Abstract
Aims
Zinc distribution at the tissue level is studied almost exclusively in lab-grown plants. It is essential to establish to what extent the patterns observed in lab-grown plants are corresponding with those in nature. To this end, we compared Zn localization in Noccaea caerulescens growing in its natural environment, a zinc/lead mine tailing, with that in hydroponically grown plants of the same origin.
Methods
Zinc concentrations in plants and soil were determined by flame AAS and Zn localization in leaf tissues was studied using Zn indicators Zincon and Zinpyr-1.
Results
The mean Zn concentration in plants at the mine tailings was around 15,000 mg/kg DW, which corresponded well with the Zn concentration in the leaves of plants grown at 1600 μM Zn in the nutrient solution. The Zn distribution patterns in leaves of plants sampled from the mine and plants grown in hydroponics were identical. Zn-dependent staining was the most intensive in water-storage epidermal cells, guard cells and vascular bundles, and less intensive in subsidiary and mesophyll cells.
Conclusions
Zinc distribution in hydroponically grown plants is representative for plants in nature. Preferential Zn sequestration in leaves, particularly in water-storage epidermal cells, restricts metal accumulation in mesophyll and contributes to Zn hypertolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Around 500 plant species of more than 30 families have been described as metal hyperaccumulators (Verbruggen et al. 2009; Krämer 2010). These often grow on metal-enriched soils and are characterized by high levels of tolerance and accumulation of one or several metals in their leaves. Most of these species hyperaccumulate nickel (Ni), and are endemic to serpentine soil. No more than 20 of them are zinc (Zn) hyperaccumulators, capable of accumulating more than 10,000 mg Zn / kg DW in their leaves (Krämer 2010).
Zn hyperaccumulation has been extensively studied in Noccaea caerulescens and Arabidopsis halleri (e.g., Assunção et al. 2003; Assunção and Schat 2003; Hanikenne et al. 2008; Milner and Kochian 2008; Pauwels et al. 2008; Roosens et al. 2008; Frerot 2011). N. caerulescens is a facultative metallophyte, which can hyperaccumulate Ni, Zn, and cadmium (Cd) (Verbruggen et al. 2009; Krämer 2010). Whereas Zn hyperaccumulation capacity is a species-wide character in N. caerulescens, Ni or Cd hyperaccumulation capacity seems to be population-specific (Assunção et al. 2003). Local N. caerulescens populations vary significantly among each other in their metal tolerance capacities (Assunção et al. 2003; Richau and Schat 2008; Seregin et al. 2014, 2015). Hypertolerance to Zn, in comparison with non-metallophytes, is a species-wide trait in N. сaerulescens, although populations from metallicolous, calamine soils are, on average, more Zn-tolerant than populations from non-metalliferous soil (Verbruggen et al. 2009). Cd hypertolerance, on the other hand, is certainly not species-wide (Assunção et al. 2003). Differences in accumulation and tolerance capacities among local N. caerulescens populations seem to be associated with differential expression of genes encoding metal transporters, such as ZNT1, IRT1, HMA3, and HMA4 (Assunção et al. 2001; Hammond et al. 2006; Halimaa et al. 2014; Visioli et al. 2014).
The N. caerulescens population from St Laurent le Minier (SLM, formerly called Ganges [GA]), grows on calamine soils between St Laurent le Minier and Ganges in South France, where mining used to take place from the Roman times until about 15 years ago (Robinson et al. 1998). The bedrock is rich in Zn/Pb sulphides and oxides, associated with barite (Robinson et al. 1998). The mine tailings host many metallophytes including N. caerulescens, Iberis intermedia, Armeria maritima and Silene latifolia (Robinson et al. 1998). The local N. caerulescens population (SLM) hyperaccumulates both Zn and Cd (Robinson et al. 1998). Moreover, Cd seems to be required for optimal growth in SLM plants (Roosens et al. 2003; Liu et al. 2008).
The distribution of heavy metals over plant tissues is insufficiently known yet. Various techniques, including histochemical methods, have been applied to compare metal distribution patterns in leaves and roots in hyperaccumulating and non-hyperaccumulating reference species (Vazquez et al. 1992, 1994; Küpper et al. 1999; Frey et al. 2000; Ma et al. 2005; Seregin and Kozhevnikova 2008; Vogel-Mikus et al. 2008; Richau et al. 2009; Seregin et al. 2011; Kozhevnikova et al. 2014a, 2014b; Dinh et al. 2014). These studies revealed that hyperaccumulators and non-hyperaccumulators do not only distinctly distribute metals over roots and leaves, but also over tissues within organs, or cell types within tissues. However, the metal distribution patterns at the tissue level have been studied almost exclusively in plants grown in greenhouses or climate rooms, usually in hydroponics, and have almost never been compared with those in plants growing in their natural environment. In fact, the foliar metal concentrations and shoot-to-root metal concentration ratios obtained in lab-grown plants can be considerably different from those in field-grown plants, which often seems to be attributable to the use of unrealistically high metal exposure levels in hydroponics or artificially spiked soils (van der Ent et al. 2013), but possibly also to climatic conditions. Likewise, it is conceivable that there may be differences between lab-grown and field-grown plants regarding their metal distribution patterns over tissues or cell types within tissues. Therefore, it is crucial to check whether the patterns obtained in hydroponics are realistic. In this study we compared the shoot Zn concentrations and the Zn distribution patterns over leaf tissues and leaf cell types between hydroponically grown plants and plants collected at the site of population origin, i.e. the mine tailings near St Laurent le Miniers.
Materials and methods
Plant material
Shoots of 30 plants of Noccaea сaerulescens F.K. Mey (formerly Thlaspi caerulescens J. & C. Presl), accession St Laurent le Minier (SLM, formerly Ganges), were collected from various tailings of abandoned mines around St Laurent le Minier, South France (43.936155, 3.671470) (Fig. 1). At the same location, we also collected seeds from 15 plants and took soil samples (10 cm3) within their immediate surroundings.
Growing plants in hydroponics
The seeds collected from the plants at the mine tailings were germinated for 2 weeks in Petri dishes on moist filter paper at 20°C in the dark. Seedlings were transferred to 1-l polyethylene pots (three seedlings per pot) filled with modified half-strength Hoagland’s nutrient solution (Assunção et al. 2003, 2008): 3 mM KNO3, 2 mM Ca(NO3)2, 1 mM NH4HPO4, 0.5 mM MgSO4, 1 μM KCl, 25 μM H3BO3, 2 μM ZnSO4, 2 μM MnSO4, 0.1 μM CuSO4 , 0.1 μM (NH4)6Mo7O24, 20 μM Fe(Na)EDTA. The pH buffer MES was added at a 2-mM concentration and the pH was set at 5.25 using KOH, to prevent Zn complexation by EDTA (Assunção et al. 2003, 2008). Plants were grown in a climate chamber (23/18°C day/night; light intensity at plant level, 200 μM m−2 s−1, 14 h d−1; relative humidity 70%). After one week of acclimation, Zn was added as Zn(NO3)2 at concentrations of 2 (control), 100, 200, 400, 800, 1000, and 1600 μM. Nine plants were used per treatment. Solutions were renewed weekly. In our experiments the solution pH did not change by more than 0.1 unit between the replacements.
Plants were harvested 8 weeks after the start of the Zn treatments. Zn toxicity was assessed from the root and shoot dry weights at harvest.
Zinc measurement
Prior to harvest, roots of lab-grown plants were desorbed from surface-bound Zn in Na2-EDTA (20 mM) for 10 min at room temperature, then the roots and shoots were separated, rinsed in demineralized water and dried superficially with filter paper. Shoots of plants harvested in the field were washed three times in demineralized water and dried superficially with filter paper. Plant and soil samples were dried to constant weight in a stove at 80°C for 48 h. Soil samples were sieved through a 1.5-mm mesh. For the analysis of acid-extractable Zn concentration in the soil, 30–50 mg of sieved soil was digested in 2 ml of a 4:1 (v/v) mixture of HNO3 (65%, v/v) and HCl (37%, v/v), in Teflon bombs at 140°C for 7 h. Plant samples were weighed and digested in the same way. Zinc concentrations in plant and soil digests were measured using flame atomic absorption spectrophotometry (Perkin Elmer 2100, the Netherlands). Prior to Zn measurements the soil extracts were filtered using a 20-μm bacterial filter. The quality of the digestion procedure and Zn concentration measurement was checked using an internal reference material (powdered poplar leaves) with a known Zn concentration (recovery 95–103%).
Histochemical staining of Zn in leaf tissues
Leaves of plants collected from the mines and plants grown at different Zn exposure levels in the laboratory were used to visualize Zn distribution patterns. We applied the metallochromic dye Zincon, as well as the fluorescent dye Zinpyr-1. Zincon is a relatively Zn-selective indicator, forming a blue-colored complex with Zn. To maximize sensitivity, we applied a procedure described in Seregin and Kozhevnikova (2011) and Seregin et al. (2011). In short, 0.013 g Zincon (sodium salt, C20H15N4NaO6S, Sigma-Aldrich Chemie GmbH) and 0.19 g borax (Na2B4O7x10H2O) were dissolved in 0.2 ml of 1 M NaOH (pH 9.8–10.4) and the volume was adjusted to 10 ml with super-demineralized water. The solution was then heated to 90°C and used after cooling down to room temperature. Thin transverse sections of leaves were made using a safety razor blade and the leaf epidermis was peeled off with tweezers. Leaf sections and epidermis samples were incubated on a glass slide in a few drops of the reagent for 10–15 min, covered with a cover glass and examined using an Olympus CX41 microscope (Olympus, Japan). Photographs were taken using a color video camera Altra 20 (Olympus, Japan).
As a more sensitive reagent, we used the membrane-permeable Zn fluorophore Zinpyr-1. Zinpyr-1 is highly selective for Zn over other metals (Kd = 0.7 ± 0.1 nM) (Burdette et al. 2001). We applied a procedure described in Sinclair et al. (2007), with slight modifications (Seregin and Kozhevnikova 2011; Seregin et al. 2011). In short, we prepared a 10-μM solution of Zinpyr-1 (C46H36Cl2N6O5, Fluka) in super-demineralised water, diluted from a 1-mM stock made up in dimethyl sulphoxide (DMSO). Leaf sections and epidermis samples were immersed in Zinpyr-1 solution (10 μM) and incubated at room temperature in darkness for 1 h. Then excess reagent was removed with filter paper, a few drops of ultra pure water were added and the samples were covered with a cover glass. Zn localization was examined using an Olympus CX41 microscope (Japan) equipped with a CX-RFL-2 reflected fluorescence attachment with a CX-DMB-2 filter set (excitation 450–490 nm, emission 500–540 nm) and an Axio Imager Z2 microscope (Zeiss) equipped with filter set 38 (excitation 450–490 nm, emission 500–550 nm). Photographs were taken using a color camera Altra 20 (Olympus, Japan) attached to the Olympus CX41 microscope, and black-and-white camera attached to the Axio Imager Z2 microscope. Black-and-white images were pseudo-coloured with ImageJ software. Six to eight plants per treatment were examined.
Statistical analysis
All experiments were replicated three times. Quantitative data were statistically analyzed using one-way ANOVA, after log-transformation of the data. Post-hoc comparison of multiple individual means was done using the minimum significant range (MSR) statistic (Sokal and Rohlf 1981).
Results
Plant growth
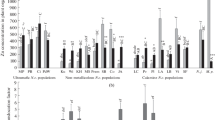
When compared to control conditions, none of the Zn concentrations applied in hydroponics caused a significant root or shoot growth inhibition (Fig. 2). However, there was a significant decrease in root dry weight at 800–1600 μM Zn in the solution compared to the plants at 200 μM, and in shoot dry weight at 1600 μM Zn compared to the plants at 100–200 μM (Fig. 2). Thus, 100–200 μM Zn in the solution was optimal for growth. Leaf chlorosis and necrosis were observed neither in lab-grown nor in field-grown plants (Fig. 1).
Effect of different Zn treatments on root (n = 9) and shoot (n = 9) dry weights of N. сaerulescens, accession SLM, grown in hydroponics (means ± SE). Treatments assigned with different letters indicate a significant difference between the means (p < 0.05). ANOVA post-hoc comparisons were performed separately for roots and shoots
Zn accumulation
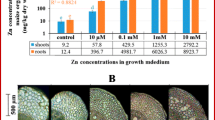
The total acid-extractable Zn concentration in soil samples collected from the mine tailings close to St Laurent le Minier was, on average, 50,000 mg/kg DW, ranging from approximately 19,000 to 92,000 mg/kg DW, while the mean Zn concentration in the shoots of the collected N. caerulescens plants was around 15,000 mg/kg DW, ranging from 10,000 to 26,000 mg/kg DW (Fig. 3). In hydroponics, the shoot Zn concentration increased significantly with increasing Zn exposure, up to the 200-μM treatment level, after which there was no significant further increase (Fig. 4). The highest Zn concentration in the leaves of the plants grown in hydroponics was around 15,000 mg/kg DW. The root Zn concentrations increased consistently with increasing Zn exposure, up to the highest exposure level. From 2 to 200 μM Zn in the nutrient solution, the Zn concentrations in the shoots were higher than those in the roots, but at 400 and 800 μM Zn they were similar, and at 1000 and 1600 μM Zn, the root metal concentrations were higher than those in shoots. Thus, the metal translocation factor, calculated as shoot-to-root Zn concentration ratio, decreased steadily with increasing Zn concentration in the solution (Fig. 5).
Zn accumulation in the roots (n = 9) and shoots (n = 9) of N. сaerulescens, accession SLM, grown in half-strength Hoagland’s solution at various concentrations of Zn(NO3)2 (means ± SE). Treatments assigned with different letters indicate a significant difference between the means (p < 0.05). ANOVA post-hoc comparisons were performed separately for roots and shoots
Zn distribution in leaf tissues
Staining with the fluorescent probe Zinpyr-1 and the metallochrome indicator Zincon yielded comparable patterns of leaf Zn localization in plants sampled from the mine and plants grown in hydroponics (Figs. 6, 7). The Zn distribution over leaf tissues was identical in plants grown at 200 up to 1600 μM Zn in the nutrient solution (Fig. 7). Zinc was found in all leaf tissues, both in cell walls and cell protoplasts. The staining was the most intensive in the leaf epidermis and vascular bundles, especially the phloem and collenchyma, whereas in mesophyll cells it was less prominent (Figs. 6a, b, 7a-c). The Zn distribution within the leaf epidermis was uneven. Zn accumulated in large water-storage cells, especially along the veins (Figs. 6c-h, 7d-h). It was also found in the guard cells of the stomata, whereas the staining of the subsidiary cells was much less intensive. This pattern was less pronounced in the adaxial epidermis compared to the abaxial epidermis (Figs. 6c-h, 7d-h).
Zn distribution over tissues in N. сaerulescens, accession SLM, in leaves that were collected from the natural population. Staining with Zinpyr-1 (a-f) and Zincon (g, h). (a) – leaf cross section in the region of the central vein; (b) – leaf cross section in the region of lateral vein; (c, d) – adaxial epidermis; (e-h) – abaxial epidermis. Epidermal cells below the vein are marked with an arrow. Bar =100 μm (a-c, e, g), 50 μm (d, f, h). E – epidermis, М – mesophyll, SC – subsidiary cells, VB – vascular bundle, WSC – water-storage epidermal cells
Zn distribution over tissues in N. сaerulescens, accession SLM, in leaves of plants grown in half-strength Hoagland’s solution at 400 (a, e), 800 (c, f, h) and 1600 (b, d, g) μM Zn(NO3)2. Staining with Zinpyr-1 (a-d) and Zincon (e-h). (a) – leaf cross section; (b) – leaf cross section in the region close to leaf edge; (c) – leaf cross section in the region of the central vein; (d-f) – abaxial epidermis; (g, h) – adaxial epidermis. Epidermal cells below (e) and above (g) the vein are marked with an arrow. Bar =200 μm (a), 100 μm (b, c, e), 50 μm (g, h), 25 μm (d, f). E – epidermis, GC – stomata guard cells, М – mesophyll, SC – subsidiary cells, VB – vascular bundle, WSC – water-storage epidermal cells
Discussion
N. caerulescens, alongside with other metallophytes (Iberis intermedia, Silene latifolia), is found on rocky slopes as well as on the sandbanks in the flood bed of the Herault river (Fig. 1). When collected in this area, the mean Zn concentration in N. caerulescens shoots ranged from 10,000 to 26,000 mg/kg DW, with a mean of 15,000 mg/kg DW (Fig. 3). The highest Zn concentration in the leaves of the plants grown in hydroponics was 15,000 mg/kg DW (Fig. 4), which compares quite well with the mean Zn concentration in plants from the mine tailings near St Laurent le Minier. Though the root Zn concentration in hydroponics continued to rise with the Zn concentration in the nutrient solution, the shoot Zn concentration leveled off at 200 μM Zn in the nutrient solution, indicating that the Zn xylem loading capacity was already close to saturation at this concentration (Fig. 4). Thus, in our experiment, the Zn concentrations from 200 to 1600 μM in the nutrient solution yielded a realistic level of Zn accumulation in the leaves.
Histochemical analysis revealed identical patterns of Zn distribution over foliar tissues and epidermal cell types in plants collected in the field and those grown in hydroponics (Figs. 6, 7), in spite of the relatively short duration of the hydroponics experiment, in comparison with the pre-harvest growing season at the population site. This result unambiguously demonstrates that the Zn distribution patterns obtained in hydroponically grown plants can be extrapolated to plants in their natural environment, at least when the foliar Zn concentrations are similar.
Zinc-dependent fluorescence was higher in leaf epidermal cells and vascular bundles than in mesophyll cells (Figs. 6a, b, 7a-c). Predominant Zn accumulation in the leaf epidermis compared to the mesophyll was also shown for other accessions of N. caerulescens, as well as for the hyperaccumulators N. japonicum, N. praecox and Sedum alfredii (Vazquez et al. 1992, 1994; Küpper et al. 1999; Frey et al. 2000; Ma et al. 2005; Vogel-Mikus et al. 2008; Monsant et al. 2010). For example, in the Prayon accession of N. caerulescens, 87,6% of the foliar Zn load was found in the epidermal cells and only 12,4% in the mesophyll cells (Monsant et al. 2010). Zinc was detected in the leaf vascular bundles of both hyperaccumulating and non-accumulating plants (Vogel-Mikus et al. 2008; Kozhevnikova et al. 2014a). Zn accumulation in the abaxial and adaxial collenchyma cells surrounding the conductive tissues in N. praecox was suggested as a mechanism to protect metabolically more active tissues from metal toxicity (Vogel-Mikus et al. 2008), while metal accumulation in the phloem seems to point to the fact that, in hyperaccumulators at least, metals are re-allocated from old to young leaves (Lu et al. 2013; Deng et al. 2016). Taken together, these data demonstrate that the general pattern of Zn localization in leaves can be similar in different Zn hyperaccumulator species.
There may be several reasons why excessive Zn is stored predominantly in the leaf epidermis: first, it may result from “passive accumulation”, along with the transpiration-driven water stream, which ends in the epidermis (Küpper et al. 2001, 2009); second, there may be “active accumulation” in the epidermis, due to a relative abundance of Zn influx transporters of the ZIP family in this tissue, e.g. NcZNT5 (Küpper and Kochian 2010; Schneider et al. 2013); third, there may be a combination of active and passive mechanisms. In any case, active accumulation most probably does play a role, such as demonstrated for Cd in N. caerulescens cell type-specific protoplasts (Leitenmaier and Küpper 2011).
The distribution of metals over different types of epidermal cells can be highly uneven (Seregin and Kozhevnikova 2008). In this study we found Zn accumulation predominantly in so-called “water-storage” (Solereder 1899; Metcalfe and Chalk 1950) or “metal-storage” (Küpper and Kochian 2010; Leitenmaier and Küpper 2013) pavement epidermal cells, characterized by a large size and a big central vacuole, and in stomatal guard cells, rather than in subsidiary cells (Figs. 6c-h, 7d-h). Preferential accumulation of Zn in large water-storage epidermal cells was also found in other N. caerulescens accessions (Frey et al. 2000; Monsant et al. 2010), however, it was not observed in the non-accumulators Lepidium ruderale and Capsella bursa-pastoris (Kozhevnikova et al. 2014a). It is important to note that the abovementioned pattern was expressed less in the adaxial epidermis compared to the abaxial epidermis (Figs. 6c-h, 7d-h), which could be related to differences in transpiration rate. The most intensive Zn-dependent staining was observed in the water-storage cells below the veins (Figs. 6g, 7e), which may be explained by the fact that they are at a shorter distance from the Zn source, i.e. the vascular bundle. Uneven Zn distribution over different epidermal cell types might be connected with a heterogeneneous localization of Zn transporters. For example, in young leaves of young N. caerulescens plants, ZNT5 mRNA levels were much higher in water-storage cells compared to stomatal guard cells or subsidiary cells, whereas in mature leaves, ZNT1 expression was higher in guard cells compared to the other types of epidermal cells (Küpper and Kochian 2010). Theoretically, differential metal accumulation capacities among epidermal cell types could also be associated with differential capacities to synthesize or accumulate particular cytoplasmic or vacuolar Zn chelators. Unfortunately, data on chelator concentrations in specific epidermal cell types are not available to date.
Several studies suggested that metal accumulation in the mesophyll occurs only after saturation of the metal accumulation capacity of the epidermis (Küpper et al. 2001, 2007; Leitenmaier and Küpper 2011, 2013). The relative metal burdens of the epidermis and the mesophyll depend evidently on their relative volumes. For example, in the hyperaccumulator A. halleri the epidermis is relatively thinner than in N. caerulescens, accounting for a much lower fraction of the total leaf volume. Thus, even when including the trichomes, A. halleri epidermal cells accumulate in total only a small fraction of the total foliar metal burden. As a result, much more Zn ends up in the mesophyll in A. halleri (Küpper et al. 2000), in comparison with N. caerulescens (Monsant et al. 2010). Consequently, in A. halleri toxic effects are observed at lower metal concentrations in the nutrient solution, in comparison with N. caerulescens (Küpper et al. 2007; Leitenmaier and Küpper 2013).
In principle, the relatively low metal-accumulating capacity of mesophyll cells can be related to altered expression levels of Zn efflux or influx transporters, in comparison with those in the epidermis, as well as a relatively low capacity for Zn chelation. In A. halleri and N. caerulescens the expression of the Zn-effluxing ATPase gene, HMA4, is not only extremely high in roots, where it plays a crucial role in Zn xylem loading (Hanikenne et al. 2008; Craciun et al. 2012), but also in the leaf vasculature and mesophyll (Craciun et al. 2012) and, possibly, even in the leaf epidermis (Schneider et al. 2013). This indicates that in the leaves HMA4 may be involved in Zn xylem unloading or in Zn efflux from mesophyll cells, thus preventing Zn to accumulate in the mesophyll, and promoting its translocation to the epidermal water storage cells (Schneider et al. 2013). In line with this, expressing NcHMA4 from an endogenous N. caerulescens HMA4 promoter in the Arabidopsis thaliana hma2/hma4 double mutant did not alleviate, but instead aggravated Zn deficiency in the leaves, although it did enhance the foliar Zn concentration (Iqbal et al. 2013). Moreover, hyperaccumulator leaf protoplasts did not seem to accumulate Zn or Cd at particularly high concentrations (Marques et al. 2004), and N. caerulescens cells in suspension accumulated less Zn or Cd than A. thaliana cells, most likely due to higher rates of efflux (Klein et al. 2008). These results clearly suggest that, except for water-storage cells, hyperaccumulator cells may in fact have lower, rather than higher capacities for metal accumulation, compared to non-hyperaccumulator cells, probably due to higher efflux rates.
In leaves of N. caerulescens, both in nature and in hydroponics, Zn was localized both in cell walls and cell protoplasts. In the mesophyll, Zn-dependent fluorescence was more intensive in the cell walls than in the protoplasts (Figs. 6, 7). Literature reports on the Zn concentrations in cell walls versus cell protoplasts are contradictory. Frey et al. (2000) found that Zn accumulated mainly in the cell wall in mesophyll cells and stomatal guard cells of N. caerulescens. However, other authors found that in mesophyll cells of N. caerulescens (Ma et al. 2005) and N. praecox (Vogel-Mikus et al. 2008) the Zn concentration in vacuoles was higher than that in cell walls. In contrast to the mesophyll, in large water-storage epidermal cells of N. praecox, Zn was mainly accumulated in the symplast (Vogel-Mikus et al. 2008). In the vacuoles of epidermal cells, metal concentrations can reach several hundreds of mM (Küpper et al. 2001, 2009). Anyway, although metal concentrations in mesophyll cell walls may occasionally reach high levels in hyperaccumulators, it is unlikely that binding to cell walls in leaves would represent the driving force of metal hyperaccumulation in the leaves. In contrast, in N. caerulescens metal hyperaccumulation in the leaf blade seems to be driven by “active” uptake across the plasma membrane of the epidermal water storage cells, which is in turn limited by the rate of metal transport from the cytoplasmic into the vacuolar compartment of these cells, such as demonstrated for Cd (Leitenmaier and Küpper 2011). Anyway, although the cell wall is probably not the most predominant metal sink in hyperaccumulator leaves, there is circumstantial evidence that Cd-induced or constitutive cell wall alterations in leaves can contribute to Cd tolerance, and the intraspecific variation therein, in A. halleri (Meyer et al. 2015; Isaure et al. 2015).
Zn transport across the tonoplast is mediated by transporters of the Cation Diffusion Facilitator family (CDF = MTP), and possibly, members of the Natural Resistance-Associated Macrophage Protein (Nramp) family, H+/Cation Exchanger family (CAX), and Heavy Metal Associated protein (HMA) family (Van der Zaal et al. 1999; Becher et al. 2004; Dräger et al. 2004; Koren’kov et al. 2007; Gustin et al. 2009; Oomen et al. 2009; Ueno et al. 2011; Tanaka et al. 2015). There is not much information on the tissue- or cell-specificity of any of these candidate tonoplast transporters. Anyway, MTP1, a member of the CDF/MTP family, was found both in mesophyll and epidermal cells and is supposed to be the only vacuolar Zn transporting CDF/MTP in the epidermal cells of N. caerulescens (Schneider et al. 2013).
Cytosolic Zn is supposed to be bound to strong chelators. In N. caerulescens, almost all of the Zn in the mesophyll cell sap was present as a Zn–nicotianamine complex, whereas in the epidermis it was bound to malate and, to a lower degree, citrate (Schneider et al. 2013). This could be taken to suggest that most of the epidermal Zn is in the vacuoles indeed, because organic acid-Zn complexes are probably too unstable to prevent toxic effects in the cytoplasm (Verbruggen et al. 2009; Leitenmaier and Küpper 2013). It is very likely that preferential accumulation of Zn in the vacuoles of the water-storage epidermal cells contributes to the exceptional degrees of Zn tolerance at the whole-plant level in Zn hyperaccumulators, most probably because it allows the plant to prevent excessive Zn accumulation in the mesophyll and, therefore, Zn interference with photosynthesis.
In conclusion, the patterns of Zn accumulation and distribution over tissues and cell types were identical in plants growing in nature and in hydroponics under laboratory conditions, suggesting that natural Zn hyperaccumulation in N. caerulescens can be effectively mimicked in the laboratory with hydroponically grown plants. In addition, our results are in support of the hypothesis that Zn hypertolerance in N. сaerulescens relies on a highly efficient Zn sequestration in large water-storage epidermal cells, and restricted accumulation in the mesophyll.
References
Assunção AGL, Schat H (2003) Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol 159:351–360
Assunção AGL, da Costa Martins P, de Folter S, Voojis R, Schat H, Aarts MGM (2001) Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 24:217–226
Assunção AGL, Bookum WM, Nelissen HJM, Vooijs R, Schat H, Ernst WHO (2003) Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types. New Phytol 159:411–419
Assunção AGL, Bleeker P, Bookum WM, Vooijs R, Schat H (2008) Intraspecific variation of metal preference patterns for hyperaccumulation in Thlaspi caerulescens: evidence from binary metal exposures. Plant Soil 303:289–299
Becher M, Talke IN, Krall L, Krämer U (2004) Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J 37:251–268
Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ (2001) Fluorescent sensors for Zn(2+) based on a fluorescein platform: synthesis, properties and intracellular distribution. J Am Chem Soc 123:7831–7841
Craciun AR, Meyer C-L, Chen J, Roosens N, Groodt RD, Hilson P, Verbruggen N (2012) Variation in HMA4 gene copy number and expression among Noccaea caerulescens populations presenting different levels of Cd tolerance and accumulation. J Exp Bot 63:4179–4189
Deng T, Tang Y, van der Ent A, Sterckeman T, Echevarria G, Morel JL, Qiu RL (2016) Nickel translocation via the phloem in the hyperaccumulator Noccaea caerulescens (Brassicaceae). Plant Soil 404:35–45
Dinh NT, Vu DT, Mulligan D, Nguyen AV (2014) Accumulation and distribution of zinc in the leaves and roots of the hyperaccumulator Noccaea caerulescens. Environ Exp Bot 110:85–95
Dräger DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Krämer U (2004) Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J 39:425–439
Frerot H (2011) A challenge for hyperaccumulating plant models: 'cycling' as fast as Arabidopsis thaliana. New Phytol 189:357–359
Frey B, Keller C, Zierold K, Schulin R (2000) Distribution of Zn in functionally different leaf epidermal cells of the hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 23:675–687
Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE (2009) MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J 57:1116–1127
Halimaa P, Lin Y-F, Ahonen VH, Blane D, Clemens S, Gyenesei A, Haikio E, Karenlampi SO, Laiho A, Aarts MGM, Pursiheimo J-P, Schat H, Schmidt H, Tuomainen MH, Tervahauta AI (2014) Gene expression differences between Noccaea caerulescens ecotypes help indentifying candidate genes for metal phytoremediation. Environ Sci Technol 48:3344–3353
Hammond JP, Bowen HC, White PJ, Mills V, Pyke KA, Baker AJ, Whiting SN, May ST, Broadley MR (2006) A comparison of the Thlaspi caerulescens and Thlaspi arvense shoot transcriptomes. New Phytol 170:239–260
Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Krämer U (2008) Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453:391–395
Iqbal M, Nawaz I, Hassan Z, Hakvoort HWJ, Bliek M, Aarts MAM, Schat H (2013) Expression of HMA4 cDNAs of the zinc hyperaccumulator Noccaea caerulescens from endogenous NcHMA4 promoters does not complement the zinc-deficiency phenotype of the Arabidopsis thaliana hma2hma4 double mutant. Front Plant Sci 4:article 404
Isaure MP, Huguet S, Meyer CL, Castillo-Michel H, Testemale D, Vantelon D, Saumitou-Laprade P, Verbruggen N, Sarret G (2015) Evidence of various mechanisms of Cd sequestration in the hyperaccumulator Arabidopsis halleri, the non-accumulator Arabidopsis lyrata, and their progenies by combined synchrotron-based techniques. J Exp Bot 66:3201–3214
Klein MA, Sekimoto H, Milner MJ, Kochian LV (2008) Investigation of heavy metal hyperaccumulation at the cellular level: development and characterization of Thlaspi caerulescens suspension cell lines. Plant Physiol 147:2006–2016
Koren’kov V, Park S, Cheng NH, Sreevidya C, Lachmansingh J, Morris J, Hirschi K, Wagner GJ (2007) Enhanced Cd2+-selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta 225:403–411
Kozhevnikova AD, Erlikh NT, Zhukovskaya NV, Obroucheva NV, Ivanov VB, Seregin IV (2014a) Nickel and zinc accumulation, distribution and effects on ruderal plants Lepidium ruderale and Capsella bursa-pastoris. Acta Physiol Plant 36:3291–3305
Kozhevnikova AD, Seregin IV, Erlikh NT, Shevyreva TA, Andreev IM, Verweij R, Schat H (2014b) Histidine-mediated xylem loading of zinc is a species-wide character in Noccaea caerulescens. New Phytol 203:508–519
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Biol 61:517–534
Küpper H, Kochian LV (2010) Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator Thlaspi caerulescens (Ganges population). New Phytol 185:114–129
Küpper H, Zhao FJ, McGrath SP (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119:305–311
Küpper H, Lombi E, Zhao FJ, McGrath SP (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212:75–84
Küpper H, Lombi E, Zhao FJ, Wieshammer G, McGrath SP (2001) Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J Exp Bot 52:2291–3000
Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Šetlík I (2007) Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol 175:655–674
Küpper H, Mijovilovich A, Götz B, Küpper FC, Meyer-Klaucke W (2009) Complexation and toxicity of copper in higher plants (I): characterisation of copper accumulation, speciation and toxicity in Crassula helmsii as a new copper hyperaccumulator. Plant Physiol 151:702–714
Leitenmaier B, Küpper H (2011) Cadmium uptake and sequestration kinetics in individual leaf cell protoplasts of the Cd/Zn hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 34:208–219
Leitenmaier B, Küpper H (2013) Compartmentation and complexation of metals in hyperaccumulator plants. Front Plant Sci 4:1–13
Liu M-Q, Yanai J, Jiang R, Zhang F, McGrath S, Zhao F (2008) Does cadmium play a physiological role in the hyperaccumulator Thlaspi caerulescens? Chemosphere 71:1276–1283
Lu L, Tian S, Zhang J, Yang X, Labavitch JM, Webb SM, Latimer M, Brown PH (2013) Efficient xylem transport and phloem remobilization of Zn in the hyperaccumulator plant species Sedum alfredii. New Phytol 198:721–731
Ma JF, Ueno D, Zhao FJ, McGrath SP (2005) Subcellular localisation of Cd and Zn in the leaves of a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Planta 220:731–736
Marques L, Cossegal M, Bodin S, Czernic P, Lebrun M (2004) Heavy metal specificity of cellular tolerance in two hyperaccumulating plants, Arabidopsis halleri and Thlaspi caerulescens. New Phytol 164:289–295
Metcalfe CR, Chalk L (1950) Anatomy of the dicotyledons. Leaves, stem, and wood in relation to taxanomy with notes on economic uses, vol 1. Clarendon Press, Oxford, pp. 83–91
Meyer CL, Juraniec M, Huguet S, Chaves-Rodriguez E, Salis P, Isaure MP, Goormaghtigh E, Verbruggen N (2015) Intraspecific variability of cadmium tolerance and accumulation, and cadmium-induced cell wall modifications in the metal hyperaccumulator Arabidopsis halleri. J Exp Bot 66:3215–3227
Milner MJ, Kochian LV (2008) Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot 102:3–13
Monsant A, Wang Y, Tang C (2010) Nitrate nutrition enhances zinc hyperaccumulation in Noccaea caerulescens (Prayon). Plant Soil 336:391–404
Oomen RJ, Wu J, Lelièvre F, Blanchet S, Richaud P, Barbier-Brygoo H, Aarts MG, Thomine S (2009) Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol 181:637–650
Pauwels M, Roosens N, Frerot H, Saumitou-Laprade P (2008) When population genetics serves genomics: putting adaptation back in a spatial and historical context. Curr Opin Plant Biol 11:129–134
Richau KH, Schat H (2008) Intraspecific variation of nickel and zinc accumulation and tolerance in the hyperaccumulator Thlaspi caerulescens. Plant Soil 314:253–262
Richau KH, Kozhevnikova AD, Seregin IV, Vooijs R, Koevoets PLM, Smith JAC, Ivanov VB, Schat H (2009) Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator, Thlaspi caerulescens. New Phytol 183:106–116
Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PEH (1998) The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 203:47–56
Roosens N, Verbruggen N, Meerts P, Ximenez-Embun P, Smith JAC (2003) Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from Western Europe. Plant Cell Environ 26:1657–1672
Roosens NH, Willems G, Saumitou-Laprade P (2008) Using Arabidopsis to explore zinc tolerance and hyperaccumulation. Trends Plant Sci 13:208–215
Schneider T, Persson DP, Husted S, Schellenberg M, Gehrig P, Lee Y, Martinoia E, Schjoerring JK, Meyer S (2013) A proteomics approach to investigate the process of Zn hyperaccumulation in Noccaea caerulescens (J & C.Presl) F. K. Meyer. Plant J 73:131–142
Seregin IV, Kozhevnikova AD (2008) Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ J Plant Physiol 55:1–22
Seregin IV, Kozhevnikova AD (2011) Histochemical methods for detection of heavy metals and strontium in the tissues of higher plants. Russ J Plant Physiol 58:721–727
Seregin IV, Kozhevnikova AD, Gracheva VV, Bystrova EI, Ivanov VB (2011) Tissue zinc distribution in maize seedling roots and its action on growth. Russ J Plant Physiol 58:109–117
Seregin IV, Erlikh NT, Kozhevnikova AD (2014) Nickel and zinc accumulation capacities and tolerance to these metals in the excluder Thlaspi arvense and the hyperaccumulator Noccaea caerulescens. Russ J Plant Physiol 61:204–214
Seregin IV, Kozhevnikova AD, Zhukovskaya NV, Schat H (2015) Cadmium tolerance and accumulation in excluder Thlaspi arvense and various accessions of hyperaccumulator Noccaea caerulescens. Russ J Plant Physiol 62:837–846
Sinclair SA, Sherson SM, Jarvis R, Camakaris J, Cobbet CS (2007) The use of the zinc-fluorophore Zinpyr-1, in the study of zinc homeostasis in Arabidopsis roots. New Phytol 174:39–45
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. W.H. Freeman & Co, San Francisco
Solereder H (1899) Systematische anatomie der dicotyledonen. Verlag von Ferdinand Enke, Stuttgart, pp. 67–77
Tanaka N, Fujiwara T, Tomioka R, Krämer U, Kawachi M, Maeshima M (2015) Characterization of the histidine-rich loop of Arabidopsis vacuolar membrane zinc transporter AtMTP1 as a sensor of zinc level in the cytosol. Plant Cell Physiol 56:510–519
Ueno D, Milner MJ, Yamaji N, Yokosho K, Koyama E, Clemencia Zambrano M, Kaskie M, Ebbs S, Kochian LV, Ma JF (2011) Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J 66:852–862
van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloiod trace elements: facts and fiction. Plant Soil 362:319–334
van der Zaal BJ, Neuteboom LW, Pinas JE, Chardonnens AN, Schat H, Verkleij JA, Hooykaas P (1999) Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol 119:1047–1055
Vazquez MD, Barcelo J, Poschenrieder C, Madico J, Hatton P, Baker AJM, Cope GH (1992) Localization of zinc and cadmium in Thlaspi caerulescens (Brassicaceae), a metallophyte that can hyperaccumulate both metals. J Plant Physiol 140:350–355
Vazquez MD, Poschenrieder C, Barcelo J, Baker AJM, Hatton P, Cope GH (1994) Compartmentation of zinc in roots and leaves of the zinc hyperaccumulator Thlaspi caerulescens. Bot Acta 107:243–250
Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181:759–776
Visioli G, Gullì M, Marmiroli N (2014) Noccaea caerulescens populations adapted to grow in metalliferousand non-metalliferous soils: Ni tolerance, accumulation and expression analysis of genes involved in metal homeostasis. Environ Exp Bot 105:10–17
Vogel-Mikus K, Simcic J, Pelicon P, Budnar M, Kump P, Necemer M, Mesjasz-Przybylowicz J, Przybylowicz WJ, Regvar M (2008) Comparison of essential and non-essential element distribution in leaves of the Cd/Zn hyperaccumulator Thlaspi praecox as revealed by micro-PIXE. Plant Cell Environ 31:1484–1496
Acknowledgements
The authors are grateful to Victor Ivanov for critical discussion of the results, to Rudo Verweij, Rob Broekman, Richard van Logtestijn and Riet Vooijs for technical support, Marc Aarts and Patrick Doumas for providing the coordinates of the mines. This work was partially supported by the grants from the Russian Foundation for Basic Research (RFBR, № 15-04-02236) and from the international scientific program GDRI LOCOMET (Transport, localization and complexation of metals in hyperaccumulating plants) funded by The National Center for Scientific Research, as well as by the French Embassy Metchnikov scholarship funded by Campus France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Rights and permissions
About this article
Cite this article
Kozhevnikova, A.D., Seregin, I.V., Gosti, F. et al. Zinc accumulation and distribution over tissues in Noccaea сaerulescens in nature and in hydroponics: a comparison. Plant Soil 411, 5–16 (2017). https://doi.org/10.1007/s11104-016-3116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3116-6