Abstract
Purpose
The objective of this paper is to assess the impact of long-term electroplating industrial activities on heavy metal contamination in agricultural soils and potential health risks for local residents.
Methods
Water, soil, and rice samples were collected from sites upstream (control) and downstream of the electroplating wastewater outlet. The concentrations of heavy metals were determined by an atomic absorption spectrophotometer. Fractionation and risk assessment code (RAC) were used to evaluate the environmental risks of heavy metals in soils. The health risk index (HRI) and hazard index (HI) were calculated to assess potential health risks to local populations through rice consumption.
Results
Hazardous levels of Cu, Cr, and Ni were observed in water and paddy soils at sites near the plant. According to the RAC analysis, the soils showed a high risk for Ni and a medium risk for Cu and Cr at certain sites. The rice samples were primarily contaminated with Ni, followed by Cr and Cu. HRI values >1 were not found for any heavy metal. However, HI values for adults and children were 2.075 and 1.808, respectively.
Conclusion
Water, paddy soil, and rice from the studied area have been contaminated by Cu, Cr, and Ni. The contamination of these elements is related to the electroplating wastewater. Although no single metal poses health risks for local residents through rice consumption, the combination of several metals may threaten the health of local residents. Cu and Ni are the key components contributing to the potential health risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
Electroplating industry plays an important role in Chinese economy and will continue to in the future (Dong et al. 2010). It is estimated that there were over 15,000 factories with more than 500,000 employees, 5,000 production lines, and an electroplating production capacity of 300,000,000 m2 in 2005 (Guan 2005). A survey by a China market research center revealed that the total industrial output value was almost 80,000,000,000 RMB in 2008. Electroplating has become an important process in industries such as automotive, manufacturing, domestic appliance, and electronics.
However, the rapid development of electroplating comes with many negative impacts. The most severe one is the production of large quantities of wastewater and sludge containing heavy metals (Fresner et al. 2007). According to one estimate, the Chinese electroplating industry annually generates about 400,000,000 t of wastewater containing heavy metals and 50,000 t of solid waste (Guan 2005). Metals used for plating include nickel, cadmium, copper, zinc, silver, chromium, and lead. It has been noted that, out of the total amount of these metals used in electroplating, 4% goes as waste in sludge and effluent (Tang and Zhang 2004). Many attempts were carried out to remove heavy metals from the electroplating effluent and sludge (Selvakumari et al. 2002; Alvarez-Ayusoa et al. 2003; Silva et al. 2006; Cavaco et al. 2007). However, heavy metals were still released into the environment via wastewater discharge (Hang et al. 2009a). Due to their nonbiodegradable and persistent nature, heavy metals are generally accumulated in the soil or sediment, and thus, pose potential risks to the ecosystem. More importantly, they can enter the food chain and subsequently threaten human health (Muchuweti et al. 2006). However, few studies show the effects of heavy metals on the area around the electroplating plants. A case study is necessary to provide insight into the potential risks of heavy metals to the surrounding environment and inhabitants.
In the present study, we investigated the levels of Cu, Cr, Ni, Pb, and Cd in water, paddy soils, and rice in a region contaminated by electroplating wastewater in Guangxi, China. The risk assessment code (RAC) was determined for the five metals in paddy soils, and the health risk index (HRI) and the hazard index (HI) of consumed rice from the contaminated region were analyzed. Our objectives are to investigate the contamination levels of heavy metals in water, paddy soils, and rice from the electroplating industrial area and to assess the potential health risks to residents through the intake of locally grown rice. The results of this study are useful for pollution control and risk management of heavy metals in similar areas.
2 Materials and methods
2.1 Studied area
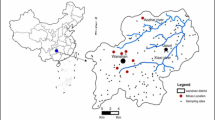
The study area (E110.38°, N24.51°) is located in northern Guangxi, China where the electroplating industrial activities have been initiated since 1996. The electroplating activities produced about 1,000 m3 wastewater daily, which mainly included Cu, Cr(VI), and Ni. After a simple chemical treatment, the effluents were discharged into a nearby river in which the water and sediments have been reported to be contaminated by heavy metals (Zhang et al. 2005). Most of the agriculture lands in this area distribute on both sides of the river and are irrigated with water from the river. Rice is the primary cultivated crop in this area and a staple crop of local residents’ diet.
2.2 Sampling and preparation
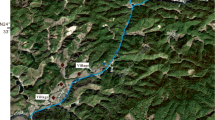
Sampling sites were chosen according to their downstream distances from the factory (Fig. 1). The sampling site upstream of the factory was selected as an uncontaminated control. All samples were collected on October 2009. At each site, one water, three soil, and three rice samples were collected. Each water sample consisted of three subsamples collected from different depths of each site and thoroughly mixed. Soil and rice grains were sampled from at least five distinct subsamples taken in a 5 × 5-m2 block for each paddy field. Each soil sample with about 1 kg was taken from the depths of 0–15 cm, which represented the plough layer. Each rice sample was collected from the corresponding soil sampling site for correlation purposes. All soil and rice grain samples were kept in clean polyethylene bags and brought to the laboratory.
Water samples were kept in clean polyethylene bottles at 4°C before chemical analysis. Soil samples were air-dried, pulverized, and passed through a 2-mm sieve. Rice grain samples were washed with deionized water and hulls were removed from the grain. The rice grain without hull was oven-dried at 70°C for 72 h, then ground with an agate mortar to fine powder.
2.3 Chemical analysis
Five hundred milliliters of water samples with 15 mL of HNO3 was evaporated until 5 mL remained. Remaining contents were digested with 15 mL of HNO3 and 20 mL HClO4 (70%) (Brar et al. 2000). The residues were diluted up to 50 mL with 2% HNO3. The solution was analyzed for Cu, Cr, Ni, Pb, and Cd using an atomic absorption spectrophotometer (AAS; PE-AA700). One gram of soil and 1 g of rice grain samples were digested after adding 15 mL of triacid mixture (HNO3, H2SO4, and HClO4 in a 5:1:1 ratio) at 80°C until a transparent solution was obtained (Allen et al. 1986). After cooling, the digested sample was diluted up to 50 mL with 2% HNO3 and concentrations of Cu, Cr, Ni, Pb, and Cd were determined by AAS.
The sequential extraction procedure described by Sutherland and Tack (2003) was performed to obtain the different fractions of heavy metals in soil. This gave partitioning in four chemical phases analogous to: F1, metals that are exchangeable and associated with carbonates; F2, metals associated with oxides of Fe and Mn; F3, metals associated with organic matter and sulfides; and F4, metals strongly associated with the crystalline structure of minerals. Cu, Cr, Ni, Pb, and Cd concentrations in the fractions were also determined using AAS.
Reagent blanks, a standard reference soil sample (GBW07403), and standard plant samples (GBW10010) were employed in the analysis to ensure accuracy and precision. Results were found within ±2% of the certified value.
2.4 Risk assessment
The RAC, defined as the fraction of metal exchangeable and/or associated with carbonates (F1%), was determined for the five heavy metals, and the values were interpreted in accordance with the RAC classifications (Martley et al. 2004). If RAC is <1%, the soil is of no risk to the environment. Low risk, medium risk, high risk, and very high risk are associated with RAC values of 1–10%, 11–30%, 31–50%, and >75%, respectively.
The HRI was calculated as the ratio of estimated exposure of rice and oral reference dose (ORD) (Hang et al. 2009b). ORDs were 4 × 10−2, 1.5, 4 × 10−3, 2 × 10−2, and 1 × 10−3 mg/kg/day for Cu, Cr, Ni, Pb, and Cd, respectively (US Environmental Protection Agency (USEPA) 1997, 2002). Estimated exposure is obtained by dividing the daily intake (DI) of heavy metals by their safe limits. An index value >1 is considered unsafe for human health (USEPA 2002). DI was calculated by the following equation:
where C (in milligrams per kilogram) is the concentration of heavy metals in the contaminated rice, Con (in grams per person per day) is the daily average consumption of rice in the region, Bw (in kilograms per person) represents body weight, EF is exposure frequency (365 days/year), ED is exposure duration (70 years, equivalent to the average lifespan), and AT is average time (365 days/year number of exposure years, assuming 70 years in this study). The average daily rice intakes of adults and children were considered to be 389.2 and 198.4 g/person/day, respectively (Zheng et al. 2007). Average adult and child body weights were considered to be 55.9 and 32.7 kg, respectively, as used in many previous studies (Wang et al. 2005; Zheng et al. 2007; Khan et al. 2008; Hang et al. 2009b).
Previous studies (Du and Dive 1990; Lim et al. 2008) reported that exposure to two or more pollutants may result in additive and/or interactive effects. HRI can, therefore, be summed across constituents to generate the HI for a specific receptor/pathway (e.g., diet) combination. The HI is a measure of the potential risk of adverse health effects from a mixture of chemical constituents in rice. The HI through daily average consumption of rice for a human being was calculated as follows:
2.5 Data analysis
All data were analyzed using Microsoft Excel and the SPSS 12.0 for Windows statistical package. Arithmetical means ± standard deviation (SD; n = 3) were used to assess the contamination levels of heavy metals in soils and rice. One-way analysis of variance was performed to determine the significance of differences between the pairs of means. When p value was <0.05, multiple range test was done according to Fisher’s least significant difference (LSD) procedure. Correlation analysis was used to determine the relationship between heavy metal concentrations in soils and rice. Independent variables were determined for a linear regression model at 95% (p < 0.05) and 99% (p < 0.01) confidence levels with regression coefficients designated as r.
3 Results and discussion
3.1 Levels of heavy metals in water samples
Six sampling sites (from W1 to W7) downstream of the electroplating wastewater outlet and one sampling site (W0) upstream of the outlet were chosen. The distances from the outlet to W0, W1, W2, W3, W4, W5, W6, and W7 were 200, 0, 50, 100, 200, 400, 800, and 1,200 m, respectively. The heavy metal concentrations are listed for each site in Table 1. Among all sampling sites, concentrations of Pb and Cd did not change evidently from sites W1 to W7. The highest heavy metal concentrations (except Pb and Cd) were in site W1 and the lowest in W0. Although the concentrations of Cu, Cr, and Ni were deceased from sites W1 to W7, concentrations at site W7 were higher than site W0.
The variations of Cu, Cr, and Ni within the water flow were similar, and the order of heavy metal concentrations in the surface water was nearly the same as that of wastewater (Zhang et al. 2005), suggesting that these metals originated from the electroplating plant. The lowest concentrations of metals were found in the water collected upstream from the plant discharge point (W0), indicating that there was no other artificial source near the river and the major source of contamination was plant wastewater. Therefore, we can conclude that the contamination of Cu, Cr, and Ni in the river could be attributed to the wastewater discharge from the plant. This can be supported by the fact that the concentrations of heavy metals were the highest in site W1 that was the closest to the outlet. Due to the dilution of river water and absorption of sediment (Vdović et al. 2006), the levels of Cu, Cr, and Ni decreased dramatically from sites W1 to W7.
According to the Environmental Quality Standards for Surface Water (Ministry of Environmental Protection of the People’s Republic of China (MEP) 2002), Cu concentrations in water exceeded the threshold value from W1 to W4. Cr in surface water was mainly Cr(VI) because wastewater from the plant contained Cr(VI) (Zhang et al. 2005). Therefore, Cr may be above the criterion maximum concentration at all the sampling sites, except for W0 (Table 1). Since the Environmental Quality Standards for Surface Water in China has not set a maximum concentration for Ni, the maximum allowed concentration for Ni was determined from the standard set by the National Recommended Water Quality Criteria in America. This standard (0.47 mg/L) is applied to assess the risk of Ni (USEPA 2006). Based on this value, Ni concentrations in water at all the sampling sites exceeded the permissible limit, except for W0. These results suggested that Cu, Cr, and Ni in the river pose a potential risk. Although the concentrations of Cu, Cr, and Ni decreased with distance, the dangers of Ni still existed far downstream (W7, 1,200 m) from the plant. This result is consistent with the previous observations that the Ni originating from an electroplating plant posed a potential risks from 0 to 1,000 m downstream of the plant (Hang et al. 2009a).
3.2 Levels of heavy metals in the soil
Table 2 lists the total heavy metal concentrations in soils. Soil Cu, Cr, and Ni concentrations in downstream sites (from S2 to S7 for Cu and Ni and S2 and S3 for Cr) were significantly higher (p < 0.05) than those in the upstream site (S1). However, there were no significant differences in Pb and Cd concentrations between the downstream sites (from S2 to S7) and the reference site S1 (p > 0.05). The mean concentrations of Cu (43.33 mg/kg), Cr (45.05 mg/kg), Ni (57.93 mg/kg), and Pb (50.05 mg/kg) in soils from downstream sites were higher than those in wastewater-irrigated soils of India (21.1, 19.1, 23.6, and 21.4 mg/kg for Cu, Cr, Ni, and Pb, respectively) (Singh et al. 2010). This indicates that the electroplating wastewater irrigation may lead to more serious soil contaminations than domestic wastewater.
The distribution of total heavy metals in soils showed that high concentrations of Cu, Cr, and Ni at site S2 gradually decreased towards site S7 where the metal levels were found to be similar to the reference soil (S1), except for Ni. This trend coincided with the distribution of dissolved heavy metals in water, which indicated that heavy metal contamination in soil is related to the electroplating wastewater. This result was supported by high correlations among Cu, Cr, and Ni (Table 3), implying they had the same pollution sources. It was noted that Ni concentration at site S7, which is far from the plant, remained at a relatively high level. This can be ascribed to the high mobility of Ni in the aquatic environment (Weiner 2008). The high mobility of Ni resulted in a long-distance transfer. This conclusion is supported by the high Ni concentration in water at site W7 (Table 2).
The concentrations of Cu in soils from two sampling sites (S2 and S3) and Ni in soils from six sampling sites (S2, S3, S4, S5, S6, and S7) exceeded class II of the national soil reference value in China (MEP 1995). The mean concentration of Ni (57.93 mg/kg) in this area was higher than that in other agricultural soils from Yangzhou (38.5 mg/kg; Huang et al. 2007), Kunsan (31.05 mg/kg; Chen and Pu 2007), and Delhi (49 mg/kg; Rattan et al. 2005). However, Pb and Cd did not exceed the maximum allowable concentrations (MAC). Although the Cr concentrations in all the soils were below the MAC, soil Cr concentration at site S2 was about 1.5-fold and 1.1-fold higher than its concentration in control soil (S1) and background values of China (Wei and Yang 2010), respectively. These results showed that soils in this area have been moderately contaminated by Cu and Cr and severely contaminated by Ni.
3.3 Fractions and RAC of heavy metals in the soil
In order to provide more information about the mobility, bioavailability, and toxicity of metals in soils, the sequential extraction procedure was introduced to determine metal fractions in paddy soils. Figure 2 shows the sequential extraction patterns for soil from the electroplating wastewater-irrigated sites (from S2 to S7) and the control site (S1). For lead and cadmium, all soils show that the main association is residual fractions (39–46% and 44–53%, respectively), indicating that these elements derive mainly from lithogenic origins (Chandra et al. 2004). However, the available Cu, Cr, and Ni (F1 + F2 + F3) were higher in electroplating wastewater-irrigated soils (from S2 to S7) than that in control soil (S1), which indicated there are anthropogenic inputs of these heavy metals (Rattan et al. 2005). Compared to the control soil (S1), the proportions of available Cu, Cr, and Ni (F1% + F2% + F3%) in the soil from site S2 increased by 61.1%, 15.8%, and 43.1%, respectively. The F1 fraction of metals is generally considered the mobile and available form, and therefore, more susceptible to be released. Compared with other heavy metals, Ni was the highest in the F1 fraction, indicating that Ni has a high potential of creating hazardous effects on the environment. The high F1 fraction of Ni was also found in the sediments originated from an electroplating plant (Hang et al. 2009a).
Metals are bound to different soil fractions, with binding strength determining their bioavailability and their risk in environment. The RAC provides an indication of the possible risks by applying a scale to the percentage of metals found in exchangeable and carbonate-bound (i.e., labile) phases (F1). According to the RAC analysis, soils showed a low risk for Pb and Cd, with RAC values <10% (Fig. 3). However, Cu showed medium risk in soils from sites S2 to S6, indicating a substantial risk of Cu mobilization from soils across almost the entire study region. Ni posed a high risk (RAC > 30) at sites S2 to S5 and is considered to be dangerous and easily able to enter the food chain (Martley et al. 2004). The RAC values of Ni in the present study were higher than those in an earlier study in the same region, which showed that 19% to 27% of Ni was present in the F1 fraction at sites S2 and S4 (Liu et al. 2006). This suggested that the risk of Ni increased with the time course. The risk of Cr was medium at site S2 and low at sites S3 to S7, which indicated that there was a relatively less transfer distance for Cr compared to Ni and Cu.
3.4 Levels of heavy metals in the rice grain
Heavy metal concentrations showed variations in rice grains from downstream and upstream sites (Table 4). The concentrations of Pb and Cd in rice grains from all sampling sites were not significantly different (p < 0.05). However, 1.9-fold, 2.1-fold, and 4.2-fold higher concentrations of Cu, Cr, and Ni, respectively, were observed in rice grains at site S2 compared to site S1. The correlations among these three metals are highly significant (Table 5), suggesting that they may be derived from the same pollution sources. Moreover, Cu, Cr, and Ni concentrations in rice grains were decreased with the increase of distance from the plant, indicating that spatial patterns of these three metals in rice grain were similar to those in soil and water (Tables 1 and 2). These results showed that the elevated levels of Cu, Cr, and Ni in rice grains from downstream sites were mainly due to the irrigation with water contaminated by the electroplating plant. Similar results were found by Kawatra and Bakhetia (2008) who reported higher concentrations of heavy metals in vegetables from sewage water-irrigated areas compared to tubewell water-irrigated areas of Ludhiana city of Punjab.
Table 4 also lists the maximum allowable levels (MAL) of Cu, Cr, Ni, Pb, and Cd in foods recommended by the Ministry of Health of China (MHC) (2005). There were no rice samples containing Cu, Pb, and Cd above their maximum level in foods. However, Cr concentrations in rice grains from three sites (S2, S3, and S4) were above its MAL. For Ni, all rice samples from downstream sites exceeded its MAL. Compared with the rice from an electronic and electric waste recycling area, mean concentrations of Ni, Cr, and Cu in rice grains from downstream sites were higher, although Pb and Cd showed lower concentrations (Fu et al. 2008). The mean concentrations of Ni, Cr, and Cu in our study were also higher than those from the lead/zinc mining area of Chenzhou (Liu et al. 2005) and the industrial area of Changshu (Hang et al. 2009b). These results indicated that rice samples from downstream sites were most significantly polluted by Ni, followed by Cr and Cu. The relatively high RAC of Ni, Cr, and Cu in soils from downstream sites (Fig. 3) can partly explain the contamination of these elements in rice.
3.5 Potential health risk of heavy metals via rice consumption
Although there are many pathways of human exposure to heavy metals, rice consumption has been identified as one of the major pathways. Table 6 shows the dietary intake of heavy metals through rice for adults and children in the study region on the assumption that the local population consumes mostly regional rice. To calculate the DI of heavy metals via rice consumption, the weighted means of heavy metal concentrations in rice grains were employed to represent the average exposure levels. The DIs of Cu, Cr, Ni, Pb, and Cd through rice are estimated to be 33.99, 6.74, 13.33, 1.50, and 0.18 mg/kg/day for adults and 29.62, 5.88, 11.61, 1.31, and 0.16 mg/kg/day for children. The DIs of heavy metals for adults are higher than those for children. This may be due to higher rice consumption of adults, which consequently increased the DIs of heavy metals. The result is in agreement with a previous study (Zheng et al. 2007). Although the DIs of heavy metals are all below the ORD for adults and children, some individuals who can consume more than twice the average amount of rice (Fu et al. 2008) may intake more heavy metals than the ORD.
The HRI is an important measure of the potential risks and adverse health effects from a single chemical compound. The HRIs of heavy metals through rice consumption for the local residents are listed in Table 6. For adults, the HRI of heavy metals from rice consumption is in decreasing order: Cu > Ni > Pb > Cd > Cr. The HRI of heavy metals for children is similar to that for adults. Our results suggest that Cu ingestion has the highest potential health risk of adverse effects for adults and children and Cr ingestion has minimum risk. There are no HRI of heavy metals beyond the value of 1; therefore, the health risk of single metal exposure through rice consumption was generally assumed to be safe. However, local residents may be at risk due to the combination of several heavy metals. The HI values for rice consumption of adults and children are 2.075 and 1.808, respectively. This indicates that adults and children may experience adverse health effects. The relative contributions of Cu, Cr, Ni, Pb, and Cd to the HI are 41.0%, 0.2%, 32.1%, 18.1%, and 8.6%, respectively. Therefore, Cu and Ni are key components contributing to the potential health risk of noncarcinogenic effects for adults and children, with Pb and Cd being second and Cr being the least contributing. It must be noted that our assessment only considered the intake of toxic elements via rice consumption. In fact, humans are also exposed to heavy metals through other foods such as wheat, vegetables, fruit, fish, meat, eggs, water, and milk (Wang et al. 2005; Zheng et al. 2007; Chary et al. 2008; Sipter et al. 2008). Furthermore, there are also other sources such as dust inhalation, dermal contact, and ingestion (for children) of metal-contaminated soils (Grasmück and Sholz 2005; Hellström et al. 2007). Therefore, potential health risks of heavy metals to local residents are far beyond our assessment.
4 Conclusion
Electroplating wastewater led to the contamination of heavy metals in water, paddy soil, and rice samples. Levels of Cu, Cr, and Ni in the surface water of the river directly correlated with the distance of the sampling sites from the electroplating plant. Ni levels in water from seven sampling sites, Cu in water from four sampling sites, and Cr in water from four sampling sites were above the permissible limits and posed an environmental risk. Significant heavy metal accumulation was found for Cu, Cr, and Ni in soils from downstream sites. The concentrations of Cu in soils from two sites and Ni from six sites exceeded the MAC of the national quality standards for soil. The RAC analysis showed that the risk of Ni in soils from downstream sites was from medium to high and Cu and Cr posed a low to medium risk. The concentrations of Cu, Cr, and Ni in rice grains from downstream sites were higher than those from upstream sites, indicating that contamination of the rice samples were related to the electroplating wastewater. Although the concentrations of Cu, Pb, and Cd in the rice grains were all below the national MAL, Cr and Ni concentrations in rice grains exceeded their MAL in three and six sites, respectively. Estimations showed that DIs of heavy metals for local adults were higher than those for local children. In general, no HRI values were >1 through rice consumption. However, the HI values through rice consumption for adults and children were 2.075 and 1.808, respectively, indicating that both adults and children may experience some adverse health effects. Cu and Ni are the key components contributing to potential health risks of noncarcinogenic effects for local residents. Due to the intake of heavy metals through other foods and other sources, potential health risks of heavy metals to the local residents may be higher than our calculations.
References
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology. Blackwell, London, pp 285–344
Alvarez-Ayusoa E, García-Sáncheza A, Querol X (2003) Purification of metal electroplating waste waters using zeolites. Water Res 37:4855–4862
Brar MS, Mahli SS, Singh AP, Arora CL, Gill KS (2000) Sewer water irrigation effects on some potentially toxic trace elements in soil and potato plants in northwestern India. Can J Soil Sci 80:465–471
Cavaco SA, Fernandes S, Quina MM, Ferreira LM (2007) Removal of chromium from electroplating industry effluents by ion exchange resins. J Hazard Mat 144(3):634–638
Chandra SK, Chary NS, Kamala CT, Suman RDS, Sreenivasa RA (2004) Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environ Int 29:1001–1008
Chary NS, Kamala CT, Raj DSS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotox Environ Safe 69:513–524
Chen F, Pu L (2007) Relationship between heavy metals and basic properties of agricultural soils in Kunshan County. Soils 39:291–296
Dong X, Li C, Li J, Wang J, Huang W (2010) A game-theoretic analysis of implementation of cleaner production policies in the Chinese electroplating industry. Res Cons Recycl 54:1442–1448
Du AL, Dive D (1990) Interaction between components of electroplating industry wastes: influence of the receiving water on the toxicity of the effluent. Environ Pollut 65:251–267
Fresner J, Schnitzer H, Gwehenberger G, Planasch M, Brunner C, Taferner K (2007) Practical experiences with the implementation of the concept of zero emissions in the surface treatment industry in Austria. J Cleaner Prod 15:1228–1239
Fu J, Zhou Q, Liu J, Liu W, Wang T, Zhang Q, Jiang G (2008) High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere 71:1269–1275
Grasmück D, Sholz RW (2005) Risk perception of heavy metal soil contamination by high-exposed and low-exposed inhabitants: the role of knowledge and emotional concerns. Risk Anal 25:611–622
Guan T (2005) The characteristics of electroplating industry in China. Met World 1:8–9 [in Chinese]
Hang X, Wang H, Zhou J, Du C, Chen X (2009a) Characteristics and accumulation of heavy metals in sediments originated from an electroplating plant. J Hazard Mater 163:922–930
Hang X, Wang H, Zhou J, Ma C, Du C, Chen X (2009b) Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River. Environ Pollut 157:2542–2549
Hellström L, Persson B, Brudin L, Petersson Grawé K, Öborn I, Järup L (2007) Cadmium exposure pathways in a population living near a battery plant. Sci Total Environ 373:447–455
Huang S, Liao Q, Hua M, Wu X, Bi K, Yan C, Chen B, Zhang X (2007) Survey of heavy metal pollution and assessment of agricultural soils in Yangzhong district, Jiangsu Province, China. Chemosphere 67:2148–2155
Kawatra BL, Bakhetia P (2008) Consumption of heavy metal and minerals by adult women through food in sewage and tube well irrigated area around Ludhiana city (Punjab, India). J Hum Ecol 23:351–354
Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soil and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152:686–692
Lim HS, Lee JS, Chon HT, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J Geochem Explor 96:223–230
Liu H, Probst A, Liao B (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166
Liu J, Zhong X, Liang Y, Luo Y, Zhu Y, Zhang X (2006) Fractionations of heavy metals in paddy soils contaminated by electroplating wastewater. J Agro-Environ Sci 25:398–401 [in Chinese]
Martley E, Gulson B, Louie H, Wu M, Di P (2004) Metal partitioning in soil profiles in the vicinity of an industrial complex, New South Wales, Australia. Geochem Explor Environ Anal 4:171–179
Ministry of Environmental Protection of the People’s Republic of China (MEP) (1995) Environmental quality standards for soil, GB 15618-1995. MEP, Beijing
Ministry of Environmental Protection of the People’s Republic of China (MEP) (2002) Environmental quality standards for surface water, GB 3838-2002. MEP, Beijing
Ministry of Health of the People’s Republic of China (MHC) (2005) Maximum levels of contaminants in foods, GB 2762-2005. MCH, Beijing
Muchuweti M, Birkett JW, Chinyanga E, Zvauya R, Scrimshaw MD, Lister JN (2006) Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: implication for human health. Agri Ecosys Environ 112:41–48
Rattan RK, Datta SP, Chhonkar PK, Suribabu K, Singh AK (2005) Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater—a case study. Agr Ecosyst Environ 109:310–322
Selvakumari G, Murugesan M, Pattabi S, Sathishkumar M (2002) Treatment of electroplating industry effluent using maize cob carbon. Bull Environ Contam Toxicol 69(2):195–202
Singh A, Sharma RK, Agrawal M, Marshal FM (2010) Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem Toxicol 48:611–619
Sipter E, Rózsa E, Gruiz K, Tátrai E, Morvai V (2008) Site-specific risk assessment in contaminated vegetable gardens. Chemosphere 71:1301–1307
Silva PTS, Mello NT, Duarte MMM, Montenegro CBSM, Araújo AN, Neto BB, Silva VL (2006) Extraction and recovery of chromium from electroplating sludge. J Hazard Mater 128(1):39–43
Tang Z, Zhang J (2004) Treatment actuality and development trend of waste water in electroplating industry. Terr Nat Resour Res 2:69–71 [in Chinese]
Sutherland R, Tack FMG (2003) Fractionation of Cu, Pb and Zn in certified reference soils SEM 2710 and SEM 2711 using the optimized BCR sequential extraction procedure. Adv Environ Res 8:37–50
US Environmental Protection Agency (USEPA) (1997) Exposure factors handbook—general factors. EPA/600/P-95/002Fa, vol. I. Office of Research and Development, National Center for Environmental Assessment, Washington
US Environmental Protection Agency (USEPA) (2002) Region 9, preliminary remediation goals. USEPA, Washington
US Environmental Protection Agency (USEPA) (2006) National recommended water quality criteria. Office of Water EPA-822-R-02-047. USEPA, Washington
Vdović N, Billon G, Gabelle C, Potdevin J (2006) Remobilization of metals from slag and polluted sediments (case study: the canal of the Deûle River, northern France). Environ Pollut 141:359–369
Wang XL, Sato T, Xing BS, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Weiner ER (2008) Applications of environmental aquatic chemistry: a practical guide. CRC Press, Taylor & Francis Group, Boca Raton
Zhang X, Luo Y, Huang H, Liu J, Chen J (2005) Heavy metal contamination in water, soil and plant near an electroplating factory. J Guilin Univ Technol 25:289–292 [in Chinese]
Zheng N, Wang QC, Zhang XW, Zheng DM, Zhang ZS, Zhang SQ (2007) Population health risk due to dietary intake of heavy metals in the industrial area of Huludao City, China. Sci Total Environ 387:96–104
Acknowledgements
This research was sponsored by the Natural Science Foundation of China (41001186 and 41063004), the Scientific Research and Technological Development Project of Guangxi (GuiKeGong 10124003-3), and the Natural Science Foundation of Guangxi (2010GXNSFA013018 and 2011GXNSFF018003). Financial assistance for this research was also provided through the Program to Sponsor Teams for Innovation in the Construction of Talent Highlands in Guangxi (GuiKeRen 2007-71). Two anonymous reviewers are thanked for their cogent reviews of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Liu, J., Zhang, XH., Tran, H. et al. Heavy metal contamination and risk assessment in water, paddy soil, and rice around an electroplating plant. Environ Sci Pollut Res 18, 1623–1632 (2011). https://doi.org/10.1007/s11356-011-0523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0523-3