Abstract
This work investigated the accumulation, allocation, and impact of zinc (Zn; 1.0 μM–10 mM) in maize (Zea mays L.) seedlings under simulated laboratory conditions. Z. mays exhibited no significant change in its habitus (the physical characteristics of plants) up to 10–1000 μM of Zn (vs 5–10 mM Zn). Zn tolerance evaluation, based on the root test, indicated a high tolerance of Z. mays to both low and intermediate (or relatively high) concentrations of Zn, whereas this plant failed to tolerate 10 mM Zn and exhibited a 5-fold decrease in its Zn tolerance. Contingent to Zn treatment levels, Zn hampered the growth of axial organs and brought decreases in the leaf area, water regime, and biomass accumulation. Nevertheless, at elevated levels of Zn (10 mM), Zn2+ was stored in the root cytoplasm and inhibited both axial organ growth and water regime. However, accumulation and allocation of Zn in Z. mays roots, studied herein employing X-ray fluorimeter and histochemical methods, were close to Zn accumulator plants. Overall, the study outcomes revealed Zn tolerance of Z. mays, and also implicate its potential role in Zn phytoextraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals/metalloids have made their entry into agricultural soils worldwide mainly as a result of rapid installation of industries and/or indiscriminate use and disposal of metal/metalloid-containing products. Zinc (Zn) belongs to the list of transition metals and stands 24th among the most abundant elements on the Earth. Low levels of Zn are significant in plants, animals/humans, and microorganisms (Alloway 2013). However, commercial fertilizers, liming materials, or manures, being added to Zn-deficient agricultural soils to achieve enhanced plant growth and productivity, have become major factors contributing elevated levels of Zn in world agricultural soils (Alloway 2013; Liu et al. 2008). Considering known toxic consequences of Zn in plants (such as inhibited plant growth and development, elevated oxidative stress, and impaired cellular metabolism) and their eventual impact on plant productivity (reviewed by Anjum et al. 2015a), sustainable minimization of Zn in agricultural soils is imperative.
Plant-based technology has emerged as a panacea for the sustainable control of elevated levels of various metals/metalloids in soils. Though the use of field crop plants for the management of the risk of a long-term pollutant dispersion (Vamerali et al. 2010), much emphasis has been given in this context to crop plants from Brassicaceae followed by Fabaceae (Leguminosae) and Poaceae (Vamerali et al. 2010; Anjum et al. 2012, 2014a; Zaidi et al. 2012). Maize (Zea mays L.) is one of important cereal crops and belongs to Poaceae. Notably, while exposed to metals/metalloids, plants have been reported to change their phenotype, and also their major growth traits. Since changes in plant growth traits can be easily visible and analyzed in metal/metalloid-exposed plants, the evaluation of plant growth traits has been used as an easily measurable parameter for monitoring the metal/metalloid impacts. Nevertheless, low and high levels of toxic metals/metalloids and high levels of plant-beneficial metals/metalloids (including Zn) can impact plant-water relation, and also bring anatomical and ultrastructural changes in roots and leaves (Poschenrieder and Barceló 2004; Mahajan and Tuteja 2005; Gajewska et al. 2006; Todeschini et al. 2011; Anjum et al. 2015b). Despite the previous facts and though Z. mays has promising attributes of a heavy metal accumulator, and its use in the phytoextraction technology has been advocated (Wuana and Okieimen 2010), literature is scarce on the research reports aimed at gaining a clear understanding of Zn accumulation, allocation, and tolerance in Z. mays employing appropriate and important traits/markers.
Given above, taking into account Z. mays (cultivar Tzaritza), exposed to varying Zn treatment levels (1.0 μM–10 mM), this study aimed to unveil Zn accumulation and allocation employing X-ray fluorimeter and histochemical methods. Additionally, efforts were also made to assess Z. mays seedling habitus (the physical characteristics of plants), tolerance (based on the root test), and impact on growth traits (such as growth of axial organs, and leaf area, water regime, and biomass accumulation).
Materials and methods
Plant material, growth conditions, and treatments
Healthy and uniform-sized seeds of Z. mays were surface-sterilized by treating them with 0.5 % KMnO4 for 5 min. Subsequently, the surface-sterilized seeds were sown in Petri dishes each having 50 Z. mays seeds and distilled water-soaked paper towels. On day 7, uniformly germinated seeds were transplanted in small glass jars with water supplemented with different Zn concentrations (1.0 μM to 10 mM), supplied as ZnSO4·7H2O, and were maintained at temperature 22–24 °С, photoperiod 16/8 h (day/night), photon flux density 80 μmol m−2 s−1 for 21 days. Z. mays seedlings were harvested on the 21st day of start of Zn exposure and used for several estimations as detailed below.
Zinc tolerance, growth traits, and water content
Wilkinson tolerance index (It) was applied to assess the tolerance of Z. mays seedlings to Zn employing the formula—It: It = (lme/lc) × 100 %, where lme indicates the increase in root growth in a metal ion solution and lc is the increase in root growth in the control (Koornneeff et al. 1997). Leaf area was measured by analyzing the scanned leaves using Scion Image for Windows v. 4.0.2. For the seedling biomass, the harvested Z. mays plants were separated into roots and shoots, and subsequently, their fresh and dry biomass were determined using an electronic balance. Roots, shoots, or whole plants were kept hot-air oven at 95 °C to till a constant weight. The water content in Z. mays plants was calculated using the formula: W = (Mf. − Md.) / Mf. × 100 %, where W is the percent water content in the plant; Mf is the fresh weight, and Md is dry weight of roots or shoots.
Zinc accumulation and its allocation
Zinc accumulation in plant was determined spectrophotometrically employing X-ray fluorescent spectrometer (Spectroscan MAX-GV, St. Petersburg, Russia) as per the methods detailed in the manual of Scientific and Production Association “Spectron” (1993). In brief, dried and milled plant material (1.0 g) was treated with 3.0 ml HNO3 and mineralized by heating on a hotplate until no smoke. Combustion was carried out in an electric furnace while gradually raising the sample temperature from 250 to 450 °C to obtain white- or light-colored powder. The obtained ash was dissolved in 10 ml of HNO3, filtered into a volumetric flask, the solution was neutralized with 1.0 N NaOH to pH 7.0, and finally the volume was made up to 100 ml with double distilled water. For the precipitation of metal ions, 0.05 ml Zr(OH)4 was added, and the solution was heated to boiling. The resultant solution (50 ml) was filtered using a vacuum pump through the filter “Vladipor” (pore size, 0.45 μm). The obtained filtrate/residue was analyzed on an X-ray fluorescent spectrometer. The Zn content in plant samples was calculated in mg kg−1 dry mass employing the mentioned below formula, where c is the concentration of the metal in the plant sample (mg); c1 is the metal concentration in blank sample (mg); m is the plant sample dry weight (g).
Histochemical studies were performed to assess Zn allocation in Z. mays plants. To prepare the diphenyltiocarbazone (ditizone) solution, just prior to slicing the Z. mays roots, 3.0 mg of a diphenyltiocarbazone was dissolved in 6.0 ml of acetone to which 2.0 ml of the distilled water and 0.2 ml of ice-cold acetic acid were added (Seryogin and Ivanov 1997). Cross-slices of Z. mays roots in the differentiation zone were prepared manually with a razor blade, maintained in ditizone solution, then rinsed well with distilled water, and were observed under a a LUMAM R8 (LOMO, Russia) microscope at ×300 magnification. However, some slices were photographed with a digital camera connecting to microscope. Localization of Zn2+ ions could be identified by red-colored tissues. The stronger the color intensity confirms the more intense Zn accumulation. Sensitivity of the method is 10 μM of Zn2+.
Statistical analysis
All experiments were conducted in triplicate, and each experiment consisted of 150 seeds or seedlings. For all measurements, averages and standard errors were calculated in Microsoft Excel 2007. Differences between means were assessed by the Tukey’s test at P = 0.05.
Results and discussion
Zinc accumulation and its allocation
Accumulation and distribution of Zn in Z. mays organs were studied in model experiments with the germination of plants in solutions of different Zn concentrations (Fig. 1a). As expected, significantly increased Zn concentrations were identified in shoots and roots of Z. mays exposed to low (10–100 μM). However, the accumulation of Zn was decreased slightly when Zn concentration in the growth medium exceeded 0.1 mM. Accumulation of Zn was comparatively higher in roots than shoots. In fact, varied metals/metalloids available in plant’s immediate vicinity can first interact with roots, where metal/metalloid sorption by plant roots helps to stabilize/immobilize noxious metals/metalloids and protects above-ground plant parts (Ali et al. 2013; Anjum et al. 2015c). Though the curve of Zn accumulation in the shoots and roots of Z. mays did not reach a plateau with increasing of Zn concentration in the growth medium up to 10 mM, the uptake curve for roots was closer to that of Zn accumulator plants (White 2012). Notably, despite the tight control of plant roots on metals/metalloids, their considerable amount can reach to above-ground plant parts (reviewed by Ali et al. 2013; Anjum et al. 2014b).
Histochemical studies performed herein confirmed the assumption that the absence or presence of inherent plant-based barriers for Zn uptake in roots and its subsequent translocation to above-ground organs (such as leaves). At 10 μM, weak coloring of cell membranes in exoderm and mesoderm cells was visible (Fig. 1b). However, in the stele cells, Zn content was almost indistinguishable from the control. Coloring of the mesoderm and endoderm cell walls and cytoplasm increased when plants exposed to 0.1 mM of Zn2+. In this case, the intensified coloration of stele cells (especially phloem and stele parenchyma). Notably, with 1.0 mM of Zn, cytoplasmic coloring enhanced in all root cells of all root tissues. Moreover, at 1.0 mM, visible coloring of conducting bundles and neighboring cells was evidenced. These indicated both a significantly increased accumulation of Zn in roots, and restricted Zn translocation to shoots, one of the major characteristics of metal/metalloid (Zn)-tolerant plants (Ali et al. 2013). Though a significant saturation of all cells of root was evidenced at 10 mM, clearly visible areas of tissue maceration in mesoderm testified the significant intoxication potential of Z. mays plants for elevated Zn levels. It is known that cell wall appeared to assume important roles in Zn fixation, which could therefore limit Zn influx into the cell. In an earlier study on tomato, exposed to 0.5–5 mM Zn, Zn fixation by the cell wall was not only due to an increase in cell wall biomass but also to an improvement of its binding capacity (Muschitz et al. 2009). At Zn level not more than 500 μM, elemental mapping using an energy-dispersive X-ray microanalysis system showed that Zn was preferentially accumulated in the idioblasts. The localization site of Zn was cell walls of a dome-shaped protrusion (cap) of idioblasts (Katayama et al. 2013). At elevated Zn, tolerance to Zn involves an expanded copy number of an ancestral MTP1 gene, encoding functional proteins that mediate the detoxification of Zn available in the cell vacuole (Drager et al. 2004). In the present research, Zn-binding capacity of cell walls appeared to have exhausted at a concentration of 0.1 mM, where further increase in Zn concentration in the growth medium led to the accumulation of Zn2+ in protoplast (cytoplasm or vacuoles).
Zinc tolerance, growth traits and water content, and Z. mays habitus
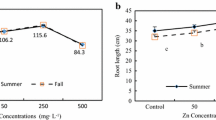
Zn tolerance assessment, based on root tests, revealed the insignificant decrease in Z. mays tolerance to Zn concentration up to 1.0 mM (Fig. 2a). However, a 5-fold decrease in Z. mays tolerance to Zn concentrations was evident and significant with 10 mM of Zn. Root-based tests were reported earlier as a major of marker of plant tolerance to different metals/metalloids (Galardi et al. 2007). Further, at the end of the first day of exposure, root and shoot length did not differ from the water control; rather, root-shoot length tended to slightly increase with 1.0 μM of Zn (Fig. 2a). Notably, the exhibition of Z. mays tolerance to Zn concentrations up to 1.0 mM, evidenced herein as slight increase in root shoot length at 1.0 μM of Zn, seem obvious because Zn is a plan beneficial trace metal required for proper functioning of many enzymes involved in numerous physiological/metabolic processes in plants (reviewed by Anjum et al. 2015a). Apart from the Zn treatment level-dependent variation in the lengths of Z. mays axial organs (Fig. 2a), roots and shoots exhibited their differential responses to Zn treatment levels, where compared to shoots, sensitivity of roots to the increasing Zn concentrations in the growth medium was displayed. In particular, increasing of Zn in the medium (from 10 μM to 0.1 mM) caused a decrease in the shoot growth of 35 % in relation to the water control. But the strongest shoot growth inhibition we observed at 10 mM of Zn. Even minor concentrations of Zn (1.0–10 μM) caused a significant inhibition of growth of the main root. However, a further increase in the concentration of Zn up to 1.0 mM had no effect on the elongation of roots. The highest concentration almost completely stops the root growth. Root sensitivity to elevated Zn level can be apparent because of the discussed above higher fraction of cytosolic Zn in root cells, where Zn load might have caused root-cellular metabolic activities and ceased root growth (reviewed by Ali et al. 2013; Anjum et al. 2015a). Regarding the growth trait modulation under Zn treatment level, the fresh and dry weight of plants is an important indicator of the growth of the plant. Zn is considered one of the essential trace elements, where its deficiency and excess can negatively impact plant growth (reviewed by Anjum et al. 2015a). This is true also in our present study, where the lowest and the highest Zn concentrations significantly inhibited the biomass accumulation (Fig. 2b). Notably, 0.1 mM (for shoots) and 1.0 mM (for roots) emerged as optimal concentrations of Zn inducing the accumulation of biomass of shoots and roots, respectively. However, the highest concentration of Zn (10 mM) inhibited the accumulation of both root and shoot biomass (Fig. 2a).
In context with leaf area, and the plant water balance, at low concentrations (10–100 μM) of Zn, Z. mays leaves exhibited increased (though insignificant) in their lamina area, whereas 1.0 mM of Zn was a threshold concentration above which leaf area significantly decreased (Fig. 2c). Leaves have been regarded as a major component of plant growth, and decrease in leaf area was evidenced as one of the strategies for conserving the internal water/moisture through the reduced rate of transpiration under “plant-water relation” impacting stresses including metal/metalloids (Mahajan and Tuteja 2005; Gajewska et al. 2006; Anjum et al. 2015b). Notably, herein, severe and increasing disorders in the water regime in seedlings were observed in Z. mays exposed up to 1.0 mM of Zn (Fig. 2c). However, the highest concentrations of Zn (10 mM) caused a significant decrease in plant-water content that was more expressed in roots than in shoots (4.3 and 2.7 %, respectively). Elevated metal/metalloid concentrations accrued alterations in water balance (in particular, changes in the water content in tissues and transpiration level) can be due to the decreases in the number and diameter of xylem vessels and phloem sieve tubes that in turn might have significantly decreased the plant-water balance with increasing Zn treatment levels (Gabbrielli et al. 1999; Pandey and Sharma 2002; Poschenrieder and Barceló 2004; Gajewska et al. 2006). Regarding plant habitus, herein, after 21 days of exposition, habitus of Z. mays plants vary greatly depending on the Zn concentration. Habitus of plants exposed to 10–1000 μM of Zn practically hardly changed, where most plants developed 3–4 leaves (Fig. 3). However, plants developed only 1–2 leaves when exposed to 5.0–10 mM Zn, whereas, at high concentrations of Zn, in spite of strong inhibition of growth, plants continued to maintain turgor and remained vital capacity. Zn treatment levels accrued changes in the growth of axial organs, and leaf area, and biomass accumulation emerged as a major factor controlling Z. mays habitus. Together, our observations on plant habitus confirmed the significant tolerance of Z. mays plants to elevated levels of Zn in the growth medium.
Conclusions
Taking together, the obtained results and their recent literature-based interpretation, main outcomes of this study can be summarized into following two main points: (a) Zn treatment levels (10–1000 μM of Zn) can improve Z. mays health by improving growth of axial organs, and leaf area, water regime, and biomass accumulation, whereas Z. mays can tolerate Zn treatment levels below 10 mM; and (b) exhibition of no significant change in the habitus (the physical characteristics) of Z. mays, Zn accumulation close to Zn accumulator plants, and the storage of maximum accumulated Zn in the root cytoplasm when exposed to 10 mM Zn can implicate its potential role in Zn phytoextraction.
References
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals - concepts and applications. Chemosphere 91:869–881
Alloway BJ (2013) Heavy metals and metalloids as micronutrients for plants and animals. In: Alloway BJ (ed) Heavy metals in soils. Springer, The Netherlands, pp 195–209
Anjum NA, Ahmad I, Pereira ME, Duarte AC, Umar S, Khan NA (2012) The plant family brassicaceae: contribution towards phytoremediation. Springer, Dordrecht
Anjum NA, Ahmad I, Valega M, Mohmood I, Gill SS, Tuteja N, Duarte AC, Pereira E (2014a) Salt marsh halophyte services to metal-metalloid remediation: assessment of the processes and underlying mechanisms. Crit Rev Environ Sci Technol 44:2038–2106
Anjum NA, Umar S, Iqbal M (2014b) Assessment of cadmium accumulation, toxicity, and tolerance in Brassicaceae and Fabaceae plants - implications for phytoremediation. Environ Sci Pollut Res 21:10286–10293
Anjum NA, Duarte AC, Pereira E, Ahmad I (2015a) Juncus maritimus root-biochemical assessment for its mercury-stabilization potential in Ria de Aveiro coastal lagoon (Portugal). Environ Sci Pollut Res 22:2231–2238
Anjum NA, Umar S, Aref IM, Iqbal M (2015b) Managing the pools of cellular redox buffers and the control of oxidative stress during the ontogeny of drought-exposed mungbean (Vigna radiata L.) - role of sulfur nutrition. Front Environ Sci 2:66. doi:10.3389/fenvs.2014.00066
Anjum NA, Singh HP, Khan MI, Masood A, Per T, Negi A, Batish D, Khan NA, Duarte AC, Pereira E, Ahmad I (2015c) Too much is bad - an appraisal of phytotoxicity of elevated plant-beneficial heavy metal ions. Environ Sci Pollut Res 22:3361–3382
Drager DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Kramer U (2004) Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high mtp1 transcript levels. Plant J 39:425–439
Gabbrielli R, Pandolfini T, Espen L, Palandri MR (1999) Growth, peroxidase activity and cytological modifications in Pisum sativum seedlings exposed to Ni2+ toxicity. J Plant Physiol 155:639–645
Gajewska E, Sklodowska M, Slaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol Plant 50:653–659
Galardi F, Corrales I, Mengoni A, Pucci S, Barletti L, Barzanti R et al (2007) Intra-specific differences in nickel tolerance and accumulation in the Ni-hyperaccumulator Alyssum bertolonii. Environ Exp Bot 60:377–384
Katayama H, Banba N, Sugimura Y, Tatsumi M, Kusakari SI, Oyama H, Nakahira A (2013) Subcellular compartmentation of strontium and zinc in mulberry idioblasts in relation to phytoremediation potential. Environ Exp Bot 85:30–35
Koornneeff M, Alonso-Blanco C, Peeters AJM (1997) Genetic approaches in plant physiology. New Phytol 137:1–8
Liu D, Li TQ, Yang XE, Islam E, Jin XF, Mahmood Q (2008) Effect of Pb on leaf antioxidant enzyme activities and ultrastructure of the two ecotypes of Sedum alfredii Hance. Russ J Plant Physiol 55:68–76
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Muschitz A, Morvan H, Faugeron C (2009) Response of cultured tomato cells subjected to excess zinc: role of cell wall in zinc compartmentation. Acta Physiol Plant 31:1197–1204
Pandey N, Sharma CP (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci 163:753–758
Poschenrieder C, Barceló J (2004) Water relations in heavy metal stressed plants. In: Prasad MNV (ed) Heavy Metal Stress in Plants, 3rd edn. Springer, Berlin, pp 249–270
Scientific and Production Association “Spectron” (1993) Method of heavy metals determination in the plant material. Methods of measurement. Environmental standard №883-93, Scientific and Production Association “Spectron”, Saint Petersburg, p.25 (in Russian)
Seryogin IV, Ivanov VB (1997) Gystochemical methods of studying of cadmium and lead allocation in plants. Plant Physiol 44:915–921 (in Russian)
Todeschini V, Linggua G, D’Agostino G, Carniato F et al (2011) Effects of high zinc concentration on poplar leaves: a morphological and biochemical study. Environ Exp Bot 71:50–56
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land - a review. Environ Chem Lett 8:1–17
White PJ (2012) Heavy metal toxicity in plants. In: Shabala S (ed) Plant Stress Physiology. CAB International, Wallingford, pp 210–237
Wuana RA, Okieimen FE (2010) Phytoremediation potential of maize (Zea mays L.) - a review. Afr J Gen Agric 6:275–287
Zaidi A, Wani PA, Khan MS (2012) Toxicity of heavy metals to legumes and bioremediation. Springer, Dordrecht
Acknowledgments
DIB and ASL thank to the Ministry of Education and Science of Russia for financing their present research through project number 6.783.2014K. NAA, IA, and EP gratefully acknowledge the financial support of both the Portuguese Foundation for Science and Technology (SFRH/BPD/64690/2009; SFRH/BPD/84671/2012), the Aveiro University Research Institute/CESAM (UID/AMB/50017/2013), and “COMPETE” through Project No. FCOMP-01-0124-FEDER-02800 (FCT PTDC/AGR-PRO/4091/2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Bashmakov, D.I., Lukatkin, A.S., Anjum, N.A. et al. Evaluation of zinc accumulation, allocation, and tolerance in Zea mays L. seedlings: implication for zinc phytoextraction. Environ Sci Pollut Res 22, 15443–15448 (2015). https://doi.org/10.1007/s11356-015-4698-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4698-x