Abstract
The effects of sewage sludge (SS) and its derived biochar (SSBC) on the availability and uptake of polycyclic aromatic hydrocarbons (PAHs) and potential toxic elements (PTEs) by Solanum lycopersicum (tomato) fruits grown in contaminated urban soil were investigated. Increasing application rates of SS and SSBC (2, 5, and 10 %) decreased PAH availability and, correspondingly, PAH accumulation (22–39 and 48–62 %, respectively) into tomato. SSBC was more effective in this regard. The available concentrations of PAHs (Σ16PAH) in the SSBC treatments were significantly reduced (from 30.0–47.3 %) as compared to the control treatment. The availability of high-molecular-weight PAHs (containing four to six benzene rings) was greatly affected, while low-molecular-weight PAHs (containing two to three benzene rings) was less affected by SSBC amendments. The addition of SSBC showed the least effect on bioaccumulation of naphthalene (two-ring PAH; 24.5–32.6 %), while the highest effect was observed for benzo(b)fluoranthene (five-ring PAH; 3.1–86.8 %) and benzo(g,h,i)perylene (six-ring PAH; 51.8–84.2 %). In contrast, increasing application rates of SS successively increased PTE (As, Cd, Cu, Pb, and Zn) availability and accumulation (15–139 %) into tomato while SSBC successively decreased PTE availability and accumulation (17–91 %). Changes in accumulation varied with PTE and the extent to which PTE concentrations in soil was elevated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Application of sewage sludge (SS) can improve soil fertility and productivity (Singh and Agrawal 2008; Roca-Pérez et al. 2009; Ahmed et al. 2010). However, it can also elevate polycyclic aromatic hydrocarbon (PAH) and potential toxic element (PTE) concentrations (EC 2001) and damage soil quality (Singh and Agrawal 2008; Creamer et al. 2008). SS production has increased with urbanization throughout the world. In light of this, there is a need to increase SS safety as a soil amendment.
SS can be converted, through pyrolysis, into SS biochar (SSBC). SSBC has been shown to enhance soil fertility (Khan et al. 2013a; Liu et al. 2013; Marks et al. 2014) and crop production (Hossain et al. 2010; Jeffery et al. 2011; Khan et al. 2013b). In addition, SSBC has been shown to reduce mobility and uptake of both PTEs and PAHs into crops (Méndez et al. 2012; Waqas et al. 2014; Ahmad et al. 2014; Luo et al. 2014). This is of interest as urban soils that are often used in food production have been reported to have elevated levels of PAHs (Wei et al. 2014; Cai et al. 2012) and PTEs (Qiao et al. 2011).
The characteristics of biochar such as structure, elemental composition, surface area, porosity, and pH play an important role in controlling the mobility of PTEs/PAHs in amended soil and their subsequent bioaccumulation into plants (Ahmad et al. 2014; Luo et al. 2014; Brennan et al. 2014). These characteristics are dependent upon biomass type and pyrolysis temperatures used in the production of biochar (Chen et al. 2008; Cao et al. 2011; Han et al. 2013). Recent studies have been shown that the addition of biochar to soil reduces PAH and PTE availability and their subsequent accumulation into plants (Jiang and Xu 2013; Beesley et al. 2013; Oleszczuk et al. 2014).
In this research, SS and SSBC materials were used to amend the urban soil highly contaminated with PAH and PTE. This study aimed to assess SS and SSBC with respect to their potential to increase crop yield while mitigating PAH and PTE transfer from contaminated urban soil to food plants.
Materials and methods
Soil sampling and preparation of biochar
SS was air-dried and SSBC prepared through its pyrolysis (Waqas et al. 2014). The detailed information is given in Supporting Information (SI). PAH- and PTE-contaminated soil (0–10 cm) was collected from an urban site in Fujian, China. The soil used in this research was contaminated owing to its irrigation with contaminated wastewater. The soil contained elevated concentrations of PAHs and PTEs. Σ16PAH (9.9 mg kg−1) and mutagenic PAHs (Σ7PAHs: 5.49 mg kg−1) exceeded the maximum permissible limits (MPL) (4.23 mg kg−1) set by the Ministry of Environmental Protection, China (MEP 2007). Zn (421 mg kg−1) and Cd (1.0 mg kg−1) concentrations exceeded the MPLs (300 mg kg−1 and 0.6 mg kg−1, respectively) set by the State Environmental Protection Administration, China (SEPA 1995), while As (4.71 mg kg−1), Cu (44 mg kg−1), and Pb (142 mg kg−1) concentrations were lower than their respective MPL limits (Table 1).
Experimental design
Soil was amended with (or without) SS or SSBC materials (<2 mm) at application rates of 2 % (SS2/SSBC2), 5 % (SS5/SSBC5), and 10 % (SS10/SSBC10) on dry weight basis (n = 4). Soil without SS and SSBC was also included as a control. Pots (4 kg) were irrigated with deionized water and cultivated with the seedlings (n = 2) of tomatoes (Solanum lycopersicum). After 1 week, these seedlings were reduced to one in each pot. The pots were kept in the control greenhouse environment and irrigated with deionized water. Ammonium nitrate solution (1 g per 100 ml) was used twice to fertilize the plants. Following a 72-day growing period, fresh tomatoes were freeze-dried and stored (−20 °C).
PAH extraction and determination
Total PAHs were determined by GC-MS (Agilent Technologies 5975C). Extracts were prepared with dichloromethane (DCM) and acetone (1:1 ratio) using an accelerated solvent extraction system (ASE, Dionex-350). A hot water (Milli Q) (200 °C) extraction (Dionex ASE) was used to assess “available” PAH concentrations (Latawiec and Reid 2009). The detailed protocols are given in SI.
PTEs were determined using ICP-OES (PerkinElmer Optima 7000 DV, USA) and ICP-MS (Agilent Technologies, 7500 CX, USA). Extracts of soil, SS, and SSBC treatments were prepared using HNO3 and HCl mix acid extraction (Khan et al. 2008), while tomato fruits were extracted using HNO3 and H2O2 in a microwave accelerated reaction system (CEM Mars, V. 194A05). EDTA was used to extract available PTEs (see SI).
Quality control
Surrogate PAH-deuterated standards and reference materials (PAH-Mix 9, XA20950009CY) were used to check the recovery efficiency of ASE extraction and further purification process. Satisfactory recovery was observed with an average recoveries ranging from 83 ± 8 to 94 ± 9 %. For PTE recovery, plant (GBW07603-GSV-2) and soil (GBW07406-GSS-6) standard reference materials (purchased from the National Research Center for Standards, China) were included in each batch. The PTE recovery was also satisfactory with an average recovery ranging from 92 ± 6 to 104 ± 4 %. The reagent blanks for both PAHs and PTEs were included to check their respective contamination during extraction and subsequent purification processes.
Data analysis
SPSS 11.5 was used to statistically analyze the data.
Results and discussion
PAH/PTE in SS and SSBC
The concentration of Σ16PAHs in SS (5.78 mg kg−1) was below the MPL (Σ9PAHs, 6 mg kg−1; CEC 2000). Pyrolysis significantly (P ≤ 0.01) reduced Σ16PAH concentrations in SSBC to 1.70 mg kg−1 (Fig. S1), this value being below the MPL (6–20 mg kg−1) set by the International Biochar Initiative (IBI 2012).
Biochars have shown different levels of PAHs dependent upon the types of feedstocks and pyrolysis conditions used in their production (Freddo et al. 2012). Longer pyrolysis time and higher temperatures of pyrolysis have been observed to reduce PAH concentrations in biochar (Freddo et al. 2012; Hale et al. 2012; Wan et al. 2014). The concentrations of PAHs in SS depend upon on the type of wastewater treated (Dai et al. 2007). Previous studies have reported PAH concentrations in SS to range from 2 to 36 mg kg−1 (Baran and Oleszczuk 2003). Thus, the SS used in the present research had a relatively lower PAH concentration (5.8 mg kg−1).
The total concentrations of individual PAHs varied greatly in SS and SSBC (Fig. S1). In SS materials, phenanthrene was the dominant compound followed by benzo(b)fluoranthene, while SSBC materials naphthalene, phenanthrene, fluoranthene, and pyrene were dominant compounds (Tables S1, S2, S3, and S4).
In contrast to the observed reductions in PAH concentrations, pyrolysis significantly (P < 0.01) increased total PTE concentrations in SSBC (Table 1). Conversion of SS to SSBC increased total PTE concentrations by the following factors: As (×5.2), Cd (×1.5), Cu (×1.4), Pb (×1.3), and Zn (×1.7). The pyrolysis process conserved PTEs and converted a large volume of the SS into liquid fuels and gaseous emissions (Fagbemi et al. 2001) which led to overall increases in PTE concentrations. However, available PTE concentrations were reduced (Table 1). Conversion of SS to SSBC decreased available PTE concentrations by the following factors: As (×21), Cd (×6.1), Cu (×8.1), Pb (×2.6), and Zn (×6.8). These results are consistent with previous work that also demonstrated increases in total PTE concentrations and decreases in their available fractions following SS conversion to SSBC (Khan et al. 2014; Waqas et al. 2014).
Influence of SS and SSBC on soil and PAH/PTE concentrations
Table 2 summarizes the influence of SS and SSBC amendments on soil basic properties and PAH/PTE concentrations. The changes observed in soil pH, EC, DOC, and total and available nutrients are discussed in SI.
Following SS amendment, the available concentrations of Σ16PAH were significantly (P < 0.05) reduced in the SS10 treatment (29 %) but not in SS2 and SS5 treatments (Fig. S2); while available Σ16PAH concentration was significantly (P < 0.05) reduced in all SSBC treatments (30–47 %) (Tables S1, S2, S3, and S4). Figure S2 shows that the sum of available Σ16PAH concentration was significantly reduced in SSBC treatments as compared to control. The available Σ16PAH concentrations in the SSBC2, SSBC5, and SSBC10 treatments were reduced by 30.0, 40.2, and 47.3 %, respectively, as compared to the control treatment (Fig. S2). The available concentrations of PAHs (Σ16PAH) were only significantly (P < 0.05) reduced (29.3 %) in the SS10 treatment but not in SS2 and SS5 treatments (Fig. S2). The possible mechanisms involved in sorption of PAHs are given later in “Bioaccumulation of PAHs/PTEs” section.
The greatest decrease in available PAHs was observed in SSBC10-amended soil. The availability of PAH compounds of low molecular weight (containing two to three benzene rings) was less affected by SSBC amendment. In contrast, high-molecular-weight PAHs (containing four to six benzene rings) were more greatly affected. This outcome is in keeping with the fact that higher-molecular-weight PAHs partition more strongly with carbonaceous sorbents than lower-molecular-weight PAHs (Xing and Pignatello 1996).
Following SSBC amendment, available PTE concentrations decreased with respect to the control soil: As (57–65 %), Pb (14–54 %), Cu (3–46 %), and Zn (7–17 %) (Cd availability was increased slightly (3–13 %)). In contrast, the addition of SS significantly (P < 0.001) increased the available concentrations of PTEs (As, Cd, Cu, and Zn) (Tables 2 and S5). As mentioned earlier, the SS material (Table 1) had several times higher concentrations of selected available PTEs than SSBC, which, upon mixing, could be released into amended soil.
These results confirm that SS conversion to SSBC represents a promising opportunity to convert higher risk SS into SSBC with lower concentrations of PAHs and less available PTEs.
These results are in keeping with our previous reports (Waqas et al. 2014; Khan et al. 2013a, 2014) and those of others (Lehmann et al. 2011; Ahmad et al. 2012) that have reported a decrease in available fractions of PTEs in biochar-amended soils. Immobilization of PTEs in biochar-amended soils has been reported to be dependent on pH, DOC, EC, and functional groups of biochars (Kookana et al. 2011).
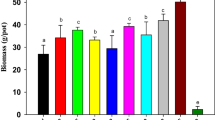
Quantification of plant biomass
SSBC addition significantly (P ≤ 0.01) increased the tomato yields as compared to the control treatment. The increases in yields were counted from 31 to 52 % in SSBC-amended soils (Fig. 1). SS addition also significantly (P ≤ 0.05) increased the tomato biomass as compared to the control soil, but no significant difference was observed between SS10 and SS2 treatments. The release of high concentrations of nutrients, particularly, NH4-N, NO3-N, and available P from SSBC and SS into respective amended soils (Table 2) could be enhanced the plant growth and high fruit yield. In previous studies, the addition of biochar derived from forest residues also increased available nutrients in soil, but no significant difference in biomass production was observed (Lucchini et al. 2014). Tomato green waste biochar addition into Ferrosol soil significantly increased corn shoot biomass, while significantly decreased its yield in Orthic Tenosol-amended soil (Smider and Singh 2014). SS-amended soil had contained higher available PTE concentrations as compared to SSBC-amended soils (Tables 1 and 2); thus, the SS10 (the highest application rate) may have caused a negative effect on biomass yield that overcomes the gains because of increases in the nutrient concentrations in plants. The data show that SSBC material has higher influence over SS for enhancing tomato fruit yield. These results indicated that SSBC had a greater influence in enhancing tomato fruit yield compared to SS.
Bioaccumulation of PAHs/PTEs
At all application rates, SSBC had greater influence than SS addition in terms of reducing PAH accumulation in tomato (Fig. 2). SSBC addition significantly (P < 0.01) reduced Σ16PAH (48–62 %) in tomato, while SS addition reduced Σ16PAH by 22–39 %. The decrease in PAH bioaccumulation of higher-molecular-weight PAHs was greater than that of lower-molecular-weight PAHs (Fig. 2).
a) Bioaccumulation of PAH compounds in tomatoes grown in soil and soil amended with SS and SSBC, while b) available PAH compounds in soils used in this study. NA naphthalene, ACN acenaphthylene, AC acenathphene, FI fluorine, PHE phenanthrene, AN anthracene, FLA fluoranthene, PY pyrene, B(b)A benzo(b)anthracene, CHR chrysene, B(b)F benzo(b)fluoranthene, B(k)F benzo(k)fluoranthene-2, B(a)P benzo(a)pyrene, I(1,2,3-c,d)P indeno(1,2,3-c,d)pyrene, D(a,h)A dibenzo(a,h)anthracene, B(g,h,i)P benzo(g,h,i)perylene. Error bars represent standard deviations (n = 4). Different letters indicate significant difference (P ≤ 0.05), while same letters indicate no significant difference between the treatments
The reductions in bioaccumulation of individual PAH compounds varied greatly. In SSBC2 treatment, the decreases in individual PAH bioaccumulation ranged from 25 to 71 %, this being higher than in the SS2 treatment (9–39 %). The addition of SSBC5 decreased individual PAHs (27–81 %) to a greater extent than in the SS5 (14–50 %). In SSBC10 treatment, the highest reduction (32–87 %) in PAH bioaccumulation was observed, while in SS10 treatment, this reduction ranged from 22 to 66 %. Results indicated that the effect of SSBC and SS on bioaccumulation of two- to three-benzene-ring PAHs was less than for four- to six-benzene-ring PAH compounds. Both SSBC and SS treatments showed the least effect on bioaccumulation of NA (two-ring PAH), while highest effect was observed for B(b)F (five-ring PAH) and B(ghi)P (six-ring PAH).
The structure (porosity and surface area) and element composition (C, N, and S) of SSBC are very different than those of SS (Table 1). These differences underpin the sorptive capacity of these materials. The degree of sorption of organic compounds like PAHs depends on the hydrophobic nature of the contaminants. Similarly, biochar surface properties including aromaticity and polarity can also play an important role in the adsoption of organic contaminants (Chen et al. 2008). Xu et al. (2011) reported that electrostatic attraction between biochars and organic contaminants can be one of the possible mechanisms for adsorption of organic compounds. The porosity of biochar based on its elemental compositions affects the sorption of PAHs. The high carbon content in biochar and the lowest polarity may be linked with larger sorption sites for PAH compounds. Basic characteristic differences such as pH, EC, DOC, available nutrients, and interactions among organic and inorganic co-contaminants could be acted as factors controlling the PAH sorption (Brennan et al. 2014).
The decreases in bioaccumulation of PAHs/PTEs in tomato fruits were consistent with the findings reported in our previous work (Waqas et al. 2014; Khan et al. 2014). Other research groups have also observed a decrease in As bioaccumulation in plant grown in biochar-amended soil (Beesley et al. 2013). The lower bioaccumulations of PAHs and PTEs in tomato grown in SSBC-amended soil as compared to the control and SS-amended soils could be linked with lower available fractions of these contaminants in SSBC-amended soil (Table 2). These findings demonstrated that SSBC addition into contaminated soil can minimize the possible health risk associated with PAH and PTE bioaccumulation and magnification into food chains.
Bioaccumulation of PTEs was significantly (P ≤ 0.05) increased in tomato grown in SS-amended soils (Fig. 3). SS10 increased PTE accumulation by As (×1.6), Cd (×2.1), Cu (×2.2), Pb (×2.0), and Zn (×2.4) (see SI). The addition of SSBC (all doses) significantly (P ≤ 0.01) decreased the accumulation of As, Cu, Pb, and Zn (but not Cd) in tomato (Fig. 3). SSBC10 decreased PTE accumulation by the following factors: As (×2.5), Cd (×1.3), Cu (×3.9), Pb (2.1), and Zn (×3.0) (see SI).
Bioaccumulation of PTEs in tomatoes grown in soil and soil amended with SS and SSBC. Error bars represent standard deviations (n = 4). Different letters indicate significant difference (P ≤ 0.05), while same letters indicate no significant difference. The horizontal lines indicate the maximum permissible limits set by SEPA (2005) for PTEs in food plants
The decreases in PTE bioaccumulation in tomato grown in SSBC-amended soil, while increases in SS-amended soil (Fig. 3) correspond to their availability in the respective soil solutions (Table 2). Strong linear relationships between available metal concentrations and their bioaccumulation into tomato were observed for As (r 2 = 0.915), Cu (r 2 = 0.980), Pb (r 2 = 0.875), and Zn (r 2 = 0.947) while Cd showed a weaker relationship (r 2 = 0.407) (Fig. 4).
Bioaccumulation factors (available PTE: accumulated PTE) were observed to be high for As (6.3) and Cd (5.9) but much lower for Cu (0.008), Pb (0.003), and Zn (0.002). The smaller pore size, higher pore volume, and higher surface area of the SSBC materials helped to reduce the mobility/bioavailability of PTEs. SSBC addition decreased the availability and bioaccumulation of PTEs which could be linked with increasing pH of soil; higher availability of DOC, P, and S; and complexion of metals and oxygen functional groups (Lehmann and Joseph 2009; Lu et al. 2014).
PTE and regulatory limits
Cu and Pb concentrations in soil were below their MPLs (Table 1) (SEPA 1995), and tomato Cu and Pb concentrations never exceed food MPLs regardless of treatment (Fig. 3). Although soil Zn concentration exceeded its MPL (Table 1) (SEPA 2005), tomato Zn concentrations never exceed its food MPLs (Fig. 3). The Cd concentration in soil exceeded its MPLs, while As soil concentration did not (Table 1) (SEPA 1995). However, both As (328 μg kg−1) and Cd (260 μg kg−1 (d.w)) in tomato grown in control soil exceeded their food MPLs (50 μg kg−1 dw and 200 μg kg−1 dw, respectively). Increasing SS application significantly (P < 0.05) increased all PTE concentrations in tomato. In contrast, increasing SSBC application significantly (P < 0.05) decreased PTE concentrations in tomato (Fig. 3). SS10 treatments greatly elevated As and Cd concentrations in tomato, these concentrations exceeding their MPLs by factors of × 100 and 2.7, respectively. In contrast, SSBC10 treatments markedly reduced As and Cd concentrations in tomato, As to within a factor of 2.5 of its food MPL and Cd to meet its food MPL (Fig. 3).
Conclusion
In conclusion, the application of SSBC to contaminated urban soil represented a promising means to decrease risks associated with SS application to soil, while increasing crop yield and improving food safety.
References
Ahmad M, Lee SS, Yang JE, Ro HM, Lee YH, Ok YS (2012) Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotox Environ Safe 79:225–231
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Ahmed HK, Fawy HA, Abdel-Hady ES (2010) Study of sewage sludge use in agriculture and its effect on plant and soil. Agric Biol J N Am 1(5):1044–1049
Baran S, Oleszczuk P (2003) The concentration of polycyclic aromatic hydrocarbons in sewage sludge in relation to the amount and origin of purified sewage. Pol J Environ Stud 12:523–529
Beesley L, Marmiroli M, Pagano L, Pigoni V, Fellet G, Fresno T, Vamerali T, Bandiera M, Marmiroli N (2013) Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci Total Environ 454–455:598–603
Brennan A, Jiménez EM, Alburquerque JA, Knapp CW, Switzer W (2014) Effects of biochar and activated carbon amendment on maize growth and the uptake and measured availability of polycyclic aromatic hydrocarbons (PAHs) and potentially toxic elements (PTEs). Environ Pollut 193:79–87
Cai C, Zhang Y, Reid BJ, Nunes LM (2012) Carcinogenic potential of soils contaminated with polycyclic aromatic hydrocarbons (PAHs) in Xiamen metropolis, China. J Environ Monit 14:3111–3117
Cao X, Ma L, Liang Y, Gao B, Harris W (2011) Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ Sci Technol 45:4884–4889
CEC (2000) Council of the European Community, Working Document on Sludge, 3rd Draft, Brussels, 27 April, 2011; P. 20
Chen Z, Chen C, Liu Y, Wu Y, Yang S, Lu C (1992) Study on soil background values in Fujian province. Chin J Environ Sci 13:70–75
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition on nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137–5143
Creamer RE, Rimmer DL, Black HIJ (2008) Do elevated soil concentrations of metals affect the diversity and activity of soil invertebrates in the long-term? Soil Use Manag 24:37–46
Dai J, Xu M, Chen J, Yang X, Ke Z (2007) PCDD/F, PAH and heavy metals in the sewage sludge from six wastewater treatment plants in Beijing, China. Chemosphere 66:353–361
EC (2001) Pollutants in urban wastewater and sewage sludge. Luxembourg: Office for Official Publications of the European Communities, UK
Fagbemi L, Khezami L, Capart R (2001) Pyrolysis products from different biomasses: application to the thermal cracking of tar. Appl Energy 69:293–306
Freddo A, Cai C, Reid BJ (2012) Environmental contextualization of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ Pollut 171:18–24
Hale SE, Lehmann J, Rutherford D, Zimmerman AR, Bachmann RT, Shitumbanuma V, ƠToole A, Sundqvist KL, Arp HPH, Cornelissen G (2012) Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ Sci Technol 46:2830–2838
Han Y, Boateng AA, Qi PX, Lim IM, Chang J (2013) Heavy metal and phenol adsorptive properties of biochars from pyrolyzed switchgrass and woody biomass in correlation with surface properties. J Environ Manag 118:196–204
Hossain MK, Strezov V, Chan KY, Ziolkowski A, Nelson PF (2010) Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 78:1167–1171
IBI (2012) The International Biochar Initiative standardized product definition and product testing guidelines for biochar that is used in soil. Final version (IBI-STD-01: revision date 15 May 2012). http://www.biochar-international.org/sites/default/files/Guidelines_for_ Biochar_That_Is_Used_in_Soil_Final.pdf. Accessed on 31.07.2012
Jeffery S, Verheijen FGA, Velde MVD, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agr Ecosyst Environ 144:175–187
Jiang J, Xu RK (2013) Application of crop straw derived biochars to Cu(II) contaminated Ultisol: evaluating role of alkali and organic functional groups in Cu(II) immobilization. Bioresour Technol 133:537–545
Khan S, Aijun L, Zhang S, Hu Q, Zhu YG (2008) Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J Hazard Mater 152:506–515
Khan S, Chao C, Waqas M, Arp HPH, Zhu YG (2013a) Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas Emissions from acidic paddy soil. Environ Sci Technol 47:8624–8632
Khan S, Wang N, Reid BJ, Freddo A, Cai C (2013b) Reduced bioaccumulation of PAHs by Lactuca satuva L. grown in contaminated soil amended with sewage sludge and sewage sludge derived biochar. Environ Pollut 175:64–68
Khan S, Reid BJ, Li G, Zhu YG (2014) Application of biochar to soil reduces cancer risk via rice consumption: a case study in Miaoqian village, Longyan, China. Environ Int 68:154–161
Kookana RS, Sarmah AK, Van Zwieten L, Krull E, Singh B (2011) Biochar application to soil: agronomic and environmental benefits and unintended consequences. Adv Agron 112:103–143
Latawiec AE, Reid BJ (2009) Beyond contaminated land assessment: on costs and benefits of bio accessibility prediction. Environ Int 35:911–919
Lehmann J, Joseph S (2009) Biochar for environmental management: an introduction. In: Lehmann J, Joseph S (eds) Biochar for Environmental Management: Science and Technology. Earthscan, London, pp 1–12
Lehmann A, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota- A review. Soil Biol Biochem 43:1812–1836
Liu X, Zhang A, Ji C, Joseph S, Bian R, Li L, Pan G, Paz-Ferreiro J (2013) Biochar’s effect on crop productivity and the dependence on experimental conditions—a meta-analysis of literature data. Plant Soil 373:583–594
Lu K, Yang X, Shen J, Robinson B, Huang H, Liu D, Bolan N, Pei J, Wang H (2014) Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric Ecosyst Environ 191:124–132
Lucchini P, Quilliam RS, DeLuca TH, Vamerali T, Jones DL (2014) Does biochar application alter heavy metal dynamics in agricultural soil? Agric Ecosyst Environ 184:149–157
Luo F, Song J, Xia W, Dong M, Chen M, Soudek P (2014) Characterization of contaminants and evaluation of the suitability for land application of maize and sludge biochars. Environ Sci Pollut Res 21(4):8707–8717
Marks EAN, Alcañiz JM, Domene X (2014) Unintended effects of biochars on short-term plant growth in a calcareous soil. Plant Soil 385(1–2):87–105
Méndez A, Gómez A, Paz-Ferreiro J, Gascó G (2012) Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 89:1354–1359
MEP (2007) Standard of soil quality assessment for exhibition sites. Ministry of Environmental Protection, the People Republic of China. (HJ 350-2007) (in Chinese). 2007; Accessed on 21.03.2013
Oleszczuk P, Zielińska A, Cornelissen G (2014) Stabilization of sewage sludge by different biochars towards reducing freely dissolved polycyclic aromatic hydrocarbons (PAHs) content. Bioresourc Technol 156:139–145
Qiao M, Cai C, Huang Y, Liu Y, Lin A, Zheng Y (2011) Characterization of soil heavy metal contamination and potential health risk in metropolitan region of northern China. Environ Monit Assess 172:353–365
Roca-Pérez L, Martínez C, Marcilla P, Boluda R (2009) Composting rice straw with sewage sludge and compost effects on the soil-plant system. Chemosphere 75:781–787
SEPA (1995) Environmental quality standard for soils. State Environmental Protection Administration, China. GB15618-1995, 1995
SEPA (2005) The limits of pollutants in food. State Environmental Protection Administration, China. GB2762-2005, 2005
Singh RP, Agrawal M (2008) Potential benefits and risks of land application of sewage sludge. Waste Manag 28:347–358
Smider B, Singh B (2014) Agronomic performance of a high ash biochar in two contrasting soils. Agric Ecosyst Environ 191:99–107
Wan Q, Yuan JH, Xu RK, Li XH (2014) Pyrolysis temperature influences ameliorating effects of biochars on acidic soil. Environ Sci Pollut Res 21(4):2486–2495
Waqas M, Khan S, Qing H, Reid BJ, Chao C (2014) The effects of sewage sludge and sewage sludge biochar on PAHs and potentially toxic element bioaccumulation in Cucumis sativa L. Chemosphere 105:53–61
Wei YL, Bao LB, Wu CC, He ZC, Zeng EY (2014) Association of soil polycyclic aromatic hydrocarbon levels and anthropogenic impacts in a rapidly urbanizing region: spatial distribution, soil-air exchange and ecological risk. Sci Total Environ 473–474:676–84
Xing B, Pignatello JJ (1996) Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol 30:1–11
Xu T, Lou LP, Luo L, Cao RK, Duan DC, Chen YX (2011) Effect of bamboo biochar on pentachlorophenol leachability and bioavailability in agricultural soil. Sci Total Environ 414:727–731
Acknowledgments
This research work was financially supported by the National High-Tech R&D Program of China (863 Program 2012AA 06A 204), National Natural Science Foundation of China (41271324), CAS-TWAS, and CAS Young International Scientist programs.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 167 kb)
Rights and permissions
About this article
Cite this article
Waqas, M., Li, G., Khan, S. et al. Application of sewage sludge and sewage sludge biochar to reduce polycyclic aromatic hydrocarbons (PAH) and potentially toxic elements (PTE) accumulation in tomato. Environ Sci Pollut Res 22, 12114–12123 (2015). https://doi.org/10.1007/s11356-015-4432-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4432-8