Abstract
The colonization dynamics in trophic-functional patterns of periphytic protist communities was studied in coastal waters of the Yellow Sea, northern China, from May to June, 2010. The periphytic protists represented different trophic-functional structures during colonization process. Only certain trophic-functional groups (e.g., photoautotrophs, algivores and non-selectives) occurred within the protist communities with low species number and abundance at the initial stage (1–3 days), while more trophic-functional groups (e.g., photoautotrophs, algivores, non-selectives and raptors) contributed to the communities with increased and peaked species number and abundance at the transitional (7–10 days) and equilibrium (14–28 days) stages, respectively. All heterotrophic groups were significantly fitted the MacArthur–Wilson model in colonization curves and represented higher species number and colonization rates at a depth of 1 m than at 3 m. These results may provide necessary understandings for ecological researches and monitoring programs using periphytic protists with different colonization ages in marine ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a primary component of biofilm, periphytic protists play a crucial role for energy flux and element cycling in many aquatic ecosystems (Fischer et al. 2002; Debenest et al. 2009; Kathol et al. 2009; Xu et al. 2011a). Pratt and Cairns (1985) classified the protists into six trophic-functional (hereafter functional) groups based on their feeding strategies: photoautotrophs (P), bacterivores (B), algivores (A), saprotrophs (S), raptors (R) and non-selectives (N). Photoautotrophic group (microalgae) is responsible for the bulk of primary production (Debenest et al. 2009; Duong et al. 2010; Xu et al. 2010). Heterotrophic protozoa can occur in high abundance and occurrence within microbial fauna, where they can form complex communities of many different taxonomic and functional types in both freshwater and marine ecosystems (Parry 2004; Gong et al. 2005; Früh et al. 2011; Kathol et al. 2011). As inhabitants of the sediment–water interface, heterotrophic consumers can potentially feed on both surface-attached and suspended prey, and thus, they can mediate the flow of both matter and energy from plankton to benthos in most aquatic ecosystems (Franco et al. 1998; Parry 2004; Kathol et al. 2009, 2011; Norf et al. 2009a, b; Xu et al. 2011b). Some bactivorous grazers can tolerate extreme environmental conditions and often play an important role in maintaining and improving water quality, mainly by their grazing activities (Patterson et al. 1989; Xu et al. 2010). Furthermore, because of their rapid responses to environmental changes, easy sampling and allowing standardization for spatiotemporal discriminations, periphytic protists have widely been used as robust bioindicators for monitoring programs and ecological conservations in aquatic environments (Gold et al. 2002; Khatoon et al. 2007; Risse-Buhl and Küsel 2009; Morin et al. 2010; Xu et al. 2011a).
The colonization of surfaces in coastal waters is advantageous for protozoan grazers (mainly ciliates) since the food supply (e.g., bacteria, algae) can be much more abundant in the biofilm than in the surrounding water (Geesey et al. 1978; Risse-Buhl and Küsel 2009). Protozoan grazers (e.g., groups B, A and R) associated with surfaces utilize a variety of food particles (e.g., bacteria, algae, flagellates, small ciliates and detritus particles) (Parry 2004; Scherwass et al. 2005). General modes of food acquisition in protozoa are either the concentration of food particles from plankton origin by filter feeders (e.g., groups B and A) or the active search for food particles in the biofilm by gulper feeders (e.g., groups B, A and R) (Franco et al. 1998; Hausmann 2002; Parry 2004; Norf et al. 2007, Norf and Weitere 2010; Kathol et al. 2011). Thus, the functional structure of the periphytic protist communities may represent significant changes during the initial colonization of biotopes due to the food supply (Kiørboe et al. 2004; Norf et al. 2007, 2009a; Wey et al. 2009; Früh et al. 2011). The colonization dynamics of periphytic protist communities, however, have been rarely investigated with respect to the functional structures although a number of relevant investigations on the colonization of the periphytic protist communities have been carried out (Beech and Landers 2002; Kiørboe et al. 2004; Duong et al. 2007; Risse-Buhl and Küsel 2009; Norf et al. 2007, 2009b; Wey et al. 2009; Morin et al. 2010; Früh et al. 2011).
In the present study, the colonization dynamics of the protist communities in functional structures were investigated using glass slide method in the coastal waters of the Yellow Sea, near Qingdao, northern China, from May to June, 2010. Our aims were as follows: (1) to document the functional composition of the periphytic protist communities; (2) to reveal the temporal variations in functional structure of periphytic protist communities during the colonization process; (3) to compare the colonization dynamics of periphytic protist communities in functional structures at different depths in coastal waters; and (4) to provide necessary understandings for both ecological researches and monitoring programs using periphytic protist communities with different colonization ages in marine ecosystems.
Materials and methods
Study area and sampling strategy
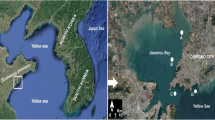
This study was carried out in the coastal waters of the Yellow Sea, near Qingdao, northern China, from May 18 to June 16, 2010 (Fig. 1). This coastal area is ~8 m deep with the following water conditions: water temperature, 14–19°C; pH ~ 8; salinity, 30–31 psu; transparency (Secchi depth), ~3 m; and dissolved oxygen, 6–7 mg l−1 during the study period. The glass slide systems were designed, deployed, anchored and sampled as described by Xu et al. (2009a, b).
A total of 140 glass slides (2.5 cm × 7.5 cm) were used as artificial substrata for collecting periphytic protist communities at two depths of 1 and 3 m below the water surface. For each depth, a total of seven PVC frames were used to hold a total of 70 slides, which were used as two parallel sampling replicates. A total of 7 sampling events were carried out, and samples were from different slides pooled. For each replicate, 5 slides were randomly collected from one PVC frame at the time interval of 1, 3, 7, 10, 14, 21 and 28 days, during the study period. From both depths, samples were collected simultaneously (Xu et al. 2011a). The slides were transferred into Petri dishes containing water from the sampling site and then stored in a cooling box before transporting to the laboratory within 2 h for identification and enumeration (Xu et al. 2009a).
Identification and enumeration
Identification and enumeration of periphytic protist species were carried out following the methods outlined by Xu et al. (2009a, b). Taxonomic classification of microalgae, ciliates and sarcodines was based on published keys and guides such as Steidinger and Tangen (1997), Song et al. (2009), Fan et al. (2010), Jiang et al. (2010) and Pan et al. (2010).
The enumeration of periphytic protists was carried out in vivo at a 100-fold magnification under an inverted microscope as soon as possible after sampling (generally within 4–6 h) (Xu et al. 2009a). For recovering all species colonizing the glass slides, one surface of an entire slide from a total of 10 slide-replicates was examined at each colonization period using bright-field illumination and occurrences were recorded. The enumeration of individual abundances was conducted as described by Xu et al. (2011a). The cell numbers were calculated to confirm the average abundance of the protist individuals (ind. cm−2).
Different periphytic protist species were assigned to the corresponding functional groups, which comprised photoautotrophs (P), bacterivores (B), algivores (A), raptors (R) and non-selectives (N), according to the literatures (Pratt and Cairns 1985; Fernandez-Leborans and Fernandez-Fernandez 2002; Xu et al. 2010). The different protist species recorded were assigned to the corresponding trophic-functional group taking into account the data from both the literatures (Fenchel 1969; Pratt and Cairns 1985; Fernandez-Leborans and Fernandez-Fernandez 2002; Xu et al. 2010) and direct observation (e.g., non-selectives Euplotes spp. feeding on both bacteria and algae and algivores Diophrys spp. mainly feeding on algae) (Table 4 in “Appendix”).
Data analyses
The colonization process in species number of each functional group of the periphytic protist communities was tested if it was fitted to the MacArthur and Wilson (1967) model:
where S t = the species number at time t; S eq = the estimated equilibrium species number of ciliate colonization; G = the colonization rate constant; and T 90% = the time taken for reaching 90% S eq. Three colonization parameters (S eq, G and T 90%) were estimated using the SIGMAPLOT. Fitness tests were conducted to determine whether the species numbers observed at each time interval fit with the MacArthur–Wilson model at the 0.05 significance level.
The increase in individual abundance of each functional group over total experimental phase was tested if it was fitted to the logistic model:
where A t = the individual abundance at time t; A max = the carrying capacity of individual abundance (maximum abundance); r = the grow rate constant; a = the coefficient constant of initial individual abundance; and T 50% = the time to 50% A max. All parameters (e.g., A max and T 50%) were estimated using the SIGMAPLOT. Fitness tests were to determine whether the individual abundance recorded at each time interval fit with the logistic model at the 0.05 significance level (Norf et al. 2007).
Species diversity (H′), evenness (J′) and species richness (D) of five functional groups in samples were calculated as follows:
where H′ = observed diversity index; P i = proportion of the total count arising from the ith species; S = total number of species; and N = total number of individuals.
All multivariate analyses were conducted using PRIMER v6.1 and the PERMANOVA+ for PRIMER (Clarke and Gorley 2006; Anderson et al. 2008). Bray–Curtis similarity matrices were computed on fourth root-transformed data. The separate clusters of samples were assigned by the routine CAP (canonical analysis of principal coordinates) of PERMANOVA+. The species distributions at different colonization stages were analyzed using the routine MDS. The significance of correlations/differences in colonization patterns of periphytic protist communities between two water depths was tested using the routine RELATE/PERMANOVA (Clarke and Gorley 2006). The contributions of ciliate species to the ciliate communities were evaluated using the SIMPER routine (Clarke and Gorley 2006).
Pearson correlation analyses were carried out to reveal the correlations among functional groups in abundance using the software SPSS (version 16.0). Data were log-transformed before analyzing. The nonparametric Kolmogorov–Smirnov test was used to evaluate the differences in the species number and colonization rates between depths of 1 m and 3 m at the 0.05 level.
Results
Taxonomic composition and functional structure
A total of 93 protist species (76 ciliates, 2 sarcodines, 14 diatoms and 1 xanthophytes) were recorded from both depths over the survey period, of which 86 (69 ciliates, 14 diatoms and 1 xanthophytes) and 79 (62 ciliates, 2 sarcodines, 14 diatoms and 1 xanthophytes) taxa were identified at depths of 1 and 3 m, respectively.
The functional composition of the periphytic protist communities collected at two depths of 1 and 3 m in the coastal waters of the Yellow Sea during the study period is summarized in “Appendix” (Table 4). The 93 protist species represented five functional groups: 15 photoautotrophs, 43 algivores, 19 bacterivores, 11 raptors and 5 non-selectives (Table 1). The algivores, bacterivores and photoautotrophs were the most common functional forms, accounting for 47, 20 and 17% at a depth of 1 m, and for 41, 20 and 19% at a depth of 3 m, respectively, of the species recorded, compared to the other two groups (Table 4 in “Appendix”). It should be noted that the algivores have higher diversity in the periphytic protist communities at 1 m than at 3 m in species number (40 vs. 32).
Temporal variations in species number and abundance
The temporal variations in species number and abundances of periphytic protist communities and all five functional groups with colonization times of 1, 3, 7, 10, 14, 21 and 28 day at two depths of 1 and 3 m during the study period are shown in Fig. 2. In terms of species number, the group P predominated the microbial communities in the initial periods (1-days), while the total protists and heterotrophic groups peeked in the colonization periods of more than 14 days at both depths (Fig. 2a, c, e). Regression fitness test showed that besides the photoautotrophs, the total protists and all heterotrophic groups were well fitted the MacArthur–Wilson model, especially the algivorous grazers (Table 1). Furthermore, the species number and colonization rates (i.e., G values) of total protists and all four heterotrophic functional groups were definitely higher at a depth of 1 m than at 3 m (P < 0.05). For example, the G values of the group A were 0.16 and 0.08 at depths 1 and 3 m, respectively (Table 1).
Colonization dynamics of species number (a, c, e) and abundance (b, d, f) of total protists (a, b) and five functional groups of periphytic protist communities collected at two depths of 1 m (c, d) and 3 m (e, f) in the coastal waters of the Yellow Sea, northern China, during the study period. P, photoautotrophs; B, bacterivores; A, algivores; R, raptors; N, non-selectives
With regard to abundance, the total protists and all functional groups peaked in the periods of more than ~14 days at both depths (Fig. 2c, d). Regression fitness test showed that the total protists and all five trophic-functional groups were well fitted the logistic model (Table 2). It should be noted that the estimated maximum abundances (A max) of total protists and functional groups, except group B, were higher at a depths of 1 m than at 3 m (P < 0.05) (Table 2).
Pearson correlation analyses revealed that the groups A and R are significantly positively correlated with the autotrophic group P, while the group N significantly positively related to the groups A and B (P < 0.05) (Table 3).
Temporal variations in functional structures
The colonization patterns of the periphytic protist communities in terms of relative species number and abundance at two depths were summarized in Fig. 3. CAP ordinations result the colonization process of both autotrophic and heterotrophic assemblages of periphytic protist communities in falling into three phases, respectively: The first canonical axis (CAP 1) separated the initial stage (1–3 days) (left) from the equilibrium stage (14–28 days) (right), while the second canonical axis (CAP 2) discriminated the samples at both stages above (upper) from those at the transitional stage (lower) (Fig. 4). PERMANOVA test revealed that there were significant differences in community patterns of both autotrophic and heterotrophic assemblages between each pair of stages (P < 0.05). However, it should be noted that no significant difference was found in colonization patterns of periphytic protist communities between two depths (P > 0.05).
Temporal variations of relative species number (a, c) and relative abundance (b, d) of five functional groups of periphytic protist communities collected at two depths of 1 m (a, b) and 3 m (c, d) in the coastal waters of the Yellow Sea, northern China, during the study period. In Fig. 4b and d, vertical scale beginning from 95% instead of zero for showing clear patterns of functional pattern in relative abundance. See Fig. 2 for abbreviations
Canonical analysis of principal coordinates (CAP) on Bray–Curtis similarities for the average species-abundance data of heterotrophs (a) and photoautotrophs (b) in 14 samples from depths of 1 and 3 m during the study period, respectively, and correlations of species numbers of five functional groups with the two CAP axes. See Fig. 2 for abbreviations. 1–28, colonization times; stage I, initial stage; stage II, transitional stage; stage III, equilibrium stage
Vector overlay of Spearman correlations of species number of all five functional groups with the CAP axes was also shown in Fig. 4. The autotrophic group predominanted the periphytic protist communities from the initial stage to the next period, while the heterotrophic assemblages showed high diversity at the equilibrium stages in terms of species number (Figs. 3, 4).
Distribution patterns of protist communities within different colonization periods
Species distribution patterns and the dominant/common taxa with a cumulative contribution of 90% to the periphytic protist communities at the three colonization stages were summarized in Fig. 5. The data point clouds of different functional groups with similar distributions represented a simple model comprising partly some functional groups (mainly the groups P, A and N) with low species number at the initial stage, followed by a complex trophic-functional pattern including all five functional groups with increasing species number at the next stages (Fig. 5).
Multidimensional scaling (MDS) ordination of dominant/common protist species at depths of 1 m (a, b, c) and 3 m (d, e, f) at the initial (1–3 days) (a, d), transitional (7–10 days) (b, e) and equilibrium stage (14–28 days) (c, f), showing similarities of species distributions. See Fig. 2 for abbreviations
SIMPER analyses showed that these dominant/common functional species with a cumulative contribution of 90% to the communities at each stage represented higher species number at a depth of 1 m than at 3 m at all three stages and accounted for increasing proportions of total species composition from the initial stage to the equilibrium (Fig. 6). For example, these dominant/common species showed higher species number at a depth of 1 m (11, 24 and 40 species) than at 3 m (10, 16 and 29 species) at the initial, transitional and equilibrium stages, respectively (Fig. 5).
Temporal variations in species richness (D) (a, d, g, j, m, p), diversity (H′) (b, e, h, k, n, q) and evenness (J′) (c, f, i, l, o, r) of total protist communities (a, b, c), and functional groups P (d, e, f), A (g, h, i), B (j, k, l), R (m, n, o) and N (p, q, o) at two depths of 1 and 3 m in the coastal waters of the Yellow Sea, northern China, during the study period. See Fig. 2 for abbreviations
Temporal variations in diversity patterns within different colonization periods
Temporal variations in species richness diversity and evenness of total protist communities and all five functional groups at two depths of 1 and 3 m in the coastal waters of the Yellow Sea during the study period were summarized in Fig. 6. In terms of species richness, groups P, A and B showed a decrease trend from the initial phase to the equilibrium phase, while the total protists and groups R and N represented a similar pattern, that is, increasing at initial phase and peaked after transitional phase (Fig. 6). In terms of species diversity and evenness, the total protists and groups P, A and B showed a similar pattern, that is, decrease from initial or transitional phase to equilibrium phase, while the values of groups R and N increased at initial phase and peaked after transitional phase (Fig. 6). Nonparametric test revealed that the species richness dynamics of total protists at a depth of 1 m were significantly different from those at a depth of 3 m (P < 0.05), while no significant differences in all three diversity indices were revealed with all five functional groups (P > 0.05).
Discussion
Simplifying community structure by grouping in functional groups allows the investigation of ecosystem-level processes mediated by functionally similar community members at different temporal scales (Pratt and Cairns 1985; Fernandez-Leborans 2001; Xu et al. 2010). In the present study, the periphytic protist communities in the coastal waters of the Yellow Sea represented five functional groups: photoautotrophs, algivores, bacterivores, raptors and non-selectives. The groups A, B, P and N were the primary contributors to the periphytic protist communities compared to the group R mainly blooming in the equilibrium communities. SIMPER analyses showed that these dominant/common functional taxa accounted for increasing proportions of total species number along colonization process at both depths, which were definitely higher at a depth of 1 m than at 3 m. This finding was consistent with the previous reports on benthic microfauna even though a large number of species may be present, only a few usually were the primary contributor to the communities (Cairns and Henebry 1982; Pratt and Cairns 1985; Azovsky 1988; Have 1993; Fernandez-Leborans and Fernandez-Fernandez 2002; Burkvskii et al. 2011). It should be noted that the consumers (A and R) were found to be significantly correlated with the group P in terms of abundance. This might imply that these functional groups have similar colonization rates due to trophic–rank relationships among them.
Microperiphyton colonization of a new artificial substratum is a dynamic process, the primary stage of which generally follows the succession: At first, bacteria colonize the slide surface, followed by diatoms and autotrophic flagellates; the next groups are bacterivorous, vagile species of amoebae and ciliates; and then larger species with a broader feeding spectrum and sessile feeders occur in freshwater biotopes (Railkin 1995; Franco et al. 1998; Strüder-Kypke 1999). Previous studies have demonstrated that during the primary colonization process, the number of species generally increases and then equilibrates, following the MacArthur and Wilson (1967) equilibrium model equation (Wang et al. 1985; Xu et al. 2005, 2009b). Once equilibrium of immigration ends, the early and late interactive phases followed, during which internal factors such as competition and predation pressure become more important (Cairns and Henebry 1982; Railkin 1995). However, it should be noted that the classic MacArthur–Wilson model does not boil down only to a saturating curve of accumulated species number, that is, it also presupposes that number of species is determined by a dynamic balance between immigration and extinction, which rates depend on the richness achieved to the moment. In addition, it also should be noted that the diversity indices generally decreased as the species number increased during colonization process in our study. This may be due to the increasing competitive exclusion among microperiphyton species as more species colonized the substrates.
In terms of functional structure with our study, multivariate analysis demonstrated that the colonization process of periphytic protist communities in coastal waters might be assigned into three phases including the initial stage (1–3 days), transitional stage (7–10 days) and the equilibrium stage (14–28 days). This finding was similar to the previous investigations on the colonization process of the benthic ciliate fauna in an estuary of the White Sea (Azovsky 1988; Have 1993; Burkovskii and Mazei 2001; Burkvskii et al. 2011). During the three colonization stages, the periphytic protist communities represented significantly differences in functional structure between each pair of stages. These findings were consistent with the previous reports on freshwater protist colonization dynamics (Railkin 1995; Strüder-Kypke 1999). Additionally, it should be noted that the abundances in 28-day samples collected at 1 m dropped sharply to low level, mainly due to the intensive immigration of metazoan consumers (e.g., copepods, coelenterates, annelids and barnacles). This implies that the protist community structure changes significantly after 28-day exposure time. Thus, our results showed that the colonization ages of more than 10 days but less than 28 days may be considered sufficiently mature communities of periphytic protists in the coastal waters of the Yellow Sea.
Colonization dynamics and functional parameters are usually used to predict the loading capacity or assimilative capacity of an aquatic ecosystem for contaminant inputs (Wang et al. 1985; Xu et al. 2005). Among the functional parameters, the S eq value (estimated equilibrium species number) is generally negatively correlated with concentrations of organic pollutants and toxic levels, while the G value (colonization rate constant) is generally high in the waters with lower environmental stress (Cairns and Henebry 1982; Xu et al. 2002, 2009b). Based on our data, besides the autotrophic assemblage, the colonization curves and the increase in abundance of all heterotrophic groups were significantly fitted the MacArthur–Wilson model and a logistic equation. These findings were consistent with the previous reports (Norf et al. 2007; Xu et al. 2005, 2009b, 2011a). It should also be noted that although the periphytic protist communities showed no significant difference in functional structure, the species numbers and colonization rates of heterotrophic functional groups were definitely higher at a depth of 1 m than at 3 m. This may be mainly due to the week-sunlight conditions and less food supply at a depth of 3 m in the water columns with a transparency of ~3 m. This may be also the reason that both accumulative species number and individual abundance were higher at a depth of 1 m than 3 m during the study period.
Recent investigations focused on freshwater have demonstrated that the planktonic and benthic resources play an important role in shaping the functional structure of biofilm-dwelling ciliates (Norf and Weitere 2010; Früh et al. 2011; Kathol et al. 2011). For example, heterotrophic consumers in biofilm are often dominated by suspension feeding (planktivorous) groups, such as peritrich ciliates, rather than groups (benthivores or periphytivores) feeding from substratum (Parry 2004; Kathol et al. 2011). Thus, the functional groups of benthic consumers should be further classified by their food resources when exploring either their role in controlling the composition of both plankton and periphyton or responses to enrichment with bacteria/algae with different resource.
It should be noted that because of the constraints of the methods used, not all protist groups could be investigated so no data are available for heterotrophic flagellates although they are very common and divers members of marine periphyton. Additionally, this study was conducted based on one dataset of the coastal waters collected in summer. Thus, further studies, however, on a range of marine waters and over extended time periods are needed in order to verify this conclusion.
In summary, the periphytic protists represented different trophic-functional structures during colonization process. Only certain trophic-functional groups (e.g., photoautotrophs, algivores and non-selectives) occurred within the protist communities with low species number and abundance at the initial stage (1–3 days), while more trophic-functional groups (e.g., photoautotrophs, algivores, non-selectives and raptors) contributed to the communities with increased and peaked species number and abundance at the transitional (7–10 days) and equilibrium (14–28 days) stages, respectively. All heterotrophic groups were significantly fitted the MacArthur–Wilson model in colonization curves and represented higher species number and colonization rates at a depth of 1 m than at 3 m. These results may provide necessary understandings for ecological researches and monitoring programs using periphytic protists with different colonization ages in marine ecosystems.
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER guide to software and statistical methods. PRIMER-E Ltd, Plymouth
Azovsky AI (1988) Colonization of sand “islands” by psammophilous ciliates: the effect of microhabitat size and stage of succession. Oikos 51:48–56
Beech CD, Landers SC (2002) Ciliated protozoan colonization of substrates from Dauphin Island, Alabama. Europ J Protistol 38:83–89
Burkovskii IV, Mazei YA (2001) A study of ciliate colonization of unpopulated substrates of an estuary in the White Sea. Oceanology 41:845–852
Burkvskii IV, Mazei YA, Esaulov AS (2011) Influence of the period of existence of a biotope on the formation of the species structure of a marine psammophilous ciliate community. Russ J Mar Biol 37:177–184
Cairns J Jr, Henebry MS (1982) Interactive and noninteractive protozoa colonization process. In: Cairns J Jr (ed) Artificial substrates. Ann Arbor Science Publishers, Ann Arbor, pp 23–70
Clarke KR, Gorley RN (2006) User manual/tutorial. PRIMER-E Ltd, Plymouth
Debenest T, Pinelli E, Coste M, Silvestre J, Mazzella N, Madigou C, Delmas F (2009) Sensitivity of freshwater periphytic diatoms to agricultural herbicides. Aquat Toxicol 93:11–17
Duong TT, Feurtet-Mazel A, Coste M, Dang DK, Boudou A (2007) Dynamics of diatom colonization process in some rivers influenced by urban pollution (Hanoi, Vietnam). Ecol Indic 7:839–851
Duong TT, Morin S, Coste M, Herlory O, Feurtet-Mazel A, Boudou A (2010) Experimental toxicity and bioaccumulation of cadmium in freshwater periphytic diatoms in relation with biofilm maturity. Sci Total Environ 408:552–562
Fan X, Chen X, Song W, AL-Rasheid KAS, Warren A (2010) Two new marine scuticociliates, Sathrophilus planus n. sp. and Pseudoplatynematum dengi n. sp., with improved definition of Pseudoplatynematum (Ciliophora, Oligohymenophora). Eur J Protistol 46:212–220
Fenchel T (1969) The ecology of marine microbenthos. IV. Structure and function of the benthic ecosystem, its chemical and physical factors and the microfauna communities with special reference to the ciliated protozoa. Ophelia 6:1–182
Fernandez-Leborans G (2001) Relative importance of protozoan functional groups in three marine sublittoral areas. J Mar Biol Ass UK 81:735–750
Fernandez-Leborans G, Fernandez-Fernandez D (2002) Protist functional groups in a sublittoral estuarine epibenthic area. Estuaries 25:382–392
Fischer H, Sachse A, Steinberg CEW, Pusch M (2002) Differential retention and utilization of dissolved organic carbon by bacteria in river sediments. Limnol Oceanogr 47:1702–1711
Franco C, Esteban G, Téllez C (1998) Colonization and succession of ciliated protozoa associated with submerged leaves in a river. Limnologica 28:275–283
Früh D, Norf H, Weitere M (2011) Response of biofilm-dwelling ciliate communities to enrichment with algae. Aquat Microb Ecol 63:299–309
Geesey GG, Mutch R, Costerton JW, Green RB (1978) Sessile bacteria—important component of microbial-population in small mountain streams. Limonol Oceanogr 23:1214–1223
Gong J, Song W, Warren A (2005) Periphytic ciliate colonization: annual cycle and responses to environmental conditions. Aquat Microb Ecol 39:159–170
Gold C, Reurtet-Mazel A, Coste M, Boudou A (2002) Field transfer of periphytic diatom communities to assess short-term structural effects of metals (Cd, Zn). Water Res 36:3654–3664
Hausmann K (2002) Food acquisition, food ingestion and food digestion by protests. Jpn J Protozool 35:85–95
Have A (1993) Effects of area and patchiness on species richness: an experimental archipelago of ciliate microcosms. Oikos 66:493–500
Jiang J, Zhang Q, Warren A, Al-Rasheid KAS, Song W (2010) Morphology and SSU rRNA gene-based phylogeny of two marine Euplotes species, E. orientalis spec. nov. and E. raikovi Agamaliev, 1966 (Ciliophora, Euplotida). Eur J Protistol 46:121–132
Kathol M, Norf H, Arndt H, Weitere M (2009) Effects of temperature increase on the grazing of planktonic bacteria by biofilm-dwelling consumers. Aquat Microb Ecol 55:65–79
Kathol M, Fischer H, Weitere M (2011) Contribution of biofilm-dwelling consumers to pelagic-benthic coupling in a large river. Freshw Biol 56:1017–1230
Khatoon H, Yusoff FM, Banerjee S, Shariff M, Mohamed S (2007) Use of periphytic cyanobacterium and mixed diatoms coated substrate for improving water qualily, survival and growth of Penaeus monodon Fabricius postlarvae. Aquaculture 27:196–205
Kiørboe T, Grossart H-P, Ploug H, Tang K, Auer B (2004) Particle-associated flagellates: swimming patterns, colonization rates, and grazing on attached bacteria. Aquat Microb Ecol 35:141–152
MacArthur R, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton, pp 1–203
Morin S, Pesce S, Tlili A, Coste M, Montuelle B (2010) Recovery potential of periphytic communities in a river impacted by a vineyard watershed. Ecol Indic 10:419–426
Norf H, Weitere M (2010) Resource quality and seasonal background alter warming effects on communities of biofilm ciliates. FEMS Microbiol Ecol 74:361–370
Norf H, Arndt H, Weitere M (2007) Impact of local temperature increase on the early development of biofilm-associated ciliate communities. Oecologia 151:341–350
Norf H, Arndt H, Weitere M (2009a) Responses of biofilm-dwelling ciliate communities to planktonic and benthic resource enrichment. Microb Ecol 57:687–700
Norf H, Arndt H, Weitere M (2009b) Effects of resource supplements on mature ciliate biofilms: an empirical test using a new type of flow cell. Biofouling 25:769–778
Pan H, Huang J, Hu X, Fan X, Al-Rasheid KAS, Song W (2010) Morphology and SSU rRNA gene sequences of three marine ciliates from Yellow Sea, China, including one new species, Uronema heteromarinum nov. spec. (Ciliophora, Scuticociliatida). Acta Protozool 49:45–59
Parry JD (2004) Protozoan grazing of freshwater biofilms. Adv Appl Microbiol 54:167–196
Patterson DJ, Larsen J, Corliss JO (1989) The ecology of heterotrophic flagellates and ciliate living in marine sediments. Prog Protistol 3:185–277
Pratt J, Cairns J Jr (1985) Functional groups in the Protozoa: roles in differing ecosystems. J Protozool 32:415–423
Railkin AI (1995) Heterotrophic flagellates on artificial substrates in the White Sea. Cytology 37:951–957
Risse-Buhl U, Küsel K (2009) Colonization dynamics of biofilm-associated ciliate morphotypes at different flow velocities. Eur J Protistol 45:64–76
Scherwass A, Fischer Y, Arndt H (2005) Detritus as a potential food source for protozoans: utilization of fine particulate plant detritus by a heterotrophic flagellate, Chilomonas paramecium, and a ciliate, Tetrahymena pyriformis. Aquat Ecol 39:439–455
Song W, Warren A, Hu X (2009) Free-living ciliates in the Bohai and Yellow Seas, China. Science Press (in both Chinese and English), Beijing, pp 1–518
Steidinger K, Tangen K (1997) Identifying marine phytoplankton. Academic Press, San Diego
Strüder-Kypke MC (1999) Periphyton and sphagnicolous protists of dystrophic bog lakes (Brandenburg, Germany). I. Annual cycles, distribution and comparison to other lakes. Limnologica 29:393–406
Wang J, Yuan Y, Shen Y (1985) Data handling in studying the process of protozoan colonization by means of PFU method. Acta Hydrobiol Sin 9:344–350
Wey JK, Norf H, Arndt H, Weitere M (2009) Role of dispersal in shaping communities of ciliates and heterotrophic flagellates within riverine biofilms. Limnol Oceanogr 54:1615–1626
Xu M, Cao H, Xie P, Deng D, Feng W, Xu J (2005) Use of PFU protozoan community structural and functional characteristics in assessment of water quality in a large, highly polluted freshwater lake in China. J Environ Monit 7:670–674 (In Chinese, with English summary)
Xu H, Min GS, Choi JK, Kim SJ, Jung JH, Lim BJ (2009a) An approach to analyses of periphytic ciliate communities for monitoring water quality using a modified artificial substrate in Korean coastal waters. J Mar Biol Assoc UK 89:669–679
Xu H, Min GS, Choi JK, Jung JH, Park MH (2009b) An approach to analyses of periphytic ciliate colonization for monitoring water quality using a modified artificial substrate in Korean coastal waters. Mar Pollut Bull 58:1278–1285
Xu H, Warren A, AL-Rasheid KAS, Zhu M, Song W (2010) Planktonic protist communities in semi-enclosed mariculture waters: temporal dynamics of functional groups and their responses to environmental conditions. Acta Oceanol Sin 29:106–115
Xu H, Zhang W, Jiang Y, Zhu M, Al-Rasheid KAS, Warren A, Song W (2011a) An approach to determining the sampling effort for analyzing biofilm-dwelling ciliate colonization using an artificial substratum in coastal waters. Biofouling 27:357–366
Xu H, Zhang W, Jiang Y, Min GS, Choi JK (2011b) An approach to identifying potential surrogates of periphytic ciliate communities for monitoring water quality of coastal waters. Ecol Indic 11:1228–1234
Acknowledgments
This work was supported by “The Natural Science Foundation of China” (project number: 41076089). Our special thanks are due to Prof. Weibo Song, Laboratory of Protozoology, Institute of Evolution and Marine Biodiversity, Ocean University of China, for his helpful discussions during the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kühl.
Appendix
Appendix
Txonomic and trophic-functional data (Table 4).
Rights and permissions
About this article
Cite this article
Zhang, W., Xu, H., Jiang, Y. et al. Colonization dynamics in trophic-functional structure of periphytic protist communities in coastal waters. Mar Biol 159, 735–748 (2012). https://doi.org/10.1007/s00227-011-1850-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1850-0