Abstract
To determine an optimal species pool for periphytic microfauna colonization surveys, a multivariate approach was used to identify the influential species from a raw dataset of periphytic microfauna. Samples were collected at two depths of 1 and 3 m in coastal waters of the Yellow Sea using a glass slide method. From the full 77-species dataset, a 23-species subset with sufficient information of the whole community was identified. The small subset maintained sufficient information of colonization pattern of entire raw communities (correlation coefficient >0.95). The colonization curves based the small subset well fitted the MacArthur-Wilson and logistic model equations in both species composition and individual abundance, respectively. Compared to the functional parameters based on the full dataset, the colonization rates (G) were significantly high and the time reaching 90 % equilibrium species number (T 90) significantly low (P < 0.05), while the growth rates (r) and the time reaching 50 % maximum abundance (T 50) showed no significant changes (P > 0.05) at both depths, respectively. The species richness, diversity, and evenness represented significantly closed linear relationships between the subset and the full dataset. The results suggest that the small subset might be used as a robust optimal species pool for colonization-based bioassessment surveys and allows developing a time-efficient protocol for marine monitoring programs using of periphytic microfauna.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Periphytic microfauna is an important component of benthic communities and plays necessary roles in energy transfer from bacteria and microalgae to the high trophic levels in microbial food webs (Azovsky 1988; Norf et al. 2009; Xu et al. 2011a, 2014a). With rapid responses to environmental changes, ease of sampling, and standardized methodologies for determining water quality status, microperiphyton has been considered as a useful bioindicator for monitoring programs to date (Cairns and Henebry 1982; Coppellotti and Matarazzo 2000; Xu et al. 2009, 2014b). Furthermore, colonization parameters of periphytic microfauna have proved to be a potentially robust indicator for assessing the loading capacity or self-purification capacity of an aquatic ecosystem for pollutant inputs (Cairns and Henebry 1982; Railkin 1995; Burkovskii and Mazei 2001; Xu et al. 2009; Zhang et al. 2013). However, the community/colonization-based bioassessments have traditionally been based on full species abundance datasets (Jiang et al. 2007; Xu et al. 2014a). This is time-consuming and laborious and significantly limits their use for monitoring research on large spatial scales.
Taxonomic sufficiency is a useful tool to reduce the time cost for monitoring research (Ellis 1985; Heino and Soininen 2007; Jiang et al. 2014). An effective approach is to identify a single taxonomic or ecological group as suitable surrogates that reflects the sensitivity of the entire species assemblage (Heino and Soininen 2007; Xu et al. 2011a; Jiang et al. 2014). Although the feasibility of these surrogates has been reported for many benthic faunas, studies on the utility of an optimal species pool of a full species set in a community for colonization-based bioassessment surveys has yet to be carried out (Stark et al. 2003; Xu et al. 2011b).
In this study, the step-best-matching analysis was used to identify an optimal species pool for colonization-based bioassessment using periphytic microfauna, based on a dataset that was collected in coastal waters of the Yellow Sea, near Qingdao, northern China. The aim of this study was to develop a feasible protocol for identifying an optimal species pool for marine bioassessment using periphytic microfauna.
Material and methods
Data collection
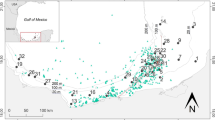
The study site was located in the harbor of the Olympic Sailing Center at Qingdao, northern China (Fig. 1). This is a typical coastal area of Yellow Sea with an average depth of ~8 m and an average tidal range of 3 m.
A total of 280 glass slides (17.5 cm2 for each) were used as artificial substrates for collecting microperiphyton communities at two depths of 1 and 3 m below the water surface. For each depth, a total of seven PVC frames were used to hold a total of 140 slides, 20 of which were randomly collected from each PVC frame at the time interval of 1, 3, 7, 10, 14, 21, and 28 days, during the study period. From both depths, samples were collected simultaneously.
Identification and enumeration of microperiphyton species were carried out following the methods outlined by Xu et al. (2009, 2011b). Taxonomic classification of ciliates was based on published keys and guides such as Song et al. (2009) and Berger (1999).
Data analyses
The colonization process of the periphytic ciliates can be followed by sampling replicate glass slides over time to follow the rate and magnitude of each species accrual process. Data can thus fit the colonization equilibrium model equation developed by MacArthur and Wilson (1967):
where S t = the taxon number at time t, S eq = the estimated equilibrium species number of the ciliate colonization, G = the colonization rate constant, and t = the colonization time. Three functional parameters (S eq, G, and T 90) were estimated using the software SIGMAPLOT (v12.5) (e.g., Zhang et al. 2013). Fitness tests were conducted to determine whether the taxon numbers observed at each time interval fit with the MacArthur-Wilson model at the 0.05 significance level. The regression analysis and fitness tests were carried out using the regression wizard-equation “Exponential Rise to Maximum (single, 2 parameter)” in the software SIGMAPLOT. The time taken to reach 90 % S eq (T 90) was calculated according to the equation T 90 = −ln (0.1)/G (e.g., Zhang et al. 2013).
The increase of individual abundance over total experimental phase was tested if it was fitted to the logistic model:
where A t = the individual abundance at time t, A max = the carrying capacity of individual abundance (maximum abundance), r = the growth rate constant, and a = the coefficient constant of initial individual abundance; T 50 = the time to 50 % A max. All parameters (e.g., A max, r, and T 50%) were estimated using the SIGMAPLOT. Fitness tests were to determine whether the individual abundance recorded at each time interval fit with the logistic model at the 0.05 significance level. The regression analysis and fitness tests were carried out using the regression wizard-equation “Sigmoidal (sigmoid, 3 parameter)” in the software SIGMAPLOT (Zhang et al. 2012).
Species diversity (H′), evenness (J′), and species richness (D) of samples were calculated as follows:
where H′ = observed diversity index, P i = proportion of the total count arising from the ith species, S = total number of species, and N = total number of individuals.
The step-best-matching analysis was used to exhaustively select the best matching subsets that were significantly correlated with the matrix of the full species abundance dataset from the same dataset by discarding the former selections using the submodule BVSTEP in the PRIMER v6.1.16 package (Clarke and Gorley 2006). The initial selection was define as best 1, second selection after discarding the first selection as best 2, third selection after discarding the first and second selection as best 3,…, and last selection with significant correlation with the matrix of full species set after peeling out selections 1–n from full species set as best n (Zhong et al. 2014). Using the selections identified at a 0.95 correlation level with the full species dataset, a smallest subset of species was determined as an optimal species pool with sufficient information of the full species set (Clarke and Gorley 2006).
The community structures of samples were analyzed using the PRIMER 6 package (Clarke and Gorley 2006). The distance-based redundancy analysis (dbRDA) of PERMANOVA+ was used to summarize temporal variations in community pattern at five taxonomic levels, using Bray-Curtis similarities from the fourth root-transformed species abundance data, respectively (Anderson et al. 2008). Mental analysis was used to summarize the relationships between pairs (the full species set and the optimal species pool) of similarity matrices using the Spearman rank correlation coefficients (p values) which were computed by the submodule RELATE (Clarke and Gorley 2006).
The nonparametric Kolmogorov-Smirnov test was used to evaluate the differences in the species number and colonization rates between depths of 1 and 3 m at the 0.05 significance level, using the software SPSS (v16.0) (e.g., Xu et al. 2012; Zhang et al. 2012). Data were log-transformed before analyzing.
Results
Full species dataset and optimal species pool
The species distribution, occurrence, and abundance in the full microperiphyton dataset studied were summarized in Supplementary Table S1 (see Appendix). A total of 77 microperiphyton species were recorded at two depths in coastal waters of the Yellow Sea, northern China, during the study period.
By successive selections of species that were generated by the BVSTEP procedure, a 23-species subset was identified from the full 77-species set (see Supplementary Table S1). Regression analyses revealed that the values of both species number and abundance represented a significantly closed relationship between the subset and the full set (R 2 > 0.98; Fig. 2).
Colonization curves based on small subset
Colonization curves in species composition based on both the small subset and the full set are plotted in Fig. 3a. Regression analyses demonstrated that all colonization curves in species composition were well fitted to MacArthur-Wilson model equation (Table 1). Three functional parameters—S eq, G, and T 90—were calculated following the MacArthur-Wilson model equation (Table 1). Compared to the values based on the full dataset, the colonization rates (G) were significantly high and the time reaching 90 % equilibrium species number (T 90 ) significantly low (P < 0.05) at both depths, respectively.
Growth curves based on both the small subset and the full set were plotted in Fig. 3b. Regression analyses revealed that all growth curves were well fitted to logistic model equation (Table 2). Three parameters—A max, r, and T 50—were obtained from the logistic model equation. However, the growth rates (r) and the time reaching 50 % maximum abundance (T 50) showed no significant changes (P > 0.05) between the two datasets.
Colonization dynamics in community structure based on small subset
The colonization dynamics in community pattern based on both the small subset and the full set during colonization period are shown in Fig. 4. The first dbRDA axis (dbRDA1) mainly separated the immature (1–7 days) communities (on the left) from the mature (10–28 days) (on the right), while the second dbRDA axis (dbRDA2) mainly discriminated the initial (1 day) (upper left) from the transitional (3–7 days) communities (lower left) (Fig. 4). It should be noted that almost all vectors (correlation coefficients >0.3) for microperiphyton species pointed toward the mature sample clouds (right; Fig. 4).
Mental analysis revealed that community pattern of the 23-species subset was significantly correlated with that of the full set (Spearman correlation coefficient >0.97; P < 0.05).
Temporal variations in biodiversity based on small subset
The linear relationships of the species richness, evenness, and diversity between the small subset and the full set are summarized in Fig. 5. The values of all three biodiversity indices represented a significantly closed relationship between the two datasets (R 2 > 0.91; Fig. 5).
Discussion
Many investigations have revealed that the colonization process of periphytic microfauna could be assigned into the initial, transitional exponential, and the equilibrium stages (Azovsky 1988; Burkovskii and Mazei 2001; Zhang et al. 2012, 2013). Zhang et al. (2012, 2013) reported that the periphytic ciliate assemblage showed significantly high similarities in community structure in the equilibrium stage but represented a significant difference from those during the initial/exponential stage (Zhang et al. 2012, 2013). Our previous works have also demonstrated that the colonization curves in species number and abundance well fitted the MacArthur-Wilson and logistic models, respectively.
In this study, a 23-species subset was identified from the full 77-species dataset of periphytic microfauna using the step-best-matching analysis. Multivariate analysis showed that this small subset maintained sufficient information of the entire communities and represented a consistent colonization dynamics with the full species dataset. Otherwise, regression analysis revealed that the community structural parameters, i.e., species richness, abundance, and biodiversity indices, represented a close linear relationship between the subset and the full species set. Thus, we propose that this subset might be used as an optimal species pool of periphytic microfauna for colonization-based bioassessment surveys.
It should be noted that the use of an optimal species pool as a surrogate for species-level identification offers two advantages: (1) more reliable since full species identification is complex and laborious, and (2) less time-consuming, reducing the costs of monitoring programs. Based on this work, the community pattern and biodiversity indices based on the optimal species pool may explain >97 and >90 % variation of those based on the full species dataset, respectively. Furthermore, the colonization rates (G) were significantly high and the time reaching 90 % equilibrium species number (T 90) significantly low based on this optimal species pool compared to the full species dataset (P < 0.05). This implies that it may shorten the exposure time to use the small species pool compared to the full species dataset and allows developing a time-efficient sampling strategy for marine bioassessment.
In summary, a 23-species subset with sufficient information of the whole community was identified. The small subset maintained sufficient information of colonization pattern of the full species set and may be used as a robust surrogate of entire periphytic microfauna in colonization surveys. This allows developing a time-efficient protocol for marine monitoring programs using of periphytic microfauna. However, further studies on a range of marine nichs and over large temporal/spatial scales are needed in order to verify this conclusion.
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER Guide to software and Statistical Methods. PRIMER-E Ltd, Plymouth
Azovsky AI (1988) Colonization of sand ‘islands’ by psammophilous ciliates: the effect of microhabitat size and stage of succession. Oikos 51:48–56
Berger H (1999) Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monographiae Biol 78:1–1080
Burkovskii IV, Mazei YA (2001) A study of ciliate colonization of unpopulated substrates of an estuary in the White Sea. Oceanology 41:845–852
Cairns J Jr, Henebry MS (1982) Interactive and noninteractive protozoa colonization processes. In: Cairns J Jr (ed) Artificial substrates. Ann Arbor Science Publishers, Ann Arbor, pp 27–30
Clarke KR, Gorley RN (2006) User Manual/Tutorial. PRIMER-E Ltd, Plymouth
Coppellotti O, Matarazzo P (2000) Ciliate colonization of artificial substrates in the Lagoon of Venice. J Mar Biol Ass UK 80:419–427
Ellis D (1985) Taxonomic sufficiency in pollution assessment. Mar Pollut Bull 16:459
Heino J, Soininen J (2007) Are higher taxa adequate surrogates for species-level assemblage patterns and species richness in stream organisms? Biol Conserv 137:78–89
Jiang J, Wu S, Shen Y (2007) Effects of seasonal succession and water pollution onthe protozoan community structure in an eutrophic lake. Chemosphere 66:523–532
Jiang Y, Xu H, Warren A (2014) Insights into discriminating environmental quality status based on a simplified taxonomic pool of ciliated protozoa in marine ecosystems. Sci Total Environ 468–469:663–670
MacArthur R, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton, p 203
Norf H, Arndt H, Weitere M (2009) Effects of resource supplements on mature ciliate biofilms: an empirical test using a new type of flow cell. Biofouling 25:769–778
Railkin AI (1995) Heterotrophic flagellates on artificial substrates in the White Sea. Cytology 37:951–957
Song W, Warren A, Hu X (2009) Free-living Ciliates in the Bohai and Yellow Seas, China. Science Press, Beijing
Stark JS, Riddle MJ, Simpson RD (2003) Human impacts in soft-sediment assemblages at Casey Station, East Antarctica: spatial variation, taxonomic resolution and data transformation. Aust Ecol 28:207–304
Xu H, Min GS, Choi JK, Jung JH, Park MH (2009) An approach to analyses of periphytic ciliate colonization for monitoring water quality using a modified artificial substrate in Korean coastal waters. Mar Pollut Bull 58:1278–1285
Xu H, Zhang W, Jiang Y, Min GS, Choi JK (2011a) An approach to identifying potential surrogates of periphytic ciliate communities for monitoring water quality of coastal waters. Ecol Indic 11:1228–1234
Xu H, Zhang W, Jiang Y, Zhu M, Al-Rasheid KAS, Warren A, Song W (2011b) An approach to determining sampling effort for analyzing biofilm-dwelling ciliate colonization using an artificial substratum in coastal waters. Biofouling 27:357–366
Xu H, Zhang W, Jiang Y, Zhu M, Al-Rasheid KAS (2012) Sampling sufficiency for analyzing taxonomic relatedness of periphytic ciliate communities using an artificial substratum in coastal waters. J Sea Res 72:22–27
Xu H, Zhang W, Jiang Y, Yang EJ (2014a) Use of biofilm-dwelling ciliate communities to determine environmental quality status of coastal water. Sci Total Environ 470–471:511–518
Xu W, Zhang W, Jiang Y (2014b) Do early colonization patterns of periphytic ciliate fauna reveal environmental quality status in coastal waters? Environ Sci Pullut Res 21:7097–7112
Zhang W, Xu H, Jiang Y, Zhu M, Al-Resheid KAS (2012) Colonization dynamics in trophic-functional structure of periphytic protist communities in coastal waters. Mar Biol 159:735–748
Zhang W, Xu H, Jiang Y, Zhu M, Al-Reshaid KAS (2013) Colonization dynamics of periphytic ciliate communities on an artificial substratum in coastal waters of the Yellow Sea. J Mar Biol Ass UK 93:57–68
Zhong X, Xu G, Wang Y, Xu H (2014) An approach to determination of functional species pool for community research. Ecol Indic 46:78–83
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 41076089), and Scholarship Award for Excellent Doctoral Student granted by Chinese Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Guangjian Xu and Xiaoxiao Zhong co-first authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 237 kb)
Rights and permissions
About this article
Cite this article
Xu, G., Zhong, X., Wang, Y. et al. An approach to determination of optimal species pool of periphytic microfauna in colonization surveys for marine bioassessment. Environ Sci Pollut Res 22, 7967–7972 (2015). https://doi.org/10.1007/s11356-015-4135-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4135-1