–53.97, 18.51–579.84, 10.04–35.60, and 5.11–60.85 mg kg−1 for As, Cd, Cr, Cu, Ni, and Pb, respectively (Szolnoki et al. 2013). Similarly, the concentrations of As, Cd, Cr, Cu, Ni, and Pb in sediments were ranged from 0.2–13.8, 0.3–8.4, 2–1000, 4–200, 2–500, and 7–150 mg kg−1, respectively (Cannon et al. 1978). Among toxic elements, As is highly carcinogenic and present in the soil of both developed and developing countries (Fig. 2). Table 1 summarizes the heavy metal concentrations in different soils, while their permissible limits in soil are given in Table 2. The permissible limits of different countries and organizations show variation in concentrations for different heavy metals. These differences may be due to different strategies adopted by these countries and organizations to set permissible limits. The variation in permissible limit may be based on soil characteristics and type. The permissible limits for As were 75, 30, and 20 mg kg−1 set by USEPA, State Environmental Protection Administration (SEPA) China, and FAO/WHO, respectively, while the permissible limits for Cu and Ni were similar for almost all countries and organizations. Other heavy metals like Cd, Cr, and Pb show great variations in their respective permissible limits set by different organizations/countries. The concentrations of all the selected heavy metals were observed above the permissible limit set by different countries and international organizations (WHO, USEPA, SEPA, etc.) for heavy metals in soils from different regions of the world (Table 2).
Heavy metal concentrations are greatly affected by soil organic contents (Khan et al. 2015). Soils with high organic waste concentrations are generally confined to heavy metal concentrations of less than 1000 mg kg−1 soil, while industrial waste-contaminated soil contains more than 10,000 mg kg−1 soil (Bader et al. 1999). Increasing concentrations and variation in distribution of heavy metal in metal-amended soil generally augment the heavy metal concentrations in plants (Castro et al. 2009). Soil is the main source of contamination of food chain with heavy metals because soil is used as an important tool for waste management and waste dumping (Zhuang et al. 2009; Rogival et al. 2007).
The prime route of heavy metal intake into the human body is through soil–crop system in agriculture area (Liu et al. 2007), where the anthropogenic activities are the primary sources of contaminations (Lim et al. 2008; Li et al. 2008; Montagne et al. 2007; Wei and Yang 2010; Yang et al. 2009a). The main route of exposure to heavy metals in urban environment is through ingestion, absorption through skin, and inhalation of dust particles (Ahmed and Ishiga 2006; Lim et al. 2008; De Miguel et al. 2007; Ferreira-Baptista and De Miguel 2005; Sindern et al. 2007), whereas the primary source of pollution in urban soil is anthropogenic one which includes vehicular emission, power plant, tire wear particles, auto repair shops, car wash centers, brake lining, coal combustion, chemical plants, weathering of building, atmospheric deposition, and house hold solid waste (Amato et al. 2009; De Miguel et al. 1997; Duzgoren-Aydin et al. 2006; Han et al. 2006; Kartal et al. 2006; Lu et al. 2009; Madrid et al. 2002; Morton-Bermea et al. 2009; Oliva and Espinosa 2007; Sezgin et al. 2003; Zhou et al. 2008).
Ecotoxicological assessment of contaminated soil is very difficult due to complex nature and diversity of soil microbiology (COM 2006). However, these test methods are considered as a good tool for obtaining information regarding bioavailability of contaminants to some specific organisms (ISO/DIS 2006).
Ecological risk assessment approach is generally applicable for representing metal-contaminated sites (US EPA 2001; Jensen et al. 2006); for this purpose, soil quality values also called guideline values are used (National Environment Protection Council (NEPC) 1999; CCME 2005; US Environmental Protection Agency (EPA) 2005; Niemeyer et al. 2010). These guideline values are based on dose–response data derived from laboratory tests on ecological process or single plant or animal species (Jones 2006). Finally, statistical applications are applied to derive these guideline values (O’Halloran 2006). Soil quality values are established on the basis of total metal concentrations in the soil. However data of total metal concentrations is not sufficient for risk assessment, and other qualities like chemical form, electric potential, and ion activity of metals are to be considered while predicting metal toxicity (Chapman et al. 2003; Stumm and Morgan 1996; Wang et al. 2013b). Soluble metals and free metal ions are more bioavailable and toxic (Lund 1990) than other form of heavy metals.
Factors affecting mobility and bioavailability of heavy metals
Heavy metal accumulation in plants is strongly influenced by different soil parameters (Wilson et al. 2014; Wang et al 2013a; Ahmad and Goni 2010) such as soil pH, OM (Fig. 1), redox potential, total metal contents, and CEC (Chlopecka 1996; Imai et al. 2002; Wang et al. 2012a). Heavy metals like Cu and Zn show a significantly negative correlation with pH (García et al. 2009; Wang et al. 2013a), while soil OM effects vary from metal to metal (Wang et al. 2013a; Khan et al. 2015). The aging factor and other soil properties are the main factors affecting the bioavailability of heavy metals (Ahmad and Goni 2010; Smolders et al. 2009). The sensitivity of heavy metals in soil is greatly affected by soil CEC and pH (Lock and Janssen 2001).
The pedotransfer function assessment is now commonly used to understand heavy metal mobility and availability in soil environment. The following regression equations are used to forecast the values of those parameters which are difficult to measure from the easily measured soil properties such as soil particle size, pH, OM, and CEC (Bouma 1989; Martin et al. 2005; Perfect 2003). The Freundlich equation is used as pedotransfer function assessment tool to assess metal availability in soil. The original model was proposed for Cd bioavailability. Springob et al. (2001) used the equation for assessment of Cd bioavailability. However, the modified Freundlich equation can be used for other metal availability in soil environment. The modified Freundlich equation is as under:
where S represents the sorbed fraction, C metals represents soil metal concentrations, and M (shape) represents the Freundlich parameters. M has an average value of 0.8 (Springob et al. 2001), while k value can be calculated through multiple regression techniques (Eqs. 2 and 3). For the assessment of k value, Springob et al. (2001) modified the Freundlich function in simple and log domain, respectively.
The modified equations are as under:
where x 1 to x n are the soil variables used to predict the value of k, while a, b… z are exponents allowing nonlinear contribution of different variables. Similarly, Eq. 2 can be expressed in log domain by multiple linear regression analysis (Eq. 3). The ability of soil to accumulate metals depends upon soil OM which acts as a main sorbent for metals (Sauvé et al. 2003; Ge and Hendershot 2005).
Soil OM
Soil OM has the capability to bind toxic metals to control heavy metal behavior and alleviate toxicity in soil (Datta et al. 2001). Wu et al. (2014) mentioned that the sequential analysis shows that Pb availability in soil gradually decreased while Cd availability increased. Similarly, the soil OM-bounded fraction of Pb increased, while that of Cd decreased. This shows that Pb has higher affinity and stability toward OM than Cd (Winter et al. 2012). This difference in relative binding affinity of Pb and Cd with soil OM is because of metal chemistry differences (Wu et al. 2014).
The heavy metals binded with dissolved OM are easily available to soil flora (Krishnamurti et al. 2004). Similarly, humic acid is an important part of organic carbon having phenolic and carboxylic functional groups which increases the heavy metal mobility and availability to plants (Fox and Comerfield 1990; Khan et al. 2006; Lagier et al. 2000; Marschner and Kalbitz 2003; Senesia et al. 2003; Evangelou and Marsi 2001; Halim et al. 2003). Therefore, soil with high humic acid concentration is not suitable for agricultural activities.
pH
Soil pH strongly influences the bioavailability of heavy metals in soil and their toxicity on living organisms (Amini et al. 2005; Basta et al. 2005; Li and Shuman 1996; Nigam et al. 2001; Prasad 1999; Seuntjens et al. 2004). At low pH, the mobility and bioavailability of metals are greater as compared to high pH (Kuo et al. 2004; Merry 2001; Tsadilas et al. 2005). The soil pH reduces the metal uptake by plants, and the availability of metals increases when the pH is below the critical value of 4. The geology, distance from roadside, industries, and type of irrigation affect the pH values in soil. Wastewater irrigation brings changes in the pH of soils (Zhuang et al. 2009). Like mobility and bioavailability of metals, the pH plays an important role in the metal speciation in soil (Luo et al. 2011). Along with other physiochemical characteristics, pH can significantly affect the heavy metal removal from contaminated soils (Di Palma et al. 2003). Heavy metal like Cu has the tendency of making Cu–OM complexes (Li et al. 2008) which leads to affect its availability. Cu bioaccumulations in plants are significantly affected by soil pH and negatively correlated with each other (García et al. 2009; Wang et al. 2006a, 2013a). Toxicological effects of free metal ions on ecological resources are the functions of soil pH (Lofts et al. 2004); therefore, it is necessary to have a basic knowledge of soil pH used for cultivation purposes. The soil with low pH should not be used for agriculture purposes due to highly availability of metals in it.
Soil texture
Soil texture along with other factor is one of the important factors that induces metal availability in soil. Clay contents can significantly affect the availability of heavy metals and their subsequent toxicity to living organisms (Beyer and Cromartie 1987). Crops grown on sandy soil are metal deficient, particularly Zn, as compared to loamy texture (Rashid and Ryan 2004; Martens and Reed 1991); this may be due to large pore size and the low retention capacity of sandy soil to retain metals.
Soil-to-plant transfer of heavy metals is strongly influenced by the soil texture. Treder and Cieslinski (2005) stated that plants cultivated on sandy soil have higher concentrations of heavy metals than those grown on clay loamy soil. The high bioaccumulation in plants is linked with higher mobility of metals in sandy soil as compared to clay soil.
Microorganisms and heavy metals have significantly negative correlationship. Effects of high heavy metal concentrations on the microbial community structure, activity, and abundance have been reported (Khan et al. 2010b; Sandaa et al. 1999; Tsezos 2009). Similarly, there is a strong association between soil microbiota and soil texture; most of microorganisms occurs in association with clay content (Alexander 1977; Sessitsch et al. 2001). Furthermore, the heavy metal movement from upper to lower soil horizon is also affected by soil texture. In sandy-textured soil, heavy metals can easily move from one horizon to other as compared to clay soil. Pore size has also effects on the metal mobility and bioavailability.
Plant species
The bioaccumulation of heavy metal is different for different plant species reflected by their growth, reproduction, occurrence, and survival in the metal-contaminated soil. It is notable that different plant species show different toxicity to the same pollutant and in the same environmental condition, because the mechanisms of elemental uptake by plants are not the same for all plant species (Garty 2001; Zechmeister et al. 2003). Heavy metal accumulation in food plants depends on metal concentrations as well as phyto-availability and phyto-variety, as different plants have different uptake rates (Medina et al. 2005: Yang et al. 2009b). The capacity of plants to uptake heavy metals is different for different heavy metals, and the same heavy metal can be accumulated at different ratio in different plant species (Singh et al. 2010b). Metal bioavailability is also affected by the presence of organic compounds of that metal in plants. As species bioavailability is considerably reduced by organo-arsenic compounds present in plant tissues (Juhasz et al. 2006). Generally, the leafy vegetable metal uptake rates are higher and more contaminated than nonleafy vegetables (Yu et al. 2006).
Heavy metals in plants
Vegetables are an important source of human diets, and their contamination can cause serious health problems (Bi et al. 2006; Khan et al. 2008a; Liu et al. 2005a; Lim et al. 2008; Pruvot et al. 2006; Radwan and Salama 2006). Leafy vegetables like lettuce are considered as potential hyperaccumulators of heavy metals (Ramos et al. 2002). One of the properties of green leafy vegetable is the accumulation of heavy metals in their tissues without exhibiting any toxicity symptoms (Intawongse and Dean 2006). Monteiro et al. (2007) reported that with increasing exposure duration, the concentrations of heavy metals in lettuce roots and shoots increased. Heavy metals can cause changes in physiology and growth of tomato at variable concentrations and result in chlorosis and necrotic symptoms on leaves (López-Millán et al. 2009). The plants grown on wastewater-irrigated soil contain high concentrations of metals in their vegetative and nonvegetative parts (Khan et al. 2008b). Garate et al. (1993) described that lettuce has higher capacity to accumulate heavy metal in different tissues. Heavy metal uptake is also affected by different plant species (Fig. 1) and within the same species by different cultivars (John and Van Laerhoven 1976). Like other food crops, potato is an important food crop grown throughout the world. It is rich with energy, dietary fibers, vitamins, carbohydrates, and essential elements such as Fe, Ca, Zn, and K (Finglas and Faulks 1984). Mineral concentrations and photosynthetic activities of plants can also be affected by toxic metals (López-Millán et al. 2009).
According to Swedish National Food Administration (1984), consumption of potato (200 g) provides carbohydrate, protein, energy, Ca, Cu, P, Fe, Mg, and Zn (36.6 g, 3.8 g, 172 kcal, 0.44 mg, 18 mg, 90 mg, 1.2 mg, 48 mg and 0.82 mg, respectively). The soil pH substantially affects the supply of these essential nutrients to plants; low pH means more supply of nutrients (Lutz et al. 1972). Moreover, the nutrient supply to plants is affected by a number of factors including soil type, climate, cultivation practices, and storage condition (Srikumar and Ockerman 1990). Concentrations of heavy metals vary in different organs of the same plant. Xu et al. (2013a) reported the heavy metal accumulation in the order of leaf > root ≈ stem > tuber. However, other scientists conveyed that the root concentrations are higher than shoot (Vanassche and Clijsters 1990).
In fruit plants, like tomato, the translocation rate of heavy metals to the fruit is rather low (Table 3 and Fig. 3), hence characterized as low-rate translocation fruit vegetable (Angelova et al. 2009). Accumulation and distribution of heavy metals like Cd have been found in different parts of tomato (Donma and Donma 2005). Tomato is an important food plant from economical as well as nutritional point of view (FAOSTAT 2007). Tomato is a rich source of minerals, vitamins, and other nutrients (Giovanelli and Paradise 2002) consumed both in raw and processed form (Martinez-Valvercle et al. 2002).
The concentrations of As in different vegetables grown in different countries. Sources: Arain et al. (2009), Sekhar et al. (2003), Patel et al. (2005), Owens et al. (2004), Alam et al. (2003), Yu et al. (2006), Chang et al. (2014), Shi et al. (2013), Williams et al. (2005), Kar et al. (2013), Ramirez-Andreotta et al. (2013), and Batista et al. (2010)
Vegetables are vulnerable to heavy metals at high concentrations, and large-scale irrigation with wastewater and application of fertilizers for commercial production increase the risk of heavy metal contamination (Gil et al. 2004). Heavy metals have significantly negative effects on plant growth (López-Millán et al. 2009); other toxic effects may include root browning, alteration of mineral concentrations, and changes in photosynthesis (López-Millán et al. 2009). At higher concentration, metal moves from roots to shoots of the plant (Rodríguez-Celma et al. 2010). Misra and Mani (1991) have suggested that metal concentrations in plant tissues were in the range of 0.02–7, 0.1–2.4, 0.2–1.0, 4-15, 1.0, and 1–13 mg kg−1 for As, Cd, Cr, Cu, Ni, and Pb, respectively.
The heavy metal concentrations in soil are given in Table 1, while their concentrations in plants are shown in Table 3 and Figs. 2 and 3. The Cd concentrations in most of the selected plants were above the permissible limits set by SEPA, EU, India, and FAO. In this review paper, we have selected different vegetables including leafy, ground stem, and fruits. The maximum concentration was found in leafy vegetables such as lettuce. The order of contaminations was leafy > fruit > ground stem vegetables (Table 3 and Fig. 3). Cr concentrations were found above the safe limit of EU (1.0 mg kg−1) and FAO (0.05 mg kg−1). Similarly, the concentrations of Pb were also above the EU (0.3 mg kg−1), FAO (0.3 mg kg−1), Indian standard (2.5 mg kg−1), and SEPA (9.0 mg kg−1) in most of the selected plants. The order of contamination was potato > spinach > cucumber > lettuce > mustard > rice (Table 3). The permissible limits for heavy metals in vegetables are summarized in Table 2. Like soil, the permissible limits set by different countries and organizations for individual metal show great variation in their respective concentrations.
Metal uptake by plants
Vegetables (leafy and nonleafy) grown on contaminated soil are considered as the major source of heavy metals. Based on heavy metal uptake, the plants are classified as accumulator, hyperaccumulator, and excluders. Plant contamination with heavy metals may occur through soil–plant, water–plant, and air–plant interfaces; however, soil–plant interface is the major source of plant metal accumulation. Literature shows that there is a strong relationship between heavy metals in soil and food crops (Bini et al. 2012; Khan et al. 2015). In general, the bioavailability of heavy metals depends on the amount of exchangeable metals in soil. Carbonate-bound and exchangeable metals are more bioavailable than other fractions (Wong et al. 2002). The bioavailability of heavy metals in plant varies for different plant organs, and the absorption and bioaccumulation rate is highest for roots as compared to other parts (Verma and Dubey 2003). Similarly, solubility and soil type also affect the metal uptake by plants (Castro et al. 2009). The mean heavy metal uptake by plants increases as the contents of these metals increase in the soil environment (Chaves et al. 2011). Galal and Shehata (2015) reported that the bioavailability of heavy metals depends upon the distance from the source; they observed that the bioavailability rate was higher in heavy-traffic roadside plants. Sludge- and compost-amended soils reduce availability of metals to plants avoiding food chain contamination (Smith 2009). Liu et al. (2005a) mentioned that the bioavailability of Cd is the highest, while that of As is the lowest for crop cultivated in heavy-metal-contaminated soil. Amaranthus dubius have the capability to remove heavy metals from soil through their root system, but shoot absorption is negligible for a number of heavy metals like As, Cr, Cu, Ni, and Pb (Mellem et al. 2009).
Accumulation, distribution, and chemical status of heavy metals in plants
Assessment of heavy metal concentrations in food crops is done through accumulation factor calculation (Table 4), which is the expression of metal concentrations in soil in relation with concentrations in plant (Eq. 4) (Khan et al. 2010a; Li et al. 2012). Heavy metals pass through a series of processes including bioaccumulation, transformation, and biomagnification after entering into food chain; therefore, it is very difficult to remove heavy metals from living organisms (Widowati 2012).
Soil-to-plant transfer and accumulation of heavy metals are a matter of concern and have been presented in a number of articles (Table 4) and a key component for health risk assessment (Cui et al. 2004; Fismes et al. 2002; Huq et al. 2001; Rattan et al. 2005; Mapanda et al. 2005; Khan et al. 2008a; Wang et al. 2006b; Liu et al. 2005c) Higher soil-to-plant transfer of heavy metals means strong accumulation of these metals in plant tissues (Khan et al. 2008a). Wang et al. (2006b) and Khan et al. (2008a) described that there is an inverse relationship between soil total metal concentrations and soil-to-plant transfer factor. However, the soil-to-plant transfer is calculated on the basis of total metal concentrations in soil (Hooda et al. 1997). The bioaccumulation factor of heavy metals is measured by the ratio of metal concentrations in plants to metal concentrations in that soil (Eq. 4) (Mattina et al. 2002).
Food plants have the tendency to accumulate heavy metals in their tissues. Nonessential elements entering our environment may lead to bioaccumulation by plants (Kashif et al. 2009). Trace elements have the capability to interact with roots through absorption in polluted soil environment and increasing risk of toxic effects on plants and animals (Rosselli et al. 2006). Plant analysis, both wild and cultivated plants, for heavy metals is one of the major sources for assessment of contaminated soil (Blaylock et al. 2003; Ernst 1996; Wenzel et al. 1993); these plants act as bioindicators for local- and large-scale soil pollution (Baker et al. 2000; Baker and Brooks 1989; Zupan et al. 2003).
The accumulation factors (Table 4) and daily intake of heavy metals (Table 5) are commonly calculated using the following formulae.
where C plant and C soil are the concentrations of heavy metal in plants and soil, respectively (Khan et al. 2010a; Cui et al. 2005; Mohamed et al. 2003). Table 4 summarizes the soil-to-plant transfer factor of heavy metals. Results show that tomato, in general, has high bioaccumulation factor among vegetables (Noor-ul-Amin et al. 2013).
DIM is the daily intake of metals, C metal represents the metal concentrations in plant; C factor is the conversion factor (fresh weight to dry weight); D food intake shows daily vegetable intake, and BWaverage weight is the average body weight. According to FAO (2000), average daily intakes of vegetables for adults and children are 0.345 and 0.232 kg person−1 day−1, respectively, whereas the average body weights for adults and children were considered as 73 (FAO 2000) and 32.7 kg, respectively (Ge 1992; Khan et al. 2010a; Wang et al. 2005). The daily food intake by a single individual is 55, 105, and 445 g day−1 for pulses, vegetables, and cereals, respectively (Tripathi et al. 1997).
Generally, heavy metals have the capability to migrate from polluted soil to plant tissues (Stasinos and Zabetakis 2013). Toxic metals can accumulate in different plant organs; however, the accumulation rate varies from organ to organ (McLaughlin et al. 1999; Wagner 1993), and some organs accumulate more than others. Bioaccumulation and transportation of heavy metals in plants are the major human exposure pathway to soil contamination through food chain (Ryan et al. 1982; Watanabe et al. 2000; Cui et al. 2004; Yu et al. 2006; Lacatutsu et al. 1996; Khan et al. 2014). Bunzl et al. (1999) reported that information regarding total metal concentrations in polluted soil is insufficient to know about the mobility and availability of heavy metals to living organism.
Heavy metals need some specific media for mobility from soil to plants and within the plants from one organ to another organ; for this purpose, they make bond with metal sulfur legends and organic acids. Grill et al. (1989) and Lugon-Moulin et al. (2004) conveyed that heavy metals have the capability to bind with phytochelatins and glutathione and accumulate in different plant organs.
Metal toxicity and tolerance
Heavy metals have shown toxicological effects on plants and animals, and their degrees of toxicities vary from species to species and from metal to metal. It is known from the literature that metal toxicity is associated with their speciation in soil; however, it is difficult to determine some specific species because of their complex nature, functioning, and distribution in soil environment (Czupyran and Levy 1989). The bioavailability and toxicity of heavy metals to living organism are dependent on soil physiochemical parameters and microbiology (Alam et al. 2003; Islam et al. 2000). Prasad (1999) discussed that the heavy metal toxicity may be influenced by changes in its characteristics such as pH.
Heavy metals interact with essential micronutrients and affect their uptake and transport by plants (Thys et al. 1991; Hernández et al. 1998) affecting plant growth and their physiological functions. Hernández et al. (1995) observed that at high Cd concentrations, plant elicited toxic symptoms and caused growth retardation. Similarly, Cd even at low concentration can exert toxicological effects on seed germination and growth (Li et al. 2005a) and Cu is also toxic to seedling growth (Munzuroglu and Geckil 2002).
The genotoxicity of heavy metals to living organisms involves interaction of heavy metals with genetic material via binding with DNA basis (Hossain and Huq 2002). A number of transgenic and bioassay techniques have been recommended for assessing the genotoxic effects of environmental pollutants on plants including random-amplified polymorphic DNAs (RAPDs), micronucleus (MCN) induction, or Comet assay (Steinkellner et al. 1998; Angelis et al. 2000; Arkhipchuk et al. 2000; DeWolf et al. 2004; Kovalchuk et al. 2001; Liu et al. 2005b).
For heavy metal toxicity assessment, various plant species are recommended depending on soil properties. Lettuce is used for toxicity assessment in moderate pH environment (pH 6–8) (ISO 2005b; Sheppard et al. 1993; Chapman et al. 2010, 2012) because it is not a good acid-tolerant species (Chapman et al. 2012; Environment Canada 2005). Among higher plants, oat is recommended for toxicity tests of heavy metals (Chang et al. 1997; ISO 2005a) under low pH conditions (Loureiro et al. 2006; Small and Jackson 1949; Bilski and Foy 1987), while Allium cepa is Pb tolerant in acidic environment (Dang et al. 1990). Heavy metal tolerance capability of plants may be associated with their chemical form (Yang and He 1995). Similarly, metal-induced reactive oxygen species (ROS) are responsible for peroxidation of fatty acid. To avoid the toxic effect of ROS, plants adopt different defense strategies including antioxidant enzymes (Gill and Tuteja 2010). Nitrogen and sulfur are essential nutrients responsible for protein and glutathione synthesis providing defensive mechanisms for heavy metal tolerance (Anjum et al. 2008; Liedschulte et al. 2010; Sarwar et al. 2010; Zechmann and Müller 2010). The recent studies conducted on plant tolerance mechanism to environmental pollutants showed that heavy metals have strong influence on leaf size and structure, and the first toxicity symptoms appear on leaves; thus, measuring length to width ratio can be very effective in evaluating plant tolerance to heavy metals (Zhang et al. 2014).
Plants can evolve an advance and complex detoxification mechanism to avoid metal toxicity which is composed of selective uptake, chelation, compartmentalization, and secretion of toxic metals (Pourrut et al. 2013). Phytochelatins play a key role in metal detoxification (Cobbett 2000). The Brassica juncea (BjCdR15) and Arabidopsis TGA3 are good tolerants to high metal concentrations because of the mechanisms such as the regulation of synthesis of phytochelatin by BjCdR15/TGA3 (Farinati et al. 2010). Similarly, different genes are involved in metal tolerance by mediating metal–glutathione conjugate transport (Kim et al. 2006). Plant protein is actively involved in heavy metal homeostasis and metal tolerance (Suzuki et al. 2002).
Effects on plant growth
Heavy metals are phytotoxic in nature and have significantly negative impacts on plant growth, and even at lower concentrations, they inhibit plant growth (Li et al. 2005a; Di Salvatore et al. 2008; Fjällborg et al. 2005). Under high concentrations of heavy metals, the plant growth drastically reduces (Chaves et al. 2011; Gopal and Khurana 2011; Kumari et al. 2011; Manivasagaperumal et al. 2011). High concentrations of heavy metals in the soil greatly affect the growth and metabolic processes of cultivated crops (John et al. 2009; Sinha et al. 2005). Heavy metals induced oxidative stresses, affect the photosynthetic process, and cause growth retardation (Le Guédard et al. 2012; Bibi and Hussain 2005; Wani et al. 2006)). Elevated concentrations of heavy metals may result in stunted growth by hampering the photosynthetic machinery and disturbing the coordination mechanism between essential elements (Astolfi et al. 2004; Gill et al. 2012), ultimately causing plant death (Sanita di Toppi and Gabbrielli 1999).
Cu is an essential element that plays an important role in various physiological functions and plant growth; however, at higher concentration, it becomes toxic and affects plant growth and interferes with normal physiological functions (Bouazizi et al. 2010; Hansch and Mendel 2009; Upadhyay and Panda 2009). Excess of heavy metals like Cu may cause root growth inhibition and damage the plasma membrane resulting in ion leakage from the cells (Bouazizi et al. 2010).
Soares et al. (2001) presented that heavy metals greatly affect the plant growth, developmental processes, and cell division. A reduction from 18 to 77 % has been observed in plant height cultivated in metal-contaminated soil (Gopal and Khurana 2011; Chaves et al. 2011). A significant decrease was observed in the leaf area of plants grown on metal-contaminated soil. This is because of changes in protein synthesis, photosynthetic activities, and respiration (Chaves et al. 2011). High concentration of Cd has significantly negative impact on lettuce shoot growth (Monteiro et al. 2007). This decrease may be due to metal-induced chromosomal aberration (Seregin and Kozhevnikova 2006).
Effects on plant structure
The effects of heavy metals on plant structure are well known. Bini et al. (2012) stated that high metal concentrations in plants have strong effect on plant morphology. Plants, under high metal stress, show clear symptoms of structural changes including absence of palisade structure, reduced leaf thickness, and structural changes in mitochondria (Bini et al. 2012). Heavy metals strongly influence the structure of plant components and cause toxicological symptoms (Lindsey and Lineberger 1981). The toxicity on cellular structure and function of organisms varies according to toxic potential of heavy metal (Saraf and Samant 2013). Trace elements elicit toxicity symptoms on plants root, shoot, and leaf structure (Mangabeira et al. 2001; Vasquez et al. 1991).
Plant cellular metabolism disruption due to heavy metals may cause structural changes in cell membrane (Prasad 1995) and chloroplast and results in reduced photosynthesis (Ramos et al. 2002; Li et al. 2005b; Mahmood et al. 2010). Similarly, high concentration of Fe produces free radicals which may damage cell membrane, protein, and DNA and alter cellular structure (Arora et al. 2002; de Dorlodot et al. 2005).
Metal exposure and human effects
Toxicological effects to human beings from consumption of metal-contaminated food are based on various factors including chemical forms of heavy metals, dose, exposure route, time, frequency, age, gender, nutritional source, and biological species (Caussy et al. 2003; Tchounwou et al. 2004). Figure 1 shows the heavy metal interaction with human health effects. Similarly, personal and environmental hygiene also plays important roles in subsequent exposure to metal toxicity. Human health and soil quality are strongly related with each other especially to the degree of pollution (Velea et al. 2009). The tendency of food plants to accumulate heavy metals in their tissues is a public health concern, because they are toxic to human health and plant tissues. Some metals are required by human body, while others (As, Cd, Pb, etc.) are toxic in nature even at trace concentrations (Yargholi and Azimi 2008; Khan et al. 2010a; Mitra et al. 2009; Gebrekidan et al. 2013) and associated with carcinogenic health risks (Abbasi et al. 2013). Similarly, consumption of As-contaminated vegetables can cause serious health risks (Batista et al. 2011). Many heavy metals are potential carcinogens (Kim et al. 2009; Lee et al. 2006; Lim et al. 2008) and can cause organ dysfunction and damage. US Department of Health and Human Services (2011), World Health Organization, International Agency for Cancer Research (2012), USEPA /IRIS (2012), and California EPA (2011) have declared As, Cd, Cr, Ni, and Pb as human carcinogens.
Food plant contamination with heavy metals and their potential impacts on plant nutritional values are an emerging health issue because of their strong links with each other (Sharma et al. 2009; Zaidi et al. 2005). Toxicological effects of metal-contaminated vegetables are mainly associated with mineral contents due to their toxicological and nutritional characteristics (Fig. 1) (Chary et al. 2008; Weldegebriel et al. 2012; Yang et al. 2011).
The common route of entrance of heavy metals to human body is through ingestion, inhalation, and dermal contacts (Kim et al. 2009), which results in minor to major health problems including diarrhea, nausea, lung diseases, anemia, kidney disorders, stomach problems, skin diseases, neurological disorders, and cancer (ATSDR 2007; NLM/HSDB 2012). Some of the disorders are caused by acute toxicity, while others through chronic exposure.
Heavy metals have the capability to accumulate in different body parts and cause adverse health impact regardless of their concentrations (Ikeda et al. 2000; Duruibe et al. 2007). The toxicological effects of toxic metals may vary from person to person. Higher concentrations of nonessential elements have effects on the reproductive capability of living organisms (Oetken et al. 2004). For health risk assessment associated with heavy metal contamination of soil, a mathematical model should be established to predict the bioaccumulation and transformation of these toxic metals (Hough et al. 2003).
Health risk is the associated health hazard of some specific chemicals and/or phenomenon in a specific environment and/or environmental conditions depending upon exposure duration and availability of receptors (Alexander 1995). Human health risk assessment is generally done through evaluating the health risk index (HRI), daily intake of metal, reference dose, and general body weight of children and adults (Eq. 6) (Cui et al. 2004; Li et al. 2012; Pandey et al. 2012; Luo et al. 2011; Singh et al. 2010a). Other techniques used for assessing carcinogenic and noncarcinogenic health risks associated with heavy metals are target cancer risk (TCR) (Eq. 9), hazard index (HI) (Eq. 8), and target hazard quotient (THQ) (Eq. 7), respectively (USEPA 2006, 2010; Yang et al. 2011). The different equations used for calculating the health risks are given as under:
where Eq. 6 C n represented heavy metal concentrations in vegetables (mg/kg, fresh weight) and D n daily intake of vegetables per year, RfD means reference oral dose (Table 5), and B w is the body weight for children and adults (Eq. 5). In Eq. 7, C is the metal concentration in vegetables, I is the ingestion rate (255 g/day/person), EFr is the exposure frequency (350 days/year), ED is the total exposure duration (70 years), BW is the average body weight, and ATn is the noncarcinogen’s averaging time, (ED × 365 day/year) (USEPA 2006; Chien et al. 2002). THQ is the target hazard quotient for heavy metals (Eq. 8). CPSo represents the carcinogenic potency slope, oral (μg/g/day)−1; ATc represents the carcinogen averaging time (70 × 365 days) (Eq. 6). The human HRIs for selected heavy metals and vegetables and different age groups are summarized in Table 6.
Studies on health risk assessment show that children are more susceptible to heavy metal pollution than adults because of their physiological and behavioral characteristics (DHAenC, Department of Health and Aging and enHealth Council 2012; Qu et al. 2012; Man et al. 2010; Zota et al. 2011). Health risk assessment is the evaluation of potential health effects to target population exposed to certain toxic media. It is the key procedure for hazardous substance management, designing remediation policies and taking control measures (Khan and Husain 2001; McGraph et al. 2004).
Heavy metal effect on nutritional values
Balance diet is the key requirement of good health. Vegetables are good sources of macro and micro nutrients that meet daily nutrient requirements. However, no single vegetable has all nutrients to fulfill the dietary requirements. Therefore, for a balance diet, a diversified nutritional regime is required to meet daily nutrient requirements (Grusak and Dellapenna 1999). FAO/WHO has set nutritional standards for children and adults at different age groups which are summarized in Table 7. Heavy metals at high concentrations in food chains and their consumption can cause depletion of essential nutrients in the body leading to serious health hazards (Arora et al. 2008; US Department of health and human services 2005).
It is agreed upon that ROS is responsible, in part, for metal contamination of plants resulting in oxidative damages of proteins, lipids, and nucleic acid, which in turn are responsible for various physiological disorders such as growth retardation, nutrient deficiency, reduced transport of nutrients, genotoxicity, and retarded photosynthesis (Le Guédard et al. 2012; Nagajyoti et al. 2010; Upadhyay and Panda 2009). Similarly, along with the above-mentioned disorders, ROS may result in enzyme inactivation and DNA damages (Pourrut et al. 2013; Saxena and Shekhawat 2013). Heavy metals alter the functions of antioxidative enzymes as well as its contents in plant cells and affect the oxidative stresses (Gallego et al. 1999; Sharma and Dietz 2009) that may lead to plant death. Heavy metals are responsible for genetic instability in plants and result in DNA damage (Liu et al. 2005b; Gichner et al. 2004; Steinkellner et al. 1998). Like other physiological disorders, toxic metals strongly influence the genetic characteristics like point mutation and recombination of plants at variable concentrations (Kovalchuk et al. 2001, 2005). It is impossible, to a specific portion of leafy vegetables, for the determination of nutritional components because the serving size varies greatly depending upon people’s knowledge, seasons, food tradition and habits, availability of specific vegetables, and economic condition of the community (Stangeland et al. 2009). Consumption of heavy-metal-contaminated food can lead to serious depletion of nutritional components like Fe and vitamins that may cause a number of physiological and pathological disorders (Iyengar and Nair 2000). The influence of heavy metals on plant nutritional components depends on soil environment, type of food crops, exposure time, and plant organs (Gonçalve et al. 2009). Literature shows that metal-contaminated-food-consuming populations were observed to be deficient of macro and micro nutrients including fats, proteins, vitamins, and minerals like Ca, Fe, and Zn (Nordberg 1996; Fox 1988). Similarly, heavy metals like Cd and micronutrients have strong interaction in gastrointestinal absorption. Fe and Zn are the most important micronutrients involved in these interactions. This absorption involves intestinal Fe transporters (Park et al. 2002; Ryu et al. 2004). Women and children have higher body Cd than men because their Fe requirements are greater than men (Nishijo et al. 2004). Therefore, adequate nutrition is vital for young children and pregnant women in the earlier stages to have optimal development and growth conditions (Christian 2010).
Effects on contents of carbohydrates
Heavy metals have strong influence on carbohydrate contents of vegetables. Excessive accumulation of toxic elements may inhibit carbohydrate synthesis by destroying the photosynthetic electron transport chain and production of ROS (Sandalio et al. 2001). High Pb concentration results in a decrease in the sucrose content of vegetables and affects the taste of rooted vegetables (Gawęda 2007). Kratovalieva and Cvetanowska (2001) reported that tomato plants cultivated in heavy-metal-polluted soil showed an increase in the total sugar contents.
Heavy metals affect the carbohydrate metabolism by different ways and produce changes in their contents, which may be due to inactivation and impairment of certain enzymes participating in carbohydrate synthesis (Vikas et al. 2002; Gawęda 2007; Nagor and Vyas 1997). At high Cd concentration, a general decrease occurs in carbohydrate metabolism, while glycolytic pathway observes an upregulation under heavy metal stresses (Rodríguez-Celma et al. 2010). Similarly, elevated Cd concentration significantly alters plant physiology that results in low carbohydrate metabolism (Chaffei et al. 2004). At lower concentration, trace metal interrupts with photosynthetic activities, carbohydrate metabolism, assimilation of essential macronutrient, and enzyme activities (Sanita di Toppi and Gabbrielli 1999; Vanassche and Clijsters 1990). Heavy metals have the capability to replace chlorophyll Mg and alter its function (Kowalewska et al. 1987). From the existing literature, it is clear that heavy metals have strong influence on carbohydrates of both leafy and nonleafy vegetables and consumption of such contaminated food can lead to a decrease in net carbohydrate contents that may result in malnutrition.
Effects on proteins and amino acids
Proteins are the basic dietary and functional component of food plants having various structural, functional, and nutritional properties and considered as essential nutrients for human beings. Nitrogen and sulfur are essential nutrients required for synthesis of proteins and amino acids (Hesse et al. 2004). The deprivation of essential nitrogen and sulfur in plants may affect the metabolic process (Carfagna et al. 2010). The total protein contents may range from 1 to 7 g/100 g of leafy vegetables (Uusiku et al. 2010). The crude protein contents of different leafy vegetables vary greatly from species to species (Odhav et al. 2007; Mosha and Gaga 1999), and the variation may be due to climatic conditions (Odhav et al. 2007) and structural and physiological characteristics. Heavy metals are responsible for low protein in plants grown on metal-contaminated soil (Widowati 2012). Heavy metals can significantly reduce protein synthesis as a result of physiological changes in plants (Chaffei et al. 2004). High metal concentration may hamper protein metabolism by altering physiological functions and synthetic activities (Sandalio et al. 2001). Heavy metals have the capability to interact with proteins and DNA which produces oxidative damages in plant molecules (Leonard et al. 2004). Nonessential toxic elements, due to oxidative stress, can affect the thylakoid and protein complex resulting in a decrease in photosynthesis (Linger et al. 2005). Higher concentrations of toxic metals have effects on the decomposition of protein contents (Wu et al. 2014). Heavy metals induced membrane damage due to changes in functions and composition of membrane lipids and proteins (Meharg 1993; Vanassche and Clijsters 1986). Similarly, high metal concentrations inhibit protein synthesis by altering the pigment–lipoprotein complex accumulation in photosystems I (Wang et al. 2009) II and effect ribulose-1,5-bisphosphate carboxylase/oxygenase enzymes (Krantev et al. 2008).
Reports regarding heavy-metal-induced changes in protein concentrations have been contradictory. A number of proteins (fructokinase, enolase, GADPH, and PGM) show decrease in concentrations in some plant species (B. juncea and poplar), while the same proteins show increase in other plants (Arabidopsis thaliana) (Alvarez et al. 2009; Sarry et al. 2006; Kieffer et al. 2009; Roth et al. 2006).
Effects on fats and fatty acids
Literature shows that most of the studies regarding adverse impacts of heavy metals on food crop are focused upon bioaccumulation, transfer, and health effects. Little knowledge is available on heavy metal impact on nutritional components, particularly lipids (Upchurch 2008).
Heavy-metal-induced oxidative stress results in chloroplast degradation and lipid peroxidation (Khanna-Chopra 2012), affecting the nutritional status of the contaminated plant. The photosynthetic activities of plants are also greatly affected by the contents of heavy metals (Hermle et al. 2006; He et al. 2011; Velikova et al. 2011; Solti et al. 2008). High Cd concentration results in lipid peroxidation (Monteiro et al. 2004), which is caused by metal-induced lipoxygenase activity resulting in displacement of metal ions and altering the enzymatic activities (Wildner and Henkel 1979).
Le Guédard et al. (2008) reported that the concentrations of tri-unsaturated fatty acids decreased when plant was cultivated in heavy-metal-contaminated soil. In the same experiment, they observed that the percentage of other fatty acids (C18:0, C18:1, and C18:2) was increased to various levels. Thus, they concluded that fatty acids can be used as a bioindicator. Heavy metals show variable effects on different fatty acids as Djebali et al. (2005) and Ben Youssef et al. (2005) have observed that heavy metals have negative impact on C18:3 percentages, while other fatty acids were affected positively.
Effects on vitamins
Green leafy vegetables are considered as a rich source of nutrients like vitamins (Gupta and Bains 2006). Vitamins are the essential nutrients for all living organisms and for plant microorganism association. Vitamin concentrations in soil have significant effects on plant growth (Bergner 1997). The vitamin contents of plants show great variation in food crops provided with organic fertilizers to those with nitrogen fertilizers, and plants grown with commercial fertilizers show significant reduction in vitamin contents (Price 1945) which may be due to the presence of some trace elements.
Environment (physical, chemical, and biological) has a strong influence on vitamin contents, and in extreme environment with high heavy metal concentrations, temperature, and pH, the vitamin contents are drastically reduced (Ipek et al. 2005). Vitamins are essential nutrients that play an important role in maintaining good health and providing protection against infectious diseases, augmenting immune system functions, and providing protection against certain malignancies. However, heavy metals have significantly negative impacts on vitamin contents and functions (Sugawara et al. 1981; Pleasants et al. 1992). Similarly, metals induced lipid peroxidation which may reduce the vitamin contents (Tatli Seven et al. 2012) in the cultivated crops. There is a negative correlation between the heavy metals and vitamins; that is, when heavy metal concentrations increased, the vitamin contents decreased and vice versa (Widowati 2012; Munzuroglu et al. 2005).
Financial implication
Soil contamination with heavy metals is one of the major environmental problems that can cause serious financial implications in the form of redevelopment issues, remediation, and reestablishment cost along with severe ecological and health concerns (Semenzin et al. 2007). Based on literature, we come to the conclusion that the major financial implications, direct and indirect, associated with heavy metals may include the following:
-
Reduction in crop production
-
Financial loss in terms of health cost
-
Loss of jobs and unemployment
-
Heavy-metal-contaminated foods are nutrient deficient that results in abnormal growth and development
-
Poverty associated with health effects and low crop yields
Conclusion
Food plants especially green leafy vegetables are the major dietary source being consumed all over the world. They play a significant role in nutritional contribution to the consumers. Green plants are the rich sources of essential nutrients; however, they are strong accumulators of heavy metals and pose great risk to human health. Similarly, nonleafy vegetables are also a good source of nutritional elements and are an important part of balance diet. But, on exposure to environmental contaminants, they accumulate heavy metals in their edible parts. The health effects of heavy metals are direct as well as indirect. Direct impacts involve direct consumption through vegetables, ingestion, and dermal contacts, while indirect impacts include reduction in nutritional components in contaminated food crops. Similarly, vegetables are the major sources of heavy metals resulting in both carcinogenic and noncarcinogenic health risks. Our findings showed that concentrations of heavy metals in food crops are much higher than their background values. The concentrations of Cd, Pb, and Cu were found above the permissible limits set by SEPA China, FAO/WHO, EU, and Indian standards that significantly affect the nutritional components of cultivated crops. Moreover, the heavy metal concentrations in plants and their nutritional composition have inverse relationship. Heavy metals have significantly negative impacts on protein, fat, and carbohydrate contents of contaminated vegetables. Therefore, it is suggested that more intensive studies are required on different aspects of heavy metal contamination on plant nutritional components and highly contaminated land should not be used for agriculture purposes prior to protective and rehabilitative measures.
References
Abbasi AM, Iqbal J, Khan MA, Shah MH (2013) Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotoxicol Environ Saf 9:237–244
Agency for Toxic Substances and Disease Registry (ATSDR) (2007) Arsenic CAS# 7440-38-2. http://www.atsdr.cdc.gov/tfacts2.pdf
Agency for Toxic Substances and Disease Registry (ATSDR) (2011) The 2011 priority list of hazardous substances. Online available at http://www.atsdr.cdc.gov/SPL/index.html
Ahmad JU, Goni MA (2010) Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environ Monit Assess 166:347–357
Ahmed F, Ishiga H (2006) Trace metal concentrations in street dusts of Dhaka city, Bangladesh. Atmos Environ 40:3835–3844
Alam MGM, Snow ET, Tanaka A (2003) Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci Total Environ 308:83–96
Alexander M (1977) Introduction to soil microbiology, 2nd edn. Wiley, New York
Alexander M (1995) How toxic are toxic chemicals in soil? Environ Sci Technol 29:2713–2717
Allaway WH (1968) Agronomic control over the environmental cycling of trace elements. Adv Agron 20:235–274
Allen HE, Hall RH, Brisbin TD (1980) Metals speciation effects on aquatic toxicity. Environ Sci Technol 14:441–443
Alloway BJ (1995) Soil processes and the behavior of metals. In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic and Professional, London, pp 11–37
Alvarez S, Berla BM, Sheffield J, Cahoon RE, Jez JM, Hicks LM (2009) Comprehensive analysis of the Brassica juncea root proteome in response to cadmium exposure by complementary proteomic approaches. J Proteome 9:2419–2431
Amaral A, Rodrigues A (2005) Metal accumulation and apoptosis in the alimentary canal of Lumbricus terrestris as a metal biomarker. BioMetals 18:199–206
Amato F, Pandolfi M, Viana M, Querol X, Alastuey A, Moreno T (2009) Spatial and chemical patterns of PM10 in road dust deposited in urban environment. Atmos Environ 43:1650–1659
Amini M, Khademi H, Afyuni M, Abbaspour KC (2005) Variability of available cadmium in relation to soil properties and landuse in an arid region in central Iran. Water Air Soil Pollut 162:205–218
Angelis KJ, McGuffie M, Menke M, Schubert I (2000) Adaptation to alkylation damage in DNA measured by the comet assay. Environ Mol Mutagen 36:146–150
Angelova VR, Babrikov TD, Ivanov KI (2009) Bioaccumulation and distribution of lead, zinc, and cadmium in crops of Solanaceae family. Commun Soil Sci Plant Anal 40:2248–2263
Anjum NA, Umar S, Ahmad A, Iqbal M, Nafees NA (2008) Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione sulphur protects mustard from cadmium toxicity. Plant Growth Regul 54:271–279
Antoine JMR, Fung LAH, Grant CN, Dennis HT, Lalor GC (2012) Dietary intake of minerals and trace elements in rice on the Jamaican market. J Food Compos Anal 26:111–121
Apostoli P (2002) Elements in environmental and occupational medicine. J Chromatogr B 778:63–97
Arain MB, Kazi TG, Baig JA, Jamali MK, Afridi HI, Shah AQ, Jalbani N, Sarfraz RA (2009) Determination of arsenic levels in lake water, sediment, and foodstuff from selected area of Sindh, Pakistan: estimation of daily dietary intake. Food Chem Toxicol 47:242–248
Arao T, Ae N (2003) Genotypic variations in cadmium levels of rice grain. Soil Sci Plant Nutr 49:473–479
Aremu MO, Atolaiye BO, Labaran L (2010) Environmental implication of metal concentrations in soil, plant foods and pond in area around the Derelict Udege mines of Nasarawa State, Nigeria. Bull Chem Soc Ethiop 24(3):351–360
Arkhipchuk VV, Malinovskaya MV, Garanko NN (2000) Cytogenetic study of organic and inorganic toxic substances on Allium cepa, Lactuca sativa, and Hydra attenuata cells. Environ Toxicol 15:338–344
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82:1227–1338
Arora M, Kiran B, Rani S, Rani A, Kaur B, Mittal N (2008) Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem 111:811–815
Arshad M, Silvestre J, Pinelli E, Kallerhoff J, Kaemmerer M, Tarigo A, Shahid M, Guiresse M, Pradere P, Dumat C (2008) A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere 71:2187–2192
Astolfi S, Zuchi S, Passera C (2004) Role of sulphur availability on cadmium-induced changes of nitrogen and sulphur metabolism in maize (Zea mays L.) leaves. J Plant Physiol 161:795–802
Awashthi SK (2000) Prevention of Food Adulteration Act no 37 of 1954. Central and State Rules as Amended for 1999. Ashoka Law House, New Delhi
Bader JL, Gonzalez G, Goodell PC (1999) Chromium-resistant bacterial populations from a site heavily contaminated with hexavalent chromium. Water Air Soil Pollut 109:263–276
Baker AMJ, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baker A, Mc Grath S, Reeves R, Smith J (2000) Metal hyper accumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soils. Lewis Publisher, London, pp 85–107
Basta NT, Ryan JA, Chaney RL (2005) Trace element chemistry in residual-treated soils: key concepts and metal bioavailability. J Environ Qual 34:49–63
Batista BL, Souza VCDO, Da Silva FG, Barbosa F Jr (2010) Survey of 13 trace elements of toxic and nutritional significance in rice from Brazil and exposure assessment. Food Addit Contam Part B Surveill 3(4):253–262
Batista BL, Souza JMO, De Souza SS, Barbosa F Jr (2011) Speciation of arsenic in rice and estimation of daily intake of different arsenic species by Brazilians through rice consumption. J Hazard Mater 191:342–348
Ben Youssef N, Nouairi I, Ben Temime S, Taamalli W, Zarrouk M, Ghorbal MH, Ben Miled Daoud D (2005) Effets du cadmium sur le métabolisme des lipides de plantules de colza (Brassica napus L.). C R Biol 328:745–757
Bergner P (1997) The healing power of minerals, special nutrients and trace elements. Prima Publishing, Rocklin
Beyer WN, Cromartie EJ (1987) A survey of Pb, Cu, Zn, Cd, Cr, As and Se in earthworms and soil from diverse sites. Environ Monit Assess 8:27–36
Bi X, Feng X, Yang Y, Qiu G, Li G, Li F, Liu T, Fu Z, Jin Z (2006) Environmental contamination of heavy metals from zinc smelting areas in Hezhang County, western Guizhou, China. Environ Int 32:883–890
Bibi M, Hussain M (2005) Effect of copper and lead on photosynthesis and plant pigments in black gram (Vigna mungo L.). Bull Environ Contam Toxicol 74:1126–1133
Bigdeli M, Seilsepour M (2008) Investigation of metals accumulation in some vegetables irrigated with waste water in Shahre Rey-Iran and toxicological implications. Am Euras J Agric Environ Sci 4(1):86–92
Bilski JJ, Foy CD (1987) Differential tolerances of oat cultivars to aluminum in nutrient solutions and in acid soils of Poland. J Plant Nutr 10:129–141
Bini C, Wahsha M, Fontana S, Maleci L (2012) Effects of heavy metals on morphological characteristics of Taraxacum officinale Web growing on mine soils in NE Italy. J Geochem Explor 123:101–108
Biswas A, Biswas S, Santra SC (2013) Arsenic in irrigated water, soil, and rice: perspective of the cropping seasons. Paddy Water Environ. doi:10.1007/s10333-013-0396-9
Blaylock MJ, Elles MP, Nuttal CY, Zdimal KL, Lee CR (2003) Treatment of As contaminated soil and water using Pteris vittata. Proc VI ICOBTE, Uppsala, Sv
Bonierbale M, Amoros W, Burgos G, Salas E, Juarez H (2007) Prospects for enhancing the nutritional value of potato by plant breeding. Afr Potato Assoc Conf Proc 7(2):26–46
Bouazizi H, Jouili H, Geitmann A, Ferjani EEI (2010) Copper toxicity in expanding leaves of Phaseolus vulgaris L.: antioxidant enzyme response and nutrient element uptake. Ecotoxicol Environ Saf 73:1304–1308
Bouma J (1989) Using soil survey data for quantitative land evalution. Adv Soil Sci 9:177–213
Brahman KD, Kazi TG, Baig JA, Afridi HI, Khan A, Arain SS, Arain MB (2014) Fluoride and arsenic exposure through water and grain crops in Nagarparkar. Pak Chemosphere 100:182–189
Bunzl K, Trautmannsheimer M, Schramel P (1999) Partitioning of heavy metals in a soil contaminated by slag: a redistribution study. J Environ Qual 28:1168–1173
California Environmental Protection Agency Office of Environmental Health Hazard Assessment (2011) Chemicals known to the state to cause cancer or reproductive toxicity, September 2, 2011. http://oehha.ca.gov/prop65/prop65_list/files/p65single090211.pdf
Cannon HL, Connally GG, Epstein JB, Parker JG, Thornton I, Wixson G (1978) Rocks: geological sources of most trace elements. In: report to the workshop at south scas plantation Captiva Island, FL, US. Geochem Environ 3:17–31
Carfagna S, Vona V, Martino VD, Esposito S, Riganoa S (2010) Nitrogen assimilation and cysteine biosynthesis in barley: evidence for root sulphur assimilation upon recovery from N deprivation. Environ Exp Bot 71:18–24
Carral E, Villares R, Puente X, Carba Ueira A (1995) Influence of watershed lithology on heavy-metal levels in estuarine sediments and organisms in Galicia (north-west Spain). Mar Pollut Bull 30:604–608
Castro E, Manas P, Heras JDL (2009) A comparison of the application of different waste products to a lettuce crop: effects on plant and soil properties. Sci Hortic 123:148–155
Caussy D, Gochfeld M, Gurzau E, Neagu C, Ruedel H (2003) Lessons from case studies of metals: investigating exposure, bioavailability, and risk. Ecotoxicol Environ Saf 56:45–51
CCME (2005) Canadian Environmental Quality Guidelines. Canadian Council of Ministers of the Environment, Winnipeg
Chaffei CK, Pageau A, Suzuki H, Gouia MH, Ghorbel, Masclaux-Daubresse C (2004) Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant Cell Physiol 45:1681–1693
Chang R (2000) Physical chemistry for the chemical and biological sciences. University Science Books, Sausalito
Chang LW, Meier JR, Smith MK (1997) Application of plant and earthworm bioassays to evaluate remediation of a lead-contaminated soil. Arch Environ Contam Toxicol 32:166–171
Chang CY, Yu HY, Chen JJ, Li FB, Zhang HH, Liu CP (2014) Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ Monit Assess 186:1547–1560
Chapman PM, Wang FY, Janssen CR, Goulet RR, Kamunde CN (2003) Conducting ecological risk assessments of inorganic metals and metalloids: current status. Hum Ecol Risk Assess 9:641–697
Chapman E, Dave G, Murimboh J (2010) Ecotoxicological risk assessment of undisturbed metal contaminated soil at two remote lighthouse sites. Ecotoxicol Environ Saf 73:961–969
Chapman EEV, Hedrei Helmer S, Dave G, Murimboh JD (2012) Utility of bioassays (lettuce, red clover, red fescue, Microtox, MetSTICK, Hyalella, bait lamina) in ecological risk screening of acid metal (Zn) contaminated soil. Ecotoxicol Environ Saf 80:161–171
Chary NS, Kamala CT, Raj DSS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf 69:513–524
Chaves LHG, Estrela MA, Sena de Souza R (2011) Effect on plant growth and heavy metal accumulation by sunflower. J Phytol 3(12):04–09
Chen HM, Zheng CR, Tu C, Zhu YG (1999) Heavy metal pollution in soils in China: status and countermeasures. Ambio 28:130–134
Cheraghi M, Lorestani B, Merrikhpour H, Rouniasi N (2013) Heavy metal risk assessment for potatoes grown in overused phosphate-fertilized soils. Environ Monit Assess 185:1825–1831
Chien LC, Hung TC, Choang KY, Choang KY, Yeh CY, Meng PJ et al (2002) Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci Total Environ 285:177–185
Chlopecka A (1996) Forms of Cd, Cu, Pb, and Zn in soil and their uptake by cereal crops when applied jointly as carbonates. Water Air Soil Pollut 87:297–309
Christian P (2010) Micronutrients, birth weight, and survival. Annu Rev Nutr 30:83–102
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123(3):825–832
Codex (1995) Codex general standard for contaminants and toxins in food and feed. Codex Alimentarius Commission, Rome, Codex Standard 193-1995
Codex Alimentarius Commission, Contaminants (1984) Joint FAO/WHO Food Standards Program, Codex Alimenturius, XVII
COM (2006) 231 final. Communication from the Commission of the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions. Thematic strategy for soil protection. 22 September 2006. Brussels
Cui YJ, Zhu YG, Zhai RH, Chen DY, Huang YZ, Qiu Y, Ling JZ (2004) Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ Int 30:785–791
Cui YJ, Zhu YG, Zhai RH, Huang Y, Qiu Y, Liang J (2005) Exposure to metal mixtures and human health impacts in a contaminated area in Nanning, China. Environ Int 31:784–790
Czupyran G, Levy RD (1989) In situ immobilization of heavy metal contaminated soil, section II. Noyes Data Corp, Park Ridge
D’Mello JPF (2003) Food safety: contaminants and toxins. CABI Publishing, Wallingford, p 480
Dang YP, Chhabra R, Verma KS (1990) Effect of Cd, Ni, Pb and Zn on growth and chemical composition of onion and fenugreek. Commun Soil Sci Plant Anal 21:717–735
Datta A, Sanyal S, Saha S (2001) A study on natural and synthetic humic acids and their complexing ability towards cadmium. Plant Soil 235(1):115–125
de Dorlodot S, Lutts S, Bertin P (2005) Effects of ferrous iron toxicity on the growth and mineral composition of an inter specific rice. J Plant Nutr 28:1–20
De Miguel E, Llamas JF, Chacón E, Berg T, Larssen S, Royset O, Vadset M (1997) Origin and patterns of distribution of trace elements in street dust: unleaded petrol and urban lead. Atmos Environ 31:2733–2740
De Miguel E, Irribarren I, Chacón E, Ordoñez A, Charlesworth S (2007) Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere 66:505–513
DeWolf H, Blust R, Backeljau T (2004) The use of RAPD in ecotoxicology. Mutat Res Rev Mutat Res 566:249–262
DHAenC (Department of Health and Aging and enHealth Council) (2012) Environmental health risk assessment: guidelines for assessing human health risks from environmental hazards [R]. ACT, Canberra
Di Palma L, Ferrantelli P, Merli C, Biancifiori F (2003) Recovery of EDTA and metal precipitation from soil flushing solutions. J Hazard Mater 103:153–168
Di Salvatore M, Carafa AM, Carratu G (2008) Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: a comparison of two growth substrates. Chemosphere 73:1461–1464
Ding C, Zhang T, Wang X, Zhou F, Yang Y, Yin Y (2013) Effects of soil type and genotype on lead concentration in rootstalk vegetables and the selection of cultivars for food safety. J Environ Manag 122:8–14
Djebali W, Zarrouk M, Brouquisse R, El Kahoui S, Limam F, Ghorbel MH, Chaibi W (2005) Ultrastructure and lipid alterations induced by cadmium in tomato (Lycopersicon esculentum) chloroplast membranes. Plant Biol 7:358–368
Donma O, Donma MM (2005) Cadmium, lead and phytochemicals. Med Hypotheses 65:699–702
Duruibe JO, Ogwuegbu MDC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Physiol Sci 2(5):112–118
Duzgoren-Aydin NS, Wong CSC, Aydin A, Song Z, You M, Li XD (2006) Heavy metal contamination and distribution in the urban environment of Guangzhou, SE China. Environ Geochem Health 28:375–391
Ebbs S, Talbott J, Sankaran R (2006) Cultivation of garden vegetables in Peoria Pool sediments from the Illinois River: a case study in trace element accumulation and dietary exposures. Environ Int 32:766–774
EC (2006) Commission Regulation No 1881/2006: setting maximum levels for certain contaminants in food stuffs. European Commission (EC)
Elbagermi MA, Edwards HGM, Alajtal AI (2012) Monitoring of heavy metal content in fruits and vegetables collected from production and market sites in the Misurata Area of Libya. Int Sch Res Net. doi:10.5402/2012/827645
Environment Canada (2005) Biological test method: test for measuring emergence and growth of terrestrial plants exposed to contaminants in soil. Method Development and Applications Section, Environmental Technology Centre
Ernst WHO (1996) Bioavailability of heavy metals and decontamination of soils by plants. Appl Geochem 11:163–167
European Union (2006) Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union 364:5–24
Evangelou VP, Marsi M (2001) Composition and metal ion complexation behavior of humic fractions derived from corn tissue. Plant Soil 229:13–24
Ewers U (1991) Standards, guidelines and legislative regulations concerning metals and their compounds. In: Merian E (ed) Metals and their compounds in the environment: occurrence, analysis and biological relevance. VCH, Weinheim, pp 458–468
Facchinelli A, Sacchi E, Mallen L (2001) Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut 114:313–324
FAO (2000) Food balance sheet, Rome. National Institute of Health (1988) National Nutrition Survey, Islamabad
FAO/WHO (2001a) Food additives and contaminants. Codex Alimentarius Commission. Joint FAO/WHO Food Standards Program, ALI-NORM 01/12A, pp 1–289
FAO/WHO (2001b) Human vitamin and mineral requirements, 2nd ed. Geneva, Switzerland
FAOSTAT (2007) FAOSTAT agriculture production database. http://faostat.fao.org/site/336/default.aspx
Farinati S, DalCorso G, Varotto S, Furini A (2010) The Brassica juncea BjCdR15, an ortholog of Arabidopsis TGA3, is a regulator of cadmium uptake, transport and accumulation in shoots and confers cadmium tolerance in transgenic plants. New Phytol 185:964–978
Ferré-Huguet N, Martí-Cid R, Schuhmacher M, Domingo JL (2008) Risk assessment of metals from consuming vegetables, fruits and rice grown on soils irrigated with waters of the Ebro River in Catalonia. Spain. Biol Trace Elem Res 123:1–14
Ferreira-Baptista L, De Miguel E (2005) Geochemistry and risk assessment of street dust in Luanda, Angola: a tropical urban environment. Atmos Environ 39:4501–4512
Finglas PM, Faulks RM (1984) The HPLC analysis of thiamin and riboflavin in potatoes. Food Chem 15:37–44
Fismes J, Perrin-Ganier C, Empereur-Biosoonnet P, Morel JL (2002) Soil to root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils. J Environ Qual 31:1649–1656
Fjällborg B, Ahlberg G, Nilsson E, Dave G (2005) Identification of metal toxicity in sewage sludge leachate. Environ Int 31:25–31
FNB (2004) Dietary reference intakes (DRIs): recommended intakes for individuals. Food and Nutrition Board, Institute of Medicine, National Academies
Fox MRS (1988) Nutritional factors that may influence bioavailability of cadmium. J Environ Qual 17:175–180
Fox TR, Comerfield NB (1990) Low molecular weight organic acid in selected forest soils of the south-eastern USA. Soil Sci Soc Am J 54:1763–1767
Galal TM, Shehata HS (2015) Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol Indic 48:244–251
Gallego SM, Benavides MP, Tomaro ML (1999) Effect of cadmium ions on antioxidative defense system in sunflower cotyledons. Biol Plant 42:49–55
Garate A, Ramos I, Manzanares M, Lucena JJ (1993) Cadmium uptake and distribution in three cultivars of Lactuca sp. Bull Environ Contam Toxicol 50:709–716
García I, Diez M, Martín F, Simón M, Dorronsoro C (2009) Mobility of arsenic and heavy metals in a sandy-loam textured and carbonated soil. Pedosphere 19:166–175
Garty J (2001) Biomonitoring atmospheric heavy metals with lichens: theory and application. Crit Rev Plant Sci 20:309–371
Gawęda M (2007) Changes in the contents of some carbohydrates in vegetables cumulating lead. Pol J Environ Stud 16(1):57–62
Ge KY (1992) The status of nutrient and meal of Chinese in the 1990s. People’s Hygiene Press, Beijing, pp 415–434
Ge Y, Hendershot W (2005) Modeling sorption of Cd, Hg and Pb in soils by the NICA [non-ideal competitive adsorption]—Donnan model. Soil Sediment Contam 14:53–69
Gebrekidan A, Weldegebriel Y, Hadera A, Bruggen BVD (2013) Toxicological assessment of heavy metals accumulated in vegetables and fruits grown in Ginfel river near Sheba Tannery, Tigray, Northern Ethiopia. Ecotoxicol Environ Saf 95:171–178
Gichner T, Patková Z, Száková J, Demnerová K (2004) Cadmium induces DNA damage in tobacco roots, but no DNA damage, somatic mutations or homologous recombination in tobacco leaves. Mutat Res Gen Toxicol Environ 559:49–57
Gichner T, Patková Z, Száková J, Demnerová K (2006) Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotoxicol Environ Saf 65:420–426
Gil C, Boluda R, Ramos J (2004) Determination and evaluation of cadmium lead and nickel in greenhouse soils of Almeria (Spain). Chemosphere 55:1027–1034
Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gonçalves JF, Antes FG, Maldaner J, Pereira LB, Tabaldi LA, Rauber R, Rossato LV, Bisognin DA, Dressler VL, Flores EMDM, Nicoloso FT (2011) Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol Biochem 47:814–821
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gill SS, Khana NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120
Giovanelli G, Paradise A (2002) Stability of dried and intermediate moisture tomato pulp during storage. Agric Food Chem 50:7277–7281
Goldbold DL, Huttermann A (1985) Effect of zinc, cadmium and mercury on root elongation of Picea abies (Karst) seedlings and the significance of these metals to forest die-back. Environ Pollut 38:375–381
Gonçalve JF, Antes FG, Maldaner J, Pereira LB, Tabaldi LA, Rauber R, Rossato LV, Bisognin DA, Dressler VL, Flores EMM, Nicoloso FT (2009) Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol Biochem 47:814–821
Gopal R, Khurana N (2011) Effect of heavy metal pollutants on sunflower. African J Plant Sci 5(9):531–536
Graham RD, Welch RM, Saunders DA, Ortiz-Monasterio I, Bouis HE, Bonierbale M (2006) Nutritious subsistence food systems. Adv Agron
Grill E, Loffler S, Winnacker E-L, Zenk MH (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci U S A 86:6838–6842
Grusak MA, Dellapenna D (1999) Improving the nutrient composition of plants to enhance human nutrition and health. Annu Rev Plant Physiol Plant Mol Biol 50:133–161
Gupta S, Bains K (2006) Traditional cooked vegetable dishes as important sources of ascorbic acid and b-carotene in the diets of Indian urban and rural families. Food Nutr Bull 27:306–310
Gupta N, Khan DK, Santra SC (2012) Heavy metal accumulation in vegetables grown in a long-term wastewater-irrigated agricultural land of tropical India. Environ Monit Assess 184:6673–6682
Halim M, Conte P, Piccolo A (2003) Potential availability of heavy metals to phytoextraction from contaminated soils induced by exogenous humic substances. Chemosphere 52:265–275
Han Y, Du P, Cao J, Posmentier ES (2006) Multivariate analysis of heavy metal contamination in urban dusts of Xi'an, Central China. Sci Total Environ 355:176–186
Hansch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol 12:259–266
Hart JJ, Welch RM, Norvell WA, Kochian LV (2006) Characterization of cadmium uptake, translocation and storage in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol 172:261–271
Hawkes JS (1997) Heavy metals. J Chem Educ 74:1369–1374
He J, Qin J, Long L, Ma Y, Li H, Li K, Jiang X, Liu X, Polle A, Liang Z, Luo ZB (2011) Net cadmium flux and accumulation oxidative stress and detoxification. Physiol Plant 143:50–63
Hermle S, Günthardt-Goerg MS, Schulin R (2006) Effects of metal-contaminated soil on the performance of young trees growing in model ecosystems under field conditions. Environ Pollut 144:703–714
Hernández LE, Gárate A, Lucena JJ, Carpena-Ruiz R (1995) Effect of cadmium on nitrogen fixing pea plants grown in perlite and vermiculite. J Plant Nutr 18:287–303
Hernández LE, Lozano E, Gárate A, Carpena R (1998) Influence of cadmium on the uptake, tissue accumulation and subcellular distribution of manganese in pea seedlings. Plant Sci 132:139–151
Hesse H, Nikiforova V, Gakiére B, Hoefgen R (2004) Molecular analysis and control of cysteine biosynthesis: integration of nitrogen and sulphur metabolism. J Exp Bot 55:1283–1292
Hooda PS, McNulty D, Alloway BJ (1997) Plant availability of heavy metals in soils previously amended with heavy applications of sewage sludge. J Sci Food Agric 73:446–454
Hossain Z, Huq F (2002) Studies on the interaction between Cd2+ ions and nucleobases and nucleotides. J Inorg Biochem 90:97–105
Hough RL, Young SD, Crout NMJ (2003) Modelling of Cd, Cu, Ni, Pb and Zn uptake, by winter wheat and forage, from a sewage disposal farm. J Soil Use Manag 19:19–27
Hu J, Wu F, Wu S, Sun X, Lin X, Wong MH (2013) Phytoavailability and phytovariety codetermine the bioaccumulation risk of heavy metal from soils, focusing on Cd-contaminated vegetable farms around the Pearl River Delta, China. Ecotoxicol Environ Saf 91:18–24
Huang JH, Fecher P, Ilgen G, Hu KN, Yang J (2012) Speciation of arsenite and arsenate in rice grain—verification of nitric acid based extraction method and mass sample survey. Food Chem 130:453–459
Huq I, Smith E, Correll R, Smith L, Smith J, Ahmed M, Roy S, Barnes M, Naidu R (2001) Arsenic transfer in water soil crop environments in Bangladesh. I: assessing potential arsenic exposure pathways in Bangladesh. In: Naidu R (ed) Arsenic in the Asia-Pacific Region workshop, ‘Managing arsenic for our future.’ 20–23 November 2001. Australia, Adelaide, p 50–51
Ikeda M, Zhang ZW, Shimbo S, Watanabe T, Nakatsuka H, Moon CS, Matsuda-Inoguchi N, Higashikawa K (2000) Urban population exposure to lead and cadmium in east and south-east Asia. Sci Total Environ 249:373–384
Imai A, Fukushima T, Matsushige K, Kim YH, Choi K (2002) Characterization of dissolved organic matter in effluents from wastewater treatment plants. Water Res 36:859–870
Intawongse M, Dean JR (2006) Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit Contam 23(1):36–48
Ipek U, Arslan EI, Öbek E, Karatas F, Erulas FA (2005) Determination of vitamin losses and degradation kinetics during composting. Process Biochem 40:621–624
Islam MR, Salminen R, Lahermo PW (2000) Arsenic and other toxic elemental contamination of groundwater, surface water and soil in Bangladesh and its possible effects on human health. Environ Geochem Health 22:33–53
ISO (2005a) Soil quality: chronic toxicity in higher plants. ISO 22030. International Organization for Standardization, Geneva
ISO (2005b) Soil quality—determination of the effects of pollutants on soil flora-screening test for emergence of lettuce seedlings (Lactuca sativa L.). ISO 17126. International Organization for Standardization, Geneva
ISO/DIS 17402 (2006) Soil quality—guidance for the selection and application of methods for the assessment of bioavailability in soil and soil materials
Iyengar V, Nair P (2000) Global outlook on nutrition and the environment: meeting the challenges of the next millennium. Sci Total Environ 249:331–346
Jensen J, Mesman M, Loibner AP, Erlacher E, Rutgers M, Archibald G, Ehlers C, Dirven-van Breemen L, Bogolte BT, Sorokin N, Celis RL, ter Laak T, Hartnik T, Bierkens J (2006) Ecological risk assessment of contaminated land: decision support for site specific investigations. RIVM
John MK, Van Laerhoven CJ (1976) Differential effects of cadmium on lettuce varieties. Environ Pollut 4:7–15
John R, Ahmad P, Gadgil K, Sharma S (2009) Heavy metal toxicity: effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int J Plant Prod 3:65–76
Jones C (2006) Förbättrade miljöriskbedömningar Naturvårdsverket, Stockholm
Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, Sansom L, Naidu R (2006) In vivo assessment of arsenic bioavailability in rice and its significance for human health risk assessment. Environ Health Perspect 114:1826–1831
Kachenko AG, Singh B (2006) Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut 169:101–123
Kar S, Das S, Jean J-S, Chakraborty S, Liu C-C (2013) Arsenic in the water-soil-plant system and the potential health risks in the coastal part of Chianan Plain, Southwestern Taiwan. J Asian Earth Sci. doi:10.1016/j.jseaes.2013.03.003
Kartal S, Aydin Z, Tokalıoğlu S (2006) Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. J Hazard Mater 132:80–89
Kashif SR, Akram M, Yaseen M, Ali S (2009) Studies on heavy metals status and their uptake by vegetables in adjoining areas of Hudiara drain in Lahore. Soil Environ 28(1):7–12
Khan FI, Husain T (2001) Risk-based monitored natural attenuation—a case study. J Hazard Mater 85:243–272
Khan S, Cao Q, Chen B-D, Zhu YG (2006) Humic acids increase the phytoavailability of Cd and Pb to wheat plants cultivated in freshly spiked, contaminated soil. J Soil Sediments 6(4):236–242
Khan S, Aijun L, Zhang S, Hu Q, Zhu YG (2008a) Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J Hazard Mater 152:506–515
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008b) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152:686–692
Khan S, Hesham AEL, Qiao M, Rehman S, He JZ (2010a) Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut Res 17:288–296
Khan S, Rehman S, Khan AZ, Khan MA, Shah MT (2010b) Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol Environ Saf 73:1820–1827
Khan S, Waqas M, Ding F, Shamshad I, Arpd HPH, Li G (2015) The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J Hazard Mater 300 (2015) 1–11 doi:10.1016/j.jhazmat.2015.06.050
Khan K, Lu Y, Khan H, Ishtiaq M, Khan S, Waqas M, Wei L, Wang T (2013a) Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food Chem Toxicol 58:449–458
Khan S, Chao C, Waqas M, Arp HPH, Zhu YG (2013b) Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environ Sci Technol 47:8624–8632
Khan S, Reid BJ, Li G, Zhu YG (2014) Application of biochar to soil reduces cancer risk via rice consumption: a case study in Miaoqian village, Longyan, China. Environ Int 68:154–161
Khanna-Chopra R (2012) Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 249:469–481
Khillare PS, Jyethi DS, Sarkar S (2012) Health risk assessment of polycyclic aromatic hydrocarbons and heavy metals via dietary intake of vegetables grown in the vicinity of thermal power plants. Food Chem Toxicol 50:1642–1652
Kieffer P, Schroder P, Dommes J, Hoffmann L, Renaut J, Hausman JF (2009) Proteomic and enzymatic response of poplar to cadmium stress. J Proteome 72:379–396
Kim D-Y, Bovet L, Kushnir S, Noh EW, Martinoia E, Lee Y (2006) AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiol 140:922–932
Kim H, Song B, Kim H, Park J (2009) Distribution of trace metals at two abandoned mine sites in Korea and arsenic-associated health risk for the residents. Toxicol Environ Heal Sci 1(2):83–90
Kovalchuk O, Titov V, Hohn B, Kovalchuk G (2001) A sensitive transgenic plant system to detect toxic inorganic compounds in the environment. Nat Biotechnol 19:568–572
Kovalchuk I, Titov V, Hohn B, Kovalchuk O (2005) Transcriptome profiling reveals similarities and differences in plant responses to cadmium and lead. Mutat Res Fundam Mol Mech Mutagen 570:149–161
Kowalewska G, Falkowski L, Hoffmann SK, Szczepaniak L (1987) Replacement of magnesium by copper (II) in the chlorophyll porphyrin ring of planktonic algae. Acta Physiol Plant 9:43–52
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–993
Kratovalieva S, Cvetanowska L (2001) Influence of different lead concentrations to some morpho-physiological parameters at tomato (Lycopersicon esculentum Mill.) in experimental conditions. Maced Agric Rev 48(1/2):35
Krishnamurti GSR, Megharaj M, Naidu R (2004) Bioavailability of cadmium-organic complexes to soil alga—an exception to the free ion model. J Agric Food Chem 52:3894–3899
Kumari MM, Sinhal VK, Srivastava A, Singh VP (2011) Zinc alleviates cadmium induced toxicity in Vigna radiata (L.) Wilczek. J Phytol 3(8):43–46
Kuo S, Huang B, Bembenek R (2004) The availability to lettuce of zinc and cadmium in a zinc fertilizer. Soil Sci 169:363–373
Lacatutsu R, Rauta C, Cârstea S, Ghelase I (1996) Soil plant-man relationships in heavy metal polluted areas in Romania. Appl Geochem 11:105–107
Lagier T, Feuillade G, Matejka G (2000) Interactions between copper and organic macromolecules: determination of conditional complexation constants. Agronomie 20:537–546
Le Guédard M, Schraauwers B, Larrieu I, Bessoule J-J (2008) Development of a biomarker for metal bioavailability: the lettuce fatty acid composition. Environ Toxicol Chem 27:1147–1151
Le Guédard M, Faure O, Bessoule J-J (2012) Early changes in the fatty acid composition of photosynthetic membrane lipids from Populus nigra grown on a metallurgical landfill. Chemosphere 88:693–698
Lee S-W, Lee B-T, Kim J-Y, Kim K-W, Lee J-S (2006) Human risk assessment for heavy metals and As contamination in the abandoned metal areas, Korea. Environ Monit Assess 11:233–244
Lee J-S, Lee S-W, Chon H-T, Kim K-W (2008) Evaluation of human exposure to arsenic due to rice ingestion in the vicinity of abandoned Myungbong Au-Ag mine site, Korea. J Geochem Explor 96:231–235
Leonard SS, Harris GK, Shi XL (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37:1921–1942
Li Z, Shuman LM (1996) Heavy metal movement in metal contaminated soil profiles. Soil Sci 161:656–666
Li W, Khan MA, Yamaguchi S, Kamiya Y (2005a) Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul 46:45–50
Li WX, Chen TB, Chen Y, Lei M (2005b) Role of trichome Pteris vittata L. in arsenic hyperaccumulation. Sci China Ser C Life Sci 48:148–154
Li BY, Zhou DM, Cang L, Zhang HL, Fan XH, Qin SW (2007) Soil micronutrient availability to crops as affected by long-term inorganic and organic fertilizer applications. Soil Tillage Res 96:166–173
Li Y, Gou X, Wang G, Zhang Q, Su Q, Xiao G (2008) Heavy metal contamination and source in arid agricultural soils in central Gansu Province, China. J Environ Sci 20:607–612
Li Q, Cai S, Mo C, Chu B, Peng L, Yang F (2010) Toxic effects of heavy metals and their accumulation in vegetables grown in a saline soil. Ecotoxicol Environ Saf 73:84–88
Li QS, Chen Y, Fu HB, Cui ZH, Shi L, Wang LL, Liu ZF (2012) Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J Hazard Mater 227:148–154
Liedschulte V, Wachter A, Zhigang AT, Rausch (2010) Exploiting plants for glutathione (GSH) production: uncoupling GSH synthesis from cellular controls results in unprecedented GSH accumulation. Plant Biotechnol J 8:1–14
Lim H-S, Lee J-S, Chon H-T, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J Geochem Explor 96:223–230
Lin AJ, Zhang XH, Wong MH, Ye ZH, Lou LQ, Wang YS, Zhu YG (2007) Increase of multi-metal tolerance of three leguminous plants by arbuscular mycorrhizal fungi colonization. Environ Geochem Health 29:473–481
Lindsey PA, Lineberger RD (1981) Toxicity, cadmium accumulation and ultrastructural alterations induced by exposure of Phaseolus seedlings to cadmium. Hortic Sci 16:434
Linger P, Ostwald A, Haensel J (2005) Cannabis sativa L. growing on heavy metal contaminated soil: growth, cadmium uptake and photosynthesis. Biol Plant 49:567–576
Liu Q, Wang Z, Tang H (1996) The progress of relationship between heavy metal fraction and biotoxicity and bioavailability (in Chinese). Environ Sci 17:89–92
Liu H, Probst A, Liao B (2005a) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166
Liu W, Li PJ, Qi XM, Zhou QX, Zheng L, Sun TH, Yang YS (2005b) DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis. Chemosphere 61:158–167
Liu WH, Zhao JZ, Ouyang ZY, Soderlund L, Liu GH (2005c) Impacts of sewage irrigation on heavy metals distribution and contamination in Beijing, China. Environ Int 31:805–812
Liu WX, Shen LF, Liu JW, Wang YW, Li SR (2007) Uptake of toxic heavy metals by rice (Oryza sativa L.) cultivated in the agricultural soils near Zhengzhou City, People's Republic of China. Bull Environ Contam Toxicol 79:209–213
Liu J, Zhang X-H, Tran H, Wang D-Q, Zhu Y-N (2011) Heavy metal contamination and risk assessment in water, paddy soil, and rice around an electroplating plant. Environ Sci Pollut Res 18:1623–1632
Lock K, Janssen CR (2001) Modeling zinc toxicity for terrestrial invertebrates. Environ Toxicol Chem 20:1901–1908
Lofts S, Spurgeon DJ, Svendsen C, Tipping E (2004) Deriving soil critical limits for Cu, Zn, Cd, and Pb: a method based on free ion concentrations. Environ Sci Technol 38:3623–3631
López-Millán A-F, Sagardoy R, Solanas M, Abadía A, Abadía J (2009) Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ Exp Bot 65:376–385
Loureiro S, Santos C, Pinto G, Costa A, Monteiro M, Nogueira AJ, Soares A (2006) Toxicity assessment of two soils from Jales mine (Portugal) using plants: growth and biochemical parameters. Arch Environ Contam Toxicol 50:182–190
Lu X, Wang L, Lei K, Huang J, Zhai Y (2009) Contamination assessment of copper, lead, zinc, manganese and nickel in street dust of Baoji, NW China. J. Hazard Mater 161:1058–1062
Lugon-Moulin N, Zhang M, Gadani F, Rossi L, Koller D, Krauss M, Wagner GJ (2004) Critical review of the science and options for reducing cadmium in tobacco (Nicotiana tabacum L.) and other plants. Adv Agron 83:111–180
Lund W (1990) Speciation analysis—why and how. Fresenius J Anal Chem 337:557–564
Luo C, Liu C, Wang Y, Liu X, Li F, Zhang G, Li X (2011) Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J Hazard Mater 186:481–490
Lutz JAJ, Genter CF, Hawkins GW (1972) Effect of soil pH on element concentration and uptake by maize. II. Cu, B, Zn, Mn, Mo, Ai & Fe. Agron J 64:583–585
LWTAP (2004) Lenntech Water treatment and air purification. Water treatment. Lenntech, Rotterdamseweg, Netherlands. http://www.excelwater.com/thp/filters/Water-Purification.htm
Madeira AC, de Varennes A, Abreu MM, Esteves C, Magalhães MCF (2012) Tomato and parsley growth, arsenic uptake and translocation in a contaminated amended soil. J Geochem Explor 123:114–121
Madrid L, Díaz-Barrientos E, Madrid F (2002) Distribution of heavy metal contents of urban soils in parks of Seville. Chemosphere 49:1301–1308
Mahalakshmi M, Balakrishnan S, Indira K, Srinivasan M (2012) Characteristic levels of heavy metals in canned tuna fish. J Toxicol Environ Health Sci 4:43–45
Mahmood Q, Ahmad R, Kwak SS, Rashid A, Anjum NA (2010) Ascorbate and glutathione: protectors of plants in oxidative stress. Ascorbate–glutathione pathway and stress tolerance in plants. Springer, pp 209–229
Man YB, Sun XL, Zhao YG, Lopez BN, Chung SS, Wu SC et al (2010) Health risk assessment of abandoned agricultural soils based on heavy metal contents in Hong Kong, the world’s most populated city. Environ Int 36:570–576
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mangabeira P, Almeida AA, Mielke M, Gomes FP, Mushrifah I, Escaig F, Laffray D, Severo MI, Oliveira AH, Galle P (2001) Ultrastructural investigations and electron probe X-ray microanalysis of chromium-treated plants. Proc. VI ICOBTE, Guelph, p 555
Manivasagaperumal R, Vijayarengan P, Balamurugan S, Thiyagarajan G (2011) Effect of copper on growth, dry matter yield and nutrient content of Vigna radiata (L.) Wilczek. J Phytol 3(3):53–62
Mansour SA, Belal MH, Abou-Arab AAK, Ashour HM, Gad MF (2009) Evaluation of some pollutant levels in conventionally and organically farmed potato tubers and their risks to human health. Food Chem Toxicol 47:615–624
Mapanda F, Mangwayana EN, Nyamangara J, Giller KE (2005) The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric Ecosyst Environ 107:151–165
Marschner B, Kalbitz K (2003) Control of bioavailability and biodegradability of dissolved organic matter in soil. Geoderma 113:211–235
Martens DC, Reed ST (1991) Zinc: unlocking agronomic potential. Solutions 29–31
Martin MA, Pachepsky YA, Rey J-M, Taguas J, Rawls WJ (2005) Balanced entropy index to characterize soil texture for soil water retention estimation. Soil Sci 170:759–766
Martinez-Valvercle I, Periage MJ, Provan G, Chesson A (2002) Phenolic compounds, lycopene and antioxidant activities in commercial varieties of tomato (Lycopersicon esculentum). J Sci Food Agric 82:323–330
Martorell I, Perelló G, Martí-Cid R, Llobet JM, Castell V, Domingo JL (2011) Human exposure to arsenic, cadmium, mercury, and lead from foods in Catalonia, Spain: temporal trend. Biol Trace Elem Res 142:309–322
Mattina MJI, White J, Eitzer B, Lannucci-Berger W (2002) Cycling of weathered chlordane residues in the environment: compositional and chiral profiles in contiguous soil, vegetation, and air compartments. Environ Toxicol Chem 21:281–288
McGraph D, Zhang CS, Carton O (2004) Geostatistical analyses and hazard assessment on soil lead in Silver mines, area Ireland. Environ Pollut 127:239–248
McLaughlin MJ, Parker DR, Clarke JM (1999) Metals and micronutrients-food safety issues. Field Crops Res 60:143–163
McLaughlin MJ, Smolders E, Degryse F, Rietra R (2011) Uptake of metals from soil into vegetables. In: Swartjes FA (ed) Dealing with contaminated sites. Springer, Netherlands, pp 325–367
Medina A, Vassilev N, Barea JM, Azcón R (2005) Application of Aspergillus niger-treated agrowaste residue and Glomus mosseae for improving growth and nutrition of Trifolium repens in a Cd-contaminated soil. J Biotechnol 116:369–378
Meers E, Ruttens A, Geebelen W, Vangronsveld J, Samson R, Vanbroekhoven K, Vandegehuchte M, Diels L, Tack FMG (2005) Potential use of the plant antioxidant network for environmental exposure assessment of heavy metals in soils. Environ Monit Assess 120:243–267
Meharg AA (1993) The role of plasmalemma in metal tolerance in angiosperm. Physiol Plant 88:191–198
Mellem JJ, Baijnath H, Odhav B (2009) Translocation and accumulation of Cr, Hg, As, Pb, Cu and Ni by Amaranthus dubius (Amaranthaceae) from contaminated sites. J Environ Sci Health Part A 44:568–575
Merry RH (2001) Environmental and ecological chemistry, acidity and alkalinity of soils. CSIRO Land and Water, Adelaide
Minh ND, Hough RL, Thuy LT, Nyberg Y, Mai LB, Vinh NC, Khai NM, Öborn I (2012) Assessing dietary exposure to cadmium in a metal recycling community in Vietnam: age and gender aspects. Sci Total Environ 416:164–171
Mishra VK, Upadhyay AR, Tripathi BD (2009) Bioaccumulation of heavy metals and two organochlorine pesticides (DDT and BHC) in crops irrigated with secondary treated waste water. Environ Monit Assess 156:99–107
Misra SG, Mani D (1991) Soil pollution. Ashish Publishing House, Punjabi Bagh
Mitra AK, Haque A, Islam M, Bashar SAMK (2009) Lead poisoning: an alarming public health problem in Bangladesh. Int J Environ Res Public Health 6:84–95
Mohamed AE, Rashed MN, Mofty A (2003) Assessment of essential and toxic elements in some kinds of vegetables. Ecotoxicol Environ Saf 55:251–260
Montagne D, Cornu S, Bourennane H, Baize D, Ratié C, King D (2007) Effect of agricultural practices on trace-element distribution in soil. Commun Soil Sci Plant Anal 38:473–491
Monteiro M, Santos C, Mann RM, Soares AMVM (2004) Physiological effects of cadmium on Lactuca sativa L. under hydroponic conditions. In: Proceedings of the Fourth SETAC World Congress and 25th Annual Meeting in North America, Portland, Oregon, USA, November 14-18, p 442
Monteiro M, Santos C, Mann RM, Soares AMVM, Lopes T (2007) Evaluation of cadmium genotoxicity in Lactuca sativa L. using nuclear microsatellites. Environ Exp Bot 60:421–427
Morton-Bermea O, Hernández-Álvarez E, González-Hernández G, Romero F, Lozano R, Beramendi-Orosco LE (2009) Assessment of heavy metal pollution in urban topsoils from the metropolitan area of Mexico City. J Geochem Explor 101:218–224
Mosha TC, Gaga HE (1999) Nutritive value and effect of blanching on the trypsin and chymotrypsin inhibitor activities of selected leafy vegetables. Plant Foods Hum Nutr 54:271–283
Moustakas NK, Akoumianakis KA, Passam HC (2001) Cadmium accumulation and its effect on yield of lettuce, radish and cucumber. Commun Soil Sci Plant Anal 32:1793–1802
Munzuroglu O, Geckil H (2002) Effects of metals on seed germination, root elongation, and coleoptile and hypocotyls growth in Triticum aestivum and Cucumis sativus. Arch Environ Contam Toxicol 43:203–213
Munzuroglu O, Obek E, Karatas F, Tatar SY (2005) Effects of simulated acid rain on vitamins A, E, and C in strawberry (Fragaria vesca). Pak J Nutr 4(6):402–406
Nabulo G, Black CR, Young SD (2011) Trace metal uptake by tropical vegetables grown on soil amended with urban sewage sludge. Environ Pollut 159:368–376
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nagor S, Vyas AV (1997) Heavy metal induced changes in growth and carbohydrate metabolism in wheat seedlings. Indian J Environ Toxicol 7(2):98
National Environment Protection Council (NEPC) (1999) Guideline on the investigation levels for soil and groundwater, Schedule B(1). Federal Register of Legislative Instruments F2008B00713, Australia
National Institute of Health, National Library of Medicine, Hazardous Substances Data Bank (NLM/HSDB) (2012) Hazardous Substances Data Bank. http://toxnet.nlm.nih.gov/cgi-bin/sis/search
Niemeyer J, Moreira-Santos M, Nogueira M, Carvalho G, Ribeiro R, Da Silva E, Sousa J (2010) Environmental risk assessment of a metal-contaminated area in the tropics Tier I: screening phase. J Soils Sediments 10:1557–1571
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750
Nigam R, Srivastava S, Prakash S, Srivastava MM (2001) Cadmium mobilization and plant availability—the impact of organic acids commonly exuded from roots. Plant Soil 230:107–113
Nishijo M, Satarug S, Honda R, Tsuritani I, Aoshima K (2004) The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Mol Cell Biochem 255:87–92
Noor-ul-Amin, Hussain A, Alamzeb S, Begum S (2013) Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, District Mardan, Pakistan. Food Chem 136:1515–1523
Nordberg G (1996) Human cadmium exposure in the general environment and related health risks—a review. In: Sources of cadmium in the environment. Organisation for Economic Co-Operation and Development, Paris, pp 94–104
O’Halloran K (2006) Toxicological considerations of contaminants in the terrestrial environment for ecological risk assessment. Human Ecol Risk Assess 12:74–83
O’Neill (1995) Arsenic. In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic and Professional, London, pp 105–121
Odhav B, Beekrum S, Akula US, Baijnath H (2007) Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J Food Compos Anal 20:430–435
Oetken M, Bachmann J, Schulte-Oehlmann U, Oehlmann J (2004) Evidence for endocrine disruption in invertebrates. Int Rev Cytol 236:1–44
Oliva SR, Espinosa AJF (2007) Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchem J 86:131–139
Oves M, Khan MS, Zaidi A, Ahmad E (2012) Soil contamination, nutritive value, and human health risk assessment of heavy metals: an overview. Toxicol Heavy Metals Leg Biorem 1–27
Owens G, Rahman MM, Heinrich T, Naidu R (2004) Bangladesh–Australia Centre for Arsenic Mitigation Program (BACAMP): program 3: safe food, sect. 1: arsenic food chain assessment. University of South Australia, Consultancy report for GHD Pty Ltd
Pandey R, Shubhashish K, Pandey J (2012) Dietary intake of pollutant aerosols via vegetables influenced by atmospheric deposition and wastewater irrigation. Ecotoxicol Environ Saf 76:200–208
Park JD, Cherrington NJ, Klaassen CD (2002) Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. Toxicol Sci 68:288–294
Patel SK, Shrivas K, Brandt R, Jakubowski N, Corns W, Hoffmann P (2005) Arsenic contamination in water, soil, sediment and rice of central India. Environ Geochem Health 27(2):131–145
Perfect E (2003) A pedotransfer function for predicting solute dispersivity: model testing and upscaling. In: Pachepsky Y, Radcliffe DE, Selim HM (eds) Scaling methods in soil physics. CRC Press, Boca Raton, pp 89–96
Piotrowska M, Kabata-Pendias A (1997) Impact of soils amended with Zn and Pb smelter dust on Cd concentrations in potatoes. J Geochem Explor 58:319–322
Pleasants E, Sandow M, Decandido S, Waslien C, Naughton B (1992) The effect of vitamin D3 and 1,25-dihydroxy vitamin D3 on the toxic symptoms of cadmium exposed rats. Nutr Res 12:1393
Pourrut B, Shahid M, Douay F, Dumat C, Pinelli E (2013) Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. Heavy Metal Stress Plants 121–147
Prasad MNV (1995) Cadmium toxicity and tolerance in vascular plants. Environ Exp Bot 35:525–545
Prasad MNV (1999) Heavy metal stress in plants from biomolecules to ecosystem. Narosa Publishing House, New Delhi
Price W (1945) Nutrition and physical degeneration. Price-Pottenger Nutrition Foundation, San Diego, p 278
Pruvot C, Douay F, Herve F, Waterlot C (2006) Heavy metals in soil, crops and grass as a source of human exposure in the former mining areas. J Soils Sediments 6:215–220
Qu CS, Sun K, Wang SR, Huang L, Bi J (2012) Monte Carlo simulation based health risk assessment of heavy metal pollution: a case study in Qixia mining area, China. Hum Ecol Risk Assess 18:733–750
Radwan MA, Salama AK (2006) Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem Toxicol 44(8):1273–1278
Ramirez-Andreotta MD, Brusseau ML, Artiola JF, Maier RM (2013) A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils. Sci Total Environ 443:299–306
Ramos I, Esteban E, Lucena JJ, Gárate A (2002) Cadmium uptake and sub cellular distribution in plants of Lactuca sp. Cd-Mn interaction. Plant Sci 162:761–767
Rashid A, Ryan J (2004) Micronutrient constraints to crop production in soils with Mediterranean type characteristic: a review. J Plant Nutr 27:959–975
Rattan RK, Datta SP, Chhonkar PK, Suribabu K, Singh AK (2005) Long-term impact of irrigation with waste water effluents on heavy metal content in soils, crops and groundwater—a case study. Agric Ecosyst Environ 109:310–322
Rodríguez-Celma J, Rellán-Álvarez R, Abadía A, Abadía J, López-Millán A-F (2010) Changes induced by two levels of cadmium toxicity in the 2-DE protein profile of tomato roots. J Proteome 73:1694–1706
Rogival D, Scheirs J, Blust R (2007) Transfer and accumulation of metals in a soil-diet-wood mouse food chain along a metal pollution gradient. Environ Pollut 145:516–528
Rosselli W, Rossi M, Sasu I (2006) Cd, Cu and Zn contents in the leaves of Taraxacum officinale. Swiss Federal Institute for Forest. Snow Landsc Res 80(3):361–366
Roth U, von Roepenack-Lahaye E, Clemens S (2006) Proteome changes in Arabidopsis thaliana roots upon exposure to Cd2+. J Exp Bot 57:4003–4013
Roychowdhury T, Tokunaga H, Ando M (2003) Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Sci Total Environ 308:15–35
Ryan JA, Pahren HR, Lucas JB (1982) Controlling cadmium in the human food chain: a review and rationale based on health effects. Environ Res 28:251–302
Ryu DY, Lee S, Park J, Choi DW, Klaassen CD, Park JD (2004) Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicol Lett 152:19–25
Sandaa RA, Torsvik V, Enger O, Daae FL, Castberg T, Hahn D (1999) Analysis of bacterial communities in heavy metal-contaminated soils at different levels of resolution. FEMS Microbiol Ecol 30:237–251
Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 522:115–126
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Saraf A, Samant A (2013) Evaluation of some minerals and trace elements in Achyranthes aspera Linn. Int J Pharma Sci 3(3):229–233
Sarry JE, Kuhn L, Ducruix C, Lafaye A, Junot C, Hugouvieux V et al (2006) The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. J Proteome 6:2180–98
Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibia S, Farid G (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90:925–929
Sauvé S, Manna S, Turmel M-C, Roy AG, Courchesne F (2003) Solid solution partitioning of Cd, Cu, Ni, Pb, and Zn in the organic horizons of a forest soil. Environ Sci Technol 37:5191–5196
Saxena I, Shekhawat GS (2013) Nitric oxide (NO) in alleviation of heavy metal induced phytotoxicity and its role in protein nitration. A review. Nitric Oxide 32:13–20
Sekhar KC, Chary NS, Kamla CT, Rao JV, Balaram V, Anjaneyulu Y (2003) Risk assessment and pathway study of arsenic in industrially contaminated sites of Hyderabad: a case study. Environ Int 29(5):601–611
Semenzin E, Critto A, Carlon C, Rutgers M, Marcomini A (2007) Development of a site-specific ecological risk assessment for contaminated sites: part II. Multicriteria based system for the selection of bioavailability assessment tools. Sci Total Environ 379:34–45
Senesia N, Orazioa VD, Riccab G (2003) Humic acids in the first generation of EUROSOILS. Geoderma 116:325–344
SEPA (1995) Environmental quality standards for soils. State Environmental Protection Administration, China, GB 15618-1995
SEPA (2005) The limits of pollutants in food. State Environmental Protection Administration, China, GB 2762-2005
Seregin IV, Kozhevnikova AD (2006) Physiological role of nickel and its toxic effects on higher plants. Russ J Plant Physiol 53:257–277
Sessitsch A, Weilharter A, Gerzabeck MH, Kirchmann H, Kandeler E (2001) Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl Environ Microbiol 67:215–224
Seuntjens P, Nowack B, Schulin R (2004) Root-zone modeling of heavy metal uptake and leaching in the presence of organic ligands. Plant Soil 265:61–73
Sezgin N, Ozcan HK, Demir G, Nemlioglu S, Bayat C (2003) Determination of heavy metal concentrations in street dusts in Istanbul E-5 highway. Environ Int 29:979–985
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Sharma RK, Agrawal M, Marshal F (2009) Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food Chem Toxicol 47:583–591
Sheppard SC, Evenden WG, Abboud SA, Stephenson M (1993) A plant life-cycle bioassay for contaminated soil, with comparison to other bioassays: mercury and zinc. Arch Environ Contam Toxicol 25:27–35
Shi Z, Tao S, Pan B, Fan W, He XC, Zuo Q, Wu SP, Li BG, Cao J, Liu WX, Xu FL, Wang XJ, Shen WR, Wong PK (2005) Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ Pollut 134:97–111
Shi GL, Lou LQ, Zhang S, Xia XW, Cai QS (2013) Arsenic, copper, and zinc contamination in soil and wheat during coal mining, with assessment of health risks for the inhabitants of Huaibei, China. Environ Sci Pollut Res 20:8435–8445
Sindern S, Lima RFS, Schwarzbauer J, Petta RA (2007) Anthropogenic heavy metal signatures for the fast growing urban area of Natal (NE-Brazil). Environ Geol 52:731–737
Singh A, Sharma RK, Agrawal M, Marshall FM (2010a) Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop Ecol 51(2):375–387
Singh R, Singh DP, Kumar N, Bhargava SK, Barman SC (2010b) Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J Environ Biol 31:421–430
Sinha S, Pandey K, Gupta AK, Bhatt K (2005) Accumulation of metals in vegetables and crops grown in the area irrigated with river water. Bull Environ Contam Toxicol 74:210–218
Sipter E, Rózsa E, Gruiz K, Tátrai E, Morvai V (2008) Site-specific risk assessment in contaminated vegetable gardens. Chemosphere 71:1301–1307
Small J, Jackson T (1949) Buffer index values in relation to soil—pH tolerances. Plant Physiol 24:75–83
Smith SR (2009) A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ Int 35:142–156
Smolders E, Oorts K, Van Sprang P, Schoeters I, Janssen CR, McGrath SP, McLaughlin MJ (2009) Toxicity of trace metals in soil as affected by soil type and aging after contamination: using calibrated bioavailability models to set ecological soil standards. Environ Toxicol Chem 28:633–1642
Soares CR, Grazziotti FS, Siquaira PH, Carvalno JO, De JH (2001) Zinc toxicity on growth and nutrition of Eucalyptus muculata and Eucalyptus urophylla. Pesq Agrop Brasileira 36:339–348
Solgi E, Esmaili-Sari A, Riyahi-Bakhtiari A, Hadipour M (2012) Soil contamination of metals in the three industrial estates, Arak, Iran. Bull Environ Contam Toxicol 88:634–638
Solti A, Gáspár L, Mészáros I, Szigeti Z, Lévai L, Sárvári E (2008) Impact of iron supply on the kinetics of recovery of photosynthesis in Cd-stressed poplar (Populus glauca). Ann Bot 102:771–782
Springob G, Tetzlaff D, Schön A, Böttcher J (2001) Quality of estimated Freundlich parameters of Cd sorption from pedotransfer functions to predict cadmium concentrations of soil solution. In: Iskandar IK, Kirkham MB (eds) Trace elements in soil. Bioavailability, flux, and transfer. Lewis Publishers, Boca Raton, pp 229–245
Srikumar TS, Ockerman PA (1990) The effects of fertilization and manuring on the content of some nutrients in potato (var. Provita). Food Chem 37:47–60
Stangeland T, Remberg SF, Lye KA (2009) Total antioxidant activity in 35 Ugandan fruits and vegetables. Food Chem 113:85–91
Stasinos S, Zabetakis I (2013) The uptake of nickel and chromium from irrigation water by potatoes, carrots and onions. Ecotoxicol Environ Saf 91:122–128
Steinkellner H, Mun-Sik K, Helma C, Ecker S, Ma TH, Horak O, Kundi M, Knasmuller S (1998) Genotoxic effects of heavy metals: comparative investigation with plant bioassays. Environ Mol Mutagen 31:183–191
Stumm W, Morgan JJ (1996) Aquatic chemistry, 3rd edn. Wiley, New York
Sugawara C, Sugawara N, Miyake H (1981) Decrease of plasma vitamin A, albumin and zinc in cadmium-treated rats. Toxicol Lett 8:323
Sun C, Liu J, Wang Y, Sun L, Yu H (2013) Multivariate and geostatistical analyses of the spatial distribution and sources of heavy metals in agricultural soil in Dehui, Northeast China. Chemosphere 92:517–523
Suzuki N, Yamaguchi Y, Koizumi N, Sano H (2002) Functional characterization of heavy metal binding protein Cdl19 from Arabidopsis. Plant J 32:165–173
Swedish National Food Administration (1984) Livsmedelskonsumtion. Uppsala, Sweden. (In Swedish.)
Szolnoki ZS, Farsang A, Puskás I (2013) Cumulative impacts of human activities on urban garden soils: origin and accumulation of metals. Environ Pollut 177:106–115
Tatli Seven P, Yilmaz S, Seven I, Tuna Kelestemur G (2012) The effects of propolis in animals exposed oxidative stress. Oxidative stress—environmental induction and dietary antioxidants. In: Volodymyr I, Lushchak (ed) (Chapter 13)/ InTECH BOOK (ISBN 978-953-51-0553-4). doi:10.5772/2536
Tchounwou PB, Centeno JA, Patlolla AK (2004) Arsenic toxicity, mutagenesis, and carcinogenesis—a health risk assessment and management approach. Mol Cell Biochem 255:47–55
Thys C, Vanthomme P, Schrevens E, De Proft M (1991) Interactions of cadmium with zinc, copper, manganese, and iron in lettuce (Lactuca sativa L.) in hydroponic culture. Plant Cell Environ 14:713–717
Tinsley IJ (1979) Chemical concepts in pollutants behavior. J. Willey and Sons Inc, NY
Tiwari KK, Singh NK, Patel MP, Tiwari MR, Rai UN (2011) Metal contamination of soil and translocation in vegetables growing under industrial wastewater irrigated agricultural field of Vadodara, Gujarat, India. Ecotoxicol Environ Saf 74(6):1670–1677
Tongesayi T, Fedick P, Lechner L, Brock C, Beau AL, Bray C (2013) Daily bioaccessible levels of selected essential but toxic heavy metals from the consumption of non-dietary food sources. Food Chem Toxicol 62:142–147
Treder W, Cieslinski G (2005) Effect of silicon application on cadmium uptake and distribution in strawberry plants grown on contaminated soils. J Plant Nutr 28:917–929
Tripathi RM, Raghunath R, Krishnamoorthy TM (1997) Dietary intake of heavy metals in Bombay City, India. Sci Total Environ 208:149–159
Tsadilas CD, Karaivazoglou NA, Tsotsolis NC, Stamatiadis S, Samaras V (2005) Cadmium uptake by tobacco as affected by liming, N form, and year of cultivation. Environ Pollut 134:239–246
Tsai PJ, Shieh HY, Lee WJ, Lai SO (2001) Health risk assessment for workers exposed to polycyclic aromatic hydrocarbons (PAHs) in carbon black manufacturing industry. Sci Total Environ 278:137–150
Tsezos M (2009) Metal-microbes interactions: beyond environmental protection. Adv Matter Res 71–73:527–532
Tufuor JK, Bentu JK, Essumang DK, Koranteng-Addo JE (2011) Analysis of heavy metals in citrus juice from the Abura–Asebu–Kwamankese District, Ghana. J Chem Pharm Res 3(2):397–402
U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program (2011) Report on carcinogens, 12 edn. http://ntp.niehs.nih.gov/ntp/roc/twelfth/roc12.pdf
U.S. Environmental Protection Agency (USEPA) (2012) Regional screening levels (Formerly PRGs)—summary table. http://www.epa.gov/region9/superfund/prg
U.S. Environmental Protection Agency Integrated Risk Assessment System (USEPA/ IRIS) (2012) Integrated risk information system. http://www.epa.gov/IRIS/
Upadhyay RK, Panda SK (2009) Copper-induced growth inhibition, oxidative stress and ultrastructural alterations in freshly grown water lettuce (Pistia stratiotes L.). C R Biol 332(7):623–632
Upchurch RG (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 30:967–977
US Department of Health and Human Services (2005) Public Health Service Agency for Toxic Substances and Disease Registry, Toxicological profile for nickel, p 32
US Environmental Protection Agency (EPA) (2001) The role of screening-level risk assessments and refining contaminants of concern in baseline ecological risk assessments. Office of Solid Waste and Emergency Response, Washington, DC
US Environmental Protection Agency (EPA) (2005) Guidance for developing ecological soil screening levels. Office of solid waste and emergency response, Washington, DC
USEPA (2006) USEPA Region III risk-based concentration table: technical background information. Unites States Environmental Protection Agency, Washington
USEPA (2010) Risk-based concentration table. Unites States Environmental Protection Agency. http://www.epa.gov/reg3hwmd/risk/human/index.htmS. Accessed 12 Aug 2011
Uusiku NP, Oelofse A, Duodu KG, Bester MJ, Faber M (2010) Nutritional value of leafy vegetables of sub-Saharan Africa and their potential contribution to human health: a review. J Food Compos Anal 23:499–509
Vanassche F, Clijsters H (1986) Inhibition of photosynthesis in Phaseolus vulgaris by treatment with toxic concentration of zinc: effect on ribulose-1,5-bisphosphate carboxylase/oxygenase. J Plant Physiol 125:355–360
Vanassche F, Clijsters H (1990) Effects of metals on enzyme-activity in plants. Plant Cell Environ 13:195–206
Vasquez MD, Poschenrieder C, Barcelo J (1991) Ultrastructural effects and localization of low cadmium concentrations in bean roots. New Phytol 120:215–226
Velea T, Gherghe L, Predica V, Krebs R (2009) Heavy metal contamination in the vicinity of an industrial area near Bucharest. Environ Sci Pollut Res. doi:10.1007/ s11356-008-0073-5
Velikova V, Tsonev T, Loreto F, Centritto M (2011) Changes in photosynthesis, mesophyll conductance to CO2, and isoprenoid emissions in Populus nigra plants exposed to excess nickel. Environ Pollut 159:1058–1066
Verma S, Dubey R (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Vikas D, Kaushalya G, Sawhney SK (2002) Effect of lead on starch mobilization in germinating chickpea seeds. J Plant Biol 29(1):85
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 51:173–212
Wang QR, Dong Y, Cui Y, Liu X (2001) Instances of soil and crop heavy metal contamination in China. Soil Sediment Contam 10:497–510
Wang X, Sato T, Xing B, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37
Wang AS, Angle JS, Chaney RL, Delorme TA, Reeves RD (2006a) Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 281:325–337
Wang G, Su MY, Chen YH, Lin FF, Luo D, Gao SF (2006b) Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ Pollut 144:127–135
Wang H, Zhao SC, Liu RL, Zhou W, Jin JY (2009) Changes of photosynthetic activities of maize (Zea mays L.) seedlings in response to cadmium stress. Photosynthetica 47(2):277–283
Wang C, Ji J, Yang Z, Chen L, Browne P, Yu R (2012a) Effects of soil properties on the transfer of cadmium from soil to wheat in the Yangtze River Delta Region, China—a typical industry–agriculture transition area. Biol Trace Elem Res. doi:10.1007/s12011-012-9367-z
Wang Y, Qiao M, Liu Y, Zhu YG (2012b) Health risk assessment of heavy metals in soils and vegetables from wastewater irrigated area, Beijing-Tianjin city cluster, China. J Environ Sci 24(4):690–698
Wang C, Yang Z, Yuan X, Browne P, Chen L, Ji J (2013a) The influences of soil properties on Cu and Zn availability in soil and their transfer to wheat (Triticum aestivum L.) in the Yangtze River delta region, China. Geoderma 193–194:131–139
Wang P, Kinraide TB, Smolders E, Zhou D-M, Menzies NW, Thakali S, Xia WW, Hao X-Z, Peijnenburg WJGM, Kopittke PM (2013b) An electrostatic model predicting Cu and Ni toxicity to microbial processes in soils. Soil Biol Biochem 57:720–730
Wani PA, Khan MS, Zaidi A (2006) An evaluation of the effects of heavy metals on the growth, seed yield and grain protein of lentil in pots. Ann Appl Biol (Suppl TAC) 27:23–24
Waqas M, Li G, Khan S, Shamshad I, Reid BJ, Qamar Z, Chao C (2015) Application of sewage sludge and sewage sludge biochar to reduce polycyclic aromatic hydrocarbons (PAH) and potentially toxic elements (PTE) accumulation in Tomato. Environ Sci Pollut Res. doi:10.1007/s11356-015-4432-8
Warren GP, Alloway BJ, Lepp NW, Singh B, Bochereau FJM, Penny C (2003) Field trials to assess the uptake of arsenic by vegetables from contaminated soils and remediation with iron oxides. Sci Total Environ 311:19–33
Watanabe T, Zhang ZW, Qu JB, Gao WP, Jian ZK, Shimbo S, Nakatsuka H, Matsuda-Inoguchi N, Higashikawa K, Ikeda M (2000) Background lead and cadmium exposure of adult women in Xian City and two farming villages in Shaanxi Province, China. Sci Total Environ 247:1–13
Waterlot C, Bidar G, Pelfrene A, Roussel H, Fourrier H, Douay F (2013) Contamination, fractionation and availability of metals in urban soils in the vicinity of former lead and zinc Smelters, France*1. Pedosphere 23(2):43–159
Weast RC (1984) CRC handbook of chemistry and physics, 64th edn. CRC Press, Boca Raton
Wei BG, Yang LS (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Weldegebriel Y, Chandravanshi BS, Wondimu T (2012) Concentration levels of metals in vegetables grown in soils irrigated with river water in Addis Ababa, Ethiopia. Ecotoxicol Environ Saf 77:57–63
Wenzel WW, Sattler H, Jockwer F (1993) Metal hyperaccumulator plants: a survey on species to be potentially used for soil remediation. Agronomy Abstracts, p 52
Widowati H (2012) The influence of cadmium heavy metal on vitamins in aquatic vegetables. Makara J Sci 16(1):33–38
Wildner GF, Henkel J (1979) The effect of divalent metal ion on the activity of Mg2 + -depleted ribulose-1, 5-bisphosphate oxygenase. Planta 146:223–228
Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39:5531–5540
Wilson SC, Tighe M, Paterson E, Ashley PM (2014) Food crop accumulation and bioavailability assessment for antimony (Sb) compared with arsenic (As) in contaminated soils. Environ Sci Pollut Res 21(20):11671–11681
Winter AR, Playle RC, George Dixon D, Borgmann U, Wilkie MP (2012) Interactions of Pb and Cd mixtures in the presence or absence of natural organic matter with the fish gill. Ecotoxicol Environ Saf 83:16–24
Wong SC, Li XD, Zhang G, Qi SH, Min YS (2002) Heavy metals in agricultural soils of the Pearl River Delta, South China. Environ Pollut 119:33–44
World Health Organization International Agency for Research on Cancer (2012) IARC monographs on the evaluation of carcinogenic risks to humans. http://www.iarc.fr/en/publications/list/monographs.index.php
Wu Z, McGrouther K, Huang J, Wu P, Wu W, Wang H (2014) Decomposition and the contribution of glomalin-related soil protein (GRSP) in heavy metal sequestration: field experiment. Soil Biol Biochem 68:283–290
Xu D, Chen Z, Sun K, Yan D, Kang M, Zhao Y (2013a) Effect of cadmium on the physiological parameters and the subcellular cadmium localization in the potato (Solanum tuberosum L.). Ecotoxicol Environ Saf 97:147–153
Xu D, Zhou P, Zhan J, Gao Y, Dou C, Sun Q (2013b) Assessment of trace metal bioavailability in garden soils and health risks via consumption of vegetables in the vicinity of Tongling mining area, China. Ecotoxicol Environ Saf 90:103–111
Yang J, He J (1995) The tolerant mechanism of crops to Cd. Chin J Appl Ecol 6(1):87–91 (In Chinese with English abstract)
Yang Q-W, Li H, Long F-Y (2007) Heavy metals of vegetables and soils of vegetable bases in Chongqing, Southwest China. Environ Monit Assess 130:271–279
Yang P, Mao R, Shao H, Gao Y (2009a) The spatial variability of heavy metal distribution in the suburban farmland of Taihang Piedmont Plain, China. C R Biol 332:558–566
Yang Y, Zhang FS, Li HF, Jiang RF (2009b) Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J Environ Manag 90:1117–1122
Yang QW, Xu Y, Liu SJ, He JF, Long FY (2011) Concentration and potential health risk of heavy metals in market vegetables in Chongqing, China. Ecotoxicol Environ Saf 74:1664–1669
Yargholi B, Azimi AA (2008) Investigation of cadmium absorption and accumulation in different parts of some vegetables. Am Eura J Agric Environ Sci 3(3):357–364
Yu L, Yan-bin W, Xin G, Yi-bing S, Gang G (2006) Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. J Environ Sci 18(6):1124–1134
Zaidi MI, Asrar A, Mansoor A, Farooqui MA (2005) The heavy metal concentrations along roadside trees of Quetta and its effects on public health. J Appl Sci 5(4):708–711
Zechmann B, Müller M (2010) Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma 246:15–24
Zechmeister HG, Grodzinska K, Szarek-Lukaszewska G (2003) Bryophytes. In: Markerts BA, Breure AM, Zechmeister HG (eds) Bioindicators and biomonitors. Elsevier, Amsterdam, pp 329–375
Zhang X, Zhang X, Gao B, Li Z, Xia H, Li H, Li J (2014) Effect of cadmium on growth, photosynthesis, mineral nutrition and metal accumulation of an energy crop, king grass (Pennisetum americanum 3 P. purpureum). Biomass Bioenergy 67:179–187
Zhou J, Ma D, Pan J, Nie W, Wu K (2008) Application of multivariate statistical approach to identify heavy metal sources in sediment and waters: a case study in Yangzhong, China. Environ Geol 54:373–380
Zhuang P, McBride MB, Xia H, Li N, Li Z (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407:1551–1561
Zota AR, Schaider LA, Ettinger AS, Wright RO, Shine JP, Spengler JD (2011) Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. J Expo Sci Environ Epidemiol 21:495–505
Zupan M, Kralj T, Grcman H, Hudnik V, Lobnik F (2003) The accumulation of Cd, Zn, Pb in Taraxacum officinale and Plantago lanceolata from contaminated soils. Proc VII ICOBTE, Uppsala Sv
Acknowledgments
The authors are thankful to the Higher Education Commission, Islamabad, Pakistan, for providing the financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Khan, A., Khan, S., Khan, M.A. et al. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 22, 13772–13799 (2015). https://doi.org/10.1007/s11356-015-4881-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4881-0