Abstract
Phthalic acid ester (PAE) pollution to soil can lead to phytotoxicity in plants and potential health risks to human being. Dibutyl phthalate (DBP) as a kind of PAE has a large usage amount and large residues in soil. To analyze antioxidant responses of plants to DBP stress, effects of varying DBP concentrations on cucumber seedlings growth had been investigated. Malonaldehyde (MDA), hydrogen peroxide (H2O2), chlorophyll, proline, glutathione (GSH), and oxidized glutathione (GSSH) contents and activities of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and peroxidase (POD) were studied. The results showed that H2O2 content increased in cucumber seedlings with the increase of DBP concentration. The chlorophyll content in the higher DBP significantly declined compared to the control. In the present study, a disturbance of the GSH redox balance was evidenced by a marked decrease in GSH/GSSG ratio in cucumber seedlings subjected DBP stress. Our results indicated that DBP treatment not only inhibited antioxidant capacity and antioxidant enzyme activity in seedlings’ leaves but might also induce chlorophyll degradation or reduce the synthesis of chlorophyll. Moreover, it could also enhance the accumulation of reactive oxygen species (ROS) which induced membrane lipid peroxidation. DBP also altered the ultrastructure of mesophyll cells, damaged membrane structure of chloroplast and mitochondrion, and increased the number and size of starch grains in chloroplasts reducing the photosynthetic capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalic acid esters (PAEs) have toxic effects on the human body and environment (Api 2001). PAEs which can destroy human or animal reproductive and genetic function through the food chain are known as environmental hormone chemicals, and the cumulative effect is obvious (Ma et al. 2013). These suggested that PAE accumulation might produce irreversible adverse effect on life reproduction and even on the continuation of species (Chen et al. 2008). As a plasticizer, PAE is widely used in the world (Ma et al. 2013). Since 1990, phthalate has been suspected of its involvement in endocrine disorder (Sultan et al. 2001). Some studies also showed a decrease in fetal testicular testosterone production and significant loss of male characteristics in mice exposed to high levels of dibutyl phthalate and phthalic acid isooctyl (two representative phthalate compounds) (Gray et al. 2000; Pan et al. 2006). These have confirmed that phthalate compounds have reproductive and developmental toxic effects to the animal (Howdeshell et al. 2008; Kondo et al. 2006; Lovekamp-Swan and Davis 2003), and also, they have the same interference effect in the human endocrine (Latini 2005; Lyche et al. 2009; Matsumoto et al. 2008). Further study has reported negative effects of prenatal exposure to low molecular weight phthalate esters, namely, at very low concentrations, they are likely to cause attention deficit hyperactivity disorder (ADHD) in children (Engel et al. 2010). PAEs’ interference with reproductive endocrine system has also been reported in human and animals in the wild (Chen et al. 2008). These reports have more confirmed that exposure to phthalate has the potential chronic effects on human health. The potential routes for human exposure to phthalates include inhalation, skin absorption, dietary intake, and so on (Guo and Kannan 2011; Schettler 2006; Wormuth et al. 2006). For the general public, the diet is a main route of contact with phthalate (Colacino et al. 2010; Fromme et al. 2007). However, in recent years, along with the rural plastic canopy promotion and application, the entry of PAE into agricultural system became deeper. Now, the effect of PAEs on crop has attracted global attention. There are six kinds of PAEs, dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP), phthalate (2-ethylhexyl) ester (DEHP), dioctyl phthalate (DOP), and butyl benzyl phthalate (BBP), that have been listed by the US EPA in the “priority monitoring pollutant list” (Wang et al. 2013), and they are called “the second global polychlorinated biphenyl (PCB) pollutants” (Wei and Xu 2005).
DBP is a kind of important PAE, and one of the main plasticizers of plastics industry, which was widely used in plastic bags, blood storage bags, intravenous catheter, children’s toys, and some drugs and pesticides (Mo et al. 2008). Because of the connection of DBP and the instability of carriers, DBP in the process of production and use will continue to be released into the air, water, soil, and biological body (Yana et al. 2014). DBP could enter into human body through breathing, drinking water, food and skin contact (cosmetics), and other ways causing potential danger to health (Kong et al. 2012). The most commonly found PAE in the soil is DBP (Kong et al. 2012; Wang et al. 2003). However, there is rare report on the damage effect of DBP to the plant.
Cucumber, one of the most common vegetables, is popular among consumers for its fresh crisp taste, nutritional value, and health care function (Wang et al. 2013). It is one of the most widely cultivated vegetable crops in the world (Wan et al. 2010). This study used cucumber as a test crop to detect the influence of different concentrations of DBP (0, 30, 50, 100, and 200 mg L−1) on cucumber seedlings. So far, few literatures have reported the influence of DBP on cucumber. Thus, this study is the first step toward elucidation of DBP effects on cucumber seedlings.
Materials and methods
Materials
The cucumber seeds (Cucumis sativus L., cv. “Jinyan” no. 4) were obtained from the Academy of Agricultural Sciences, Tianjin, China. DBP (99.5 % purity) was purchased from Tianjin Fuyu Chemical Co. DBP was dissolved in ethyl alcohol to establish 100 mg mL−1 stock solution.
Plant culture
The cucumber seeds were washed clearly with distilled water and soaked in warm water (50 °C) overnight, and then, seeds were sown in a nursery box and incubated in the dark, at 27 ± 1 °C, to allow germination (Zhang et al. 2014). After 4 days of germination, seedlings were placed in 300-mL pots (one seedling per pot) containing 100 g vermiculites per pot, and relative humidity which ranged from 80 to 90 % was maintained. Cultivation of cucumber seeds was modified by Hoagland’s solution (pH 6.0). After the emergence of at least three leaves per seedling, the cucumber seedlings were treated with different concentrations of DBP (0, 30, 50, 100, and 200 mg L−1), respectively.
The experiment was carried out in the greenhouse of Northeast Agricultural University, Harbin, Heilongjiang Province.
Determination of hydrogen peroxide (H2O2) content and lipid peroxidation
Malonaldehyde content
Malonaldehyde (MDA) content was measured following the method of Zhou et al. (2013) and expressed as nanomoles per gram of fresh weight. Fresh leaves (0.5 g) were homogenized at 4 °C and then added to 5 mL 50 mM PBS-Na (pH 7.8) and 2.5 mL thiobarbituric acid. The mixture was heated at 100 °C for 10 min and then quickly cooled in an ice bath. Then, it was centrifuged at 3,000×g for 15 min. The absorbance of the supernatant was measured at 450, 532, and 600 nm, respectively. The MDA was determined by the following equation:
MDA = [6.452 × (D532 − D600) − 0.559 × D450] × Vt/(Vs × FW), where Vt is total volume of extract (mL), Vs is the determination of extraction volume (mL), and Fw is the sample fresh weight (g).
Determination of H2O2 content in homogenized cucumber seedlings
The content of H2O2 was determined using the method described by Deng et al. (2014) with minor modifications. Plant material (2 g) was homogenized in 5 mL of precooled acetone at 4 °C. Maintained at 4 °C, the homogenate was centrifugated for 10 min at 15,000×g, and the supernatant was collected for sample extraction. Of supernatant, 0.5 mL was mixed thoroughly with 0.1 mL of 5 % titanium sulfate and 0.2 mL ammonium solution, and the mixture was then centrifuged at 3,000×g for 10 min at room temperature. The absorbance of H2O2 was determined using a spectrophotometer at 415 nm; the content of H2O2 was calculated from a standard curve.
Chlorophyll content
Chlorophyll was extracted from the leaves of cucumber seedlings with 80 % acetone and absolute ethyl alcohol (1:1, V/V), and chlorophyll content was determined spectrophotometrically according to Ge et al. (2012).
Proline content
Proline content was measured by ninhydrin chromogenic methods with modification (Tijen and Ìsmail 2005). Fresh leaves (0.1 g) were placed in a glass tube; 5 mL of 3 % sulfosalicylic acid was transferred to the glass tube. The glass tube was incubated for 10 min in a water bath at 100 °C. Two milliliters of the filtrate was digested in another glass tube after the addition of 4 mL chromogenic solution (2 mL of 2.5 % ninhydrin and 2 mL of glacial acetic acid). The glass tube was put into a 100 °C water bath for 30 min. Then, the glass tube was put into an ice bath to end the reaction. Toluene (5 mL) was put into the glass tube and then vortexed and left to stand by. The absorption of the toluene layer was measured at 520 nm with a spectrophotometer. A standard curve was made by detecting 0–20 μg of l-proline.
Glutathione and oxidized glutathione contents
Glutathione (GSH) content was measured by the 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB)-oxidized GSH (oxidized glutathione (GSSH)) recycling assay. An amount of 1 g fresh leaves was ground with 5 mL of chilled 50 g/L trichloroacetic acid (TCA) containing 5 mM EDTA. The solution was centrifuged for 20 min at 12,000×g; the supernatant obtained was used to measure the content of GSH according to the method of Guri (Collier et al. 2006). The reaction mixtures contained 1 mL of the extraction, 1 mL of 0.1 M PBS-Na (pH 7.7), and 0.5 mL of 4 mM DTNB. The content of GSSG was measured at 412 nm.
Activities of antioxidant enzymes
Superoxide dismutase
Measurement of superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium (NBT) according to Zhang et al. (2005). The reaction medium contained 50 mM PBS-Na (pH 7.8), 77.12 μM NBT, 0.1 M EDTA, 13.37 mM methionine, and 20 μM riboflavin. The reaction mixture contained 3.9 mL of the reaction medium and 0.1 mL enzyme extract. Reaction mixtures were illuminated for 10 min at a light intensity of 60 μmol (m2 s)−1, and absorbance of solution was measured at 560 nm. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of reduction of NBT.
Catalase
Catalase (CAT, EC 1.11.1.6) activity was determined using a modified method based on the method of Jebara et al. (2005) by monitoring the decomposition of H2O2 at 240 nm with a spectrometer (Shimadzu UV-1800). The reaction mixture contained 0.2 mL enzyme extract, 1.5 mL 0.2 M PBS-Na (pH 7.8), and 1 mL distilled water. The reaction was started by addition of 0.3 mL 0.1 M H2O2, and its consumption was measured per minute compared to a control reaction without substrate at 240 nm for about 4 min.
Ascorbate peroxidase
Plant material (0.5 g) was homogenized with 7.5 mL of 50 mM PBS-K (pH 7.8) containing 5 mM EDTA, 2 mM ascorbate (ASA), and a little of PVPP. After centrifugation for 20 min at 10,000×g, the supernatant was collected for the measurement of ascorbate peroxidase (APX, EC 1.11.1.11) activity according to the method of Shah and Nahakpamb (2012) with some modifications. The assay was carried out in a reaction mixture consisting of 50 mM PBS-K (pH 7.0), 0.5 mM ASA, 0.1 mM EDTA, and enzyme extraction. The changes in the absorbance at 290 nm were recorded for 10 s after the addition of 0.1 M H2O2. One unit of APX activity was defined as an absorbance change of 0.1 unit min−1.
Peroxidase
The peroxidase (POD, EC 1.11.1.7) activity was determined as described by Shah and Nahakpamb (2012). The reaction mixture contained 50 mL of 200 mM PBS-Na (pH 5.0), 28 μL of guaiacol, and 19 μL of 30 % H2O2. Oxidation of guaiacol in the presence of H2O2 was measured by an increase in the absorbance at 470 nm. The increase was recorded for 5 min. One unit POD activity was defined as the absorbance change of 1 unit/min.
Transmission electron microscopy
Ultrastructural studies were performed on leaf tissues of cucumber from control and each treatment plants. The material treatment was embedded and double stained according to the method described by Zhang et al. (2014). Tissue samples from leaves in the different experimental conditions were fixed in 2.5 % glutaraldehyde overnight and postfixed in 2 % osmium tetroxide (OsO4) for 2 h. After dehydrating and embedding, ultra-thin section samples were double stained with 1.0 % uranyl acetate followed by 5.0 % lead citrate. Four replicates were made for each experimental group, and two samples per replication were then examined and photographed under H-600IV transmission electron microscope (Hitachi, Tokyo, Japan, at 90 kV).
Data analysis
To test the effects of DBP stress on the activities of enzymes and contents of MDA, H2O2, chlorophyll, proline, GSH, and GSSH, the experimental design used was a completely randomized design. All the experimental data were the means from ten replicate leaves of five random seedlings under the same treatment conditions, each done with five replicates, according to the modified method of Zhang et al. (2014). Values were expressed as means ± standard error (SE). Statistical comparisons were carried out using SPSS 19.0 software, and significant differences were indicated by different letters (P < 0.05).
Results
Effects of DBP on non-enzymatic substances
Different parameters, MDA, H2O2, chlorophyll, proline, GSH, and GSSH content, were investigated at 1, 3, 5, and 7-day interval, and data are presented in Figs. 1, 2, 3, and 4.
a, b Contents of MDA and H2O2 in leaves of Cucumis sativus L. under DBP exposure. Bars are standard errors. Means with different small letters are significantly different from one another under the DBP different concentration treatment, and different capital letters are significantly different from one another under the DBP different treatment times according to ANOVA test (P < 0.05)
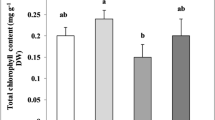
Chlorophyll content (mg L−1 FW) in the Cucumis sativus L. exposed to 30, 50, 100, and 200 mg L−1 of DBP. Bars are standard errors. Means with different small letters are significantly different from one another under the DBP different concentration treatment, and different capital letters are significantly different from one another under the DBP different treatment times according to ANOVA test (P < 0.05)
Content of proline in leaves of Cucumis sativus L.. Bars are standard errors. Means with different small letters are significantly different from one another under the DBP different concentration treatment, and different capital letters are significantly different from one another under the DBP different treatment times according to ANOVA test (P < 0.05)
Contents of GSH and GSSH and GSH/GSSH in leaves of Cucumis sativus L. (on days 1, 3, 5, and 7 after DBP exposure). Bars are standard errors. Means with different small letters are significantly different from one another under the DBP different concentration treatment, and different capital letters are significantly different from one another under the DBP different treatment times according to ANOVA test (P < 0.05)
Effects of DBP on lipid peroxidation and H2O2 production
The level of lipid peroxidation in DBP-treated cucumber seedlings was measured as the content of MDA. On DBP exposure for 1 day, MDA content was virtually unchanged in leaves of cucumber seedlings. However, after 3 days of DBP exposure to 50, 100, and 200 mg L−1, the MDA content of cucumber seedlings increased significantly (P < 0.05) compared to the control (Fig. 1a). Thereafter, MDA contents changed little with DBP concentration and time.
An increase in H2O2 content was enhanced with increasing DBP concentration and prolonged exposure duration (Fig. 1b). With the DBP concentration increased, an increase of H2O2 content was obtained gradually at 1, 3, and 5 days. The H2O2 content remained high in DBP stress at seventh day. And when exposed to 200 mg L−1 DBP for 7 days, H2O2 content increased by about 89 % (P < 0.05) compared to control.
Effects of DBP on chlorophyll content
The effects of DBP stress on the content of chlorophyllin on the leaves of cucumber seedlings for 1, 3, 5, and 7 days of exposure were obviously changed (Fig. 2). With the increase of DBP concentration and time, chlorophyll content of cucumber seedlings decreased. The chlorophyll contents in cucumber seedlings under all DBP treatment groups decreased significantly (P < 0.05) compared to control at 3, 5, and 7 days. In the presence of applied DBP for 7 days, chlorophyll content was decreased by 36.14, 49.66, 56.13, and 57.18 % (P < 0.05) at concentration of 30, 50, 100, and 200 mg L−1 in comparison to the control, respectively.
Effects of DBP on proline content
Proline content expressed increased trend with the increase of DBP concentration and treatment time (Fig. 3), and the high levels of DBP concentration groups compared with the control significantly increased (P < 0.05). With DBP treatment for 1 day, the proline content was increased, compared to the control, by 14.99, 27.59, 47.28, and 57.21 % (P < 0.05), respectively. After 7 days, proline content of DBP (30, 50, 100, and 200 mg L−1) treatment increased by 36.91, 46.80, 92.71, and 107.98 % (P < 0.05) compared to control, respectively.
Effects of DBP on the levels of thiol compounds
We analyzed the contents of GSH, GSSH, and GSH/GSSH ratio in DBP-treated cucumber plants (Fig. 4). Our results showed that GSH content decreased significantly (P < 0.05) after exposure to DBP (30, 50, 100, and 200 mg L−1) for 3 days, and with further exposure to DBP, GSH content declined. With an exposure to 30, 50, 100, and 200 mg L−1 DBP for 7 days, GSH contents decreased by 54.24, 68.06, 78.16, and 92.46 % (P < 0.05) compared with control, respectively (Fig. 4a). Moreover, in comparison with the control, we observed the increased contents of GSSH (Fig. 4b) and the dropped ratios of GSH/GSSG (Fig. 4c) in leaves of cucumber seedlings at 3, 5, and 7 days under DBP stress. The highest content of GSSH (867.5691 ± 8.8596 μmol g−1 FW) and the lowest ratio of GSH/GSHH (0.0263 ± 0.0016) in cucumber leaves were found at 200 mg L−1 DBP treatment after 7 days. Values for the GSH/GSSG ratio remained at low level in DBP treatment groups compared with control (Fig. 4c), and with long-term exposure to DBP, values reduced more.
Effects of DBP on antioxidant enzyme activity
The effects of DBP on the activities of APX, CAT, SOD, and POD in seedlings of cucumber are shown in Fig. 5. The activity of SOD increased in cucumber seedling leaves with the increase of DBP concentration with time. However, the activity of SOD under high DBP (100 and 200 mg L−1) concentration increased before decreasing (Fig. 5a). And after 7 days, the SOD activity decreased under high DBP concentrations (100 and 200 mg L−1), by 12.40 and 47.14 % (P < 0.05) reductions, respectively (Fig. 5a).
a–d Effects of DBP application on superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) activities in seedling leaves of Cucumis sativus L. The data shown are the average of five replicates. Error bars represent ±SE. Means with different small letters are significantly different from one another under the DBP different concentration treatment, and different capital letters are significantly different from one another under the DBP different treatment times according to ANOVA test (P < 0.05)
APX activity decreased significantly (P < 0.05) in cucumber seedling leaves exposed to DBP after 1 day, while APX activity in leaves was increased under low DBP concentrations (30 and 50 mg L−1) after 3 days, and decreased under high DBP concentrations (100 and 200 mg L−1), compared with the control (Fig. 5b). Among the DBP-treated plants and with time, there was a fluctuation in the APX activity. APX activity decreased significantly (P < 0.05) under 100 and 200 mg L−1 DBP concentrations, which were 53.44 and 58.17 % lower compared to the control after 7 days.
The change of the CAT activity was not obvious at the first day. However, under the 200-mg L−1 DBP concentration (at 3, 5, and 7 days) compared with the control, CAT activity decreased significantly (P < 0.05), indicating 26, 49, and 52 % reductions, respectively (Fig. 5c).
As shown in Fig. 5d, POD activity is a parabolic trend with the increase of DBP concentration with time. The POD activity increased significantly (P < 0.05) in cucumber seedling leaves exposed to DBP after 3 days. The POD activity decreased significantly (P < 0.05) at 3 days compared with 5 days under same level of DBP concentrations (100 and 200 mg L−1) but was higher compared with control. However, the POD activity of cucumber seedling leaves under high DBP concentrations (100 and 200 mg L−1) was lower than the control at seventh day, and POD activities were decreased to 75 % (P < 0.05) and 51 % (P < 0.05) of the control group treated with 100 and 200 mg L−1 DBP, respectively.
Observations of leaf ultrastructure
When observed by transmission electron microscopy, the mesophyll cells of the control plant showed typical cells with a definite cell wall, containing chloroplasts, mitochondria, and a clear nucleolus. The chloroplasts exhibited a typical chloroplast structure, ellipsoidal shape, with stacked thylakoids formed by lamellae with grana and distinct membrane. Small ellipse mitochondria with distinct cristae and free ribosomes were present in association with the chloroplasts and nuclei (Fig. 6a).
Transmission electron microscopy of mesophyll cell of Cucumis sativus L., cv. “Jinyan” no. 4. a Control plant (bar = 2 μm). b The plant treated with 30 mg L−1 of DBP for 7 days (bar = 2 μm). c The plant treated with milligrams per liter of DBP for 7 days (bar = 2 μm). d The plant treated with 100 mg L−1 of DBP for 7 days (bar = 5 μm). e The plant treated with 200 mg L−1 of DBP for 7 days (bar = 5 μm). C chloroplast, CW cell wall, M mitochondrion, N nucleus, S starch grain, T thylakoid lamellae, V vacuole
After 7-day exposure to 30 and 50 mg L−1 of DBP, chloroplasts showed few changes in ultrastructural organization (Fig. 6b, c). Some smaller starch grains were observed in the chloroplast. In addition, mitochondria showed no changes in ultrastructural organization. Higher concentration treatments caused more dramatic ultrastructure changes in cucumber, with a large increase in mesophyll cell volume. After culturing cucumber in a concentration of 100 and 200 mg L−1 of DBP for 7 days, an increase of vacuole volume could be observed (Fig. 6d, e). The number of starch grains increased in the chloroplasts and the nuclei became invisible. The amount of chloroplast decreased under higher DBP concentration, especially exposure to 200 mg L−1 of DBP. Moreover, some thylakoids in chloroplasts were grouped with irregular morphology. Mitochondria were disrupted and swollen, and they were gradually swollen and inner cristae become invisible.
Discussion
Environmental stress could induce production of reactive oxygen species (ROS) in the plant cells, and their accumulations are toxic for plants due to membrane lipid oxidation and metabolism reduction (Halliwell 2006). The ROS posing strong oxidizing capacities can attack nucleic acids, pigments, and proteins, cause membrane lipid peroxidation, alter activities of antioxidative enzymes and antioxidant content, and finally lead to cell death (Gomes et al. 2013; Li et al. 2013). In this study, we selected cucumber seedling leaves as the test material, and the selection widely cultivated the plants in greenhouse. And our results indicated that the MDA and H2O2 levels in the DBP treatment groups were higher than that in the control (Fig. 1), which might cause the damage of DBP to cucumber leaf organelles.
To cope with ROS, plants are endowed with a complex enzymatic antioxidant system including SOD, which catalyzes the reaction from O2·− to H2O2 (Apel and Hirt 2004), and APX, CAT, and POD, which function to detoxify the H2O2 produced (Mittler 2002; Gill and Tuteja 2010). Under low DBP concentration (30 and 50 mg L−1) conditions, SOD activity in the cucumber seedlings increased adaptability with increasing of treatment time, but at higher DBP concentrations (100 and 200 mg L−1), SOD activity in the cucumber seedlings decreased, when both conditions were compared with the control groups (Fig. 5a). This result indicated cucumber seedlings might be resistant to low concentration of DBP stress, but the increase in pollutant concentrations might lead to the breakdown of growth process and the stimulation of other relevant responses.
We observed that in the DBP-treated plants, there was a fluctuation in the APX and POD activities with time, but only CAT activity of cucumber seedlings decreased significantly (P < 0.05) under high DBP (100 and 200 mg L−1) treatments (Figs. 5c, d), which might be due to the absorption and accumulation of DBP by the plants resulting in damage of normal growth of the protective enzyme system of plant cells. However, almost all studied antioxidant enzymes (SOD, CAT, APX, and POD) were inhibited following exposure to high DBP (100 and 200 mg L−1) in the current study. The related reports indicated a decrease in antioxidant enzyme activity due to generation of O2 and H2O2 which exceeds the elimination ability of enzymes (Apel and Hirt 2004; Rao et al. 2006). Inhibition in antioxidant enzyme activity was consistent with increased H2O2 production and lipid peroxidation in the current study (Figs. 1 and 5). Previously, we observed that exposure to high DEP decreased SOD activity in cucumber (Zhang et al. 2014). Miguel et al. (2012) also observed similar phenomenon in maize where exposure to 80 mg L−1 dichlorobenzene significantly decreased POD activity in leaves. Antioxidant enzymes like CAT and POD activities were decreased under nickel stress in Raphanus sativus L., which is in agreement with our current findings (Sharma and Dietz 2006).
Of the various detoxification pathways, glutathione (GSH), its redox state (GSH/GSSG), and proline were found to play an indispensable role in detoxification of ROS (Anjum et al. 2012). Proline can protect plant cells under adverse conditions by maintaining the integrity of cell membranes and preventing water loss from the cells (Zhang and Li 2003). The proline content in plants can be used as a response to heavy metal pollution (Sharma and Dietz 2006; Rai et al. 2004) and climate change (Dobra et al. 2010). In our study, the increase of proline content under DBP stress (Fig. 3), compared with control, is consistent with those in plants in adverse conditions (Dai et al. 2012). Higher accumulation of proline in the leaves of cucumber seedlings under DBP stress might indicate its greater influence on growth of cucumber seedlings (Fig. 3).
The changes in GSH level, GSH/GSSG ratio, can affect the resistance and adaptation of plants to various environmental stresses (Rausch et al. 2007). GSH is also one of major antioxidants in photosynthetic and non-photosynthetic tissue of plants (Nehnevajova et al. 2012). GSH not only performs as substrates in the ascorbate–glutathione cycle but also acts as redox buffering in the apoplast (Foyer et al. 2001; Matés et al. 2002). It is able to detoxify ROS by direct scavenging or by the action of enzymes as substrates in the ascorbate–glutathione cycle, which operates both in chloroplasts and the cytosol. GSH is oxidized to GSSG as part of its cellular antioxidant defense (Foyer and Noctor 2011; Seth et al. 2012). In the present study, a disturbance of the GSH redox balance was evidenced by a marked decrease in GSH/GSSG ratio in cucumber seedlings subjected to DBP stress. The reduction of GSH and accumulation of GSSG would help explain the decreased GSH/GSSG ratios in seedlings under DBP stress conditions (Fig. 4). The study suggested the disruption of the GSH and GSSH balance in the plant system, and this might be because of inactivation of the enzyme by the larger ROS produced in plant cells under DBP stress in the presence of thiol groups at active sites (Nagalakshmi and Prasad 2001).
Chlorophyll content is considered as an important indicator to evaluate the damage of plant under stress condition. So, in order to evaluate the damage of DBP stress on cucumber seedlings, the chlorophyll content was selected among the indices. In this study, DBP application decreased chlorophyll content (Fig. 2), which was an indication of DBP-induced damage in chloroplast. Chlorophyll contents have been reported to be decreased under exposure to organic pollutants (Oguntimehin et al. 2010; Ahammed et al. 2012). This similar conclusion was reported by Kumar et al. (2012) on maize and rice stressed by heat and Zhang et al. (2014) on cucumber stressed by DEP and DEHP. This could be an indication that leaf tissues from DBP stress conditions suffered from oxidative stress. Otherwise, our results on the membrane lipid peroxidation and the increase of the content of H2O2 with the changes of antioxidant systems have confirmed this phenomenon.
Ultrastructural investigation revealed that DBP stress damaged the ultrastructure of cucumber mesophyll cells, in this study. Chloroplast and mitochondria exhibited swollen grana/stroma lamellae, degraded grana stacking and the stroma, and/or lose thylakoid membranes (Fig. 6a–e). These ultrastructural alterations suggest that DBP induced important disturbances in metabolic functions and lipid composition of membranes. Among all treatments, mesophyll cell treated with 200 mg L−1 of DBP showed the highest morphological deformations (Fig. 6e).
In present study, there was a significant increase in the number, length, and width of starch grains per chloroplast under the increasing DBP treatment, resulting in distorted and swollen chloroplasts. Chloroplasts were often observed fully occupied with big starch grains under stress condition, such as heavy metals (dos Santos et al. 2013; Sanchez-Pardo et al. 2014a), elevated CO2 (Sun et al. 2011), chilling (Vella et al. 2012), and salinity (Barhoumi et al. 2007). On the contrary, Sanchez-Pardo et al. (2014b) suggested the content of starch grains was found to be reduced in chloroplast of soybean, and white lupin leaves were under Cu excess. It seems that toxicity of condition stress to the amount of starch grains in higher plants was condition and species specific. Sun et al. (2011) demonstrated that the accumulation of starch grains in chloroplast could damage the thylakoids and grana and consequently inhibited photosynthesis.
The reduction in photosynthetic apparatus could lead to a fall in the biosynthesis of chlorophyll, caused by the destruction of the internal structure of the chloroplast and thylakoid membrane damage (Quartacci et al. 2000). A decrease in chlorophyll content (Chl a, Chl b, and total Chl) was also discovered to be more severely affected under higher concentrations of DBP stress compared to the control.
Notably, chloroplasts were filled with a great number of small vacuoles after 7-day exposure to 200 mg L−1 of DBP (Fig. 6e). It has been known that the increase in vacuolization and in the number of vacuole in treated cells may be due to a cellular detoxification mechanism. Cytoplasmic vacuolization was also reported for Dunaliella tertiolecta after Pb exposure (Saçan et al. 2007) and for barley following UV exposure (Pichrtová et al. 2013). Vacuolization could contribute to compartmentalization of toxic materials, and numerous vacuoles may represent the breakdown site of accumulated by-products (Nishikawa et al. 2003; Saçan et al. 2007).
In conclusion, this work showed that DBP treatment not only inhibited antioxidant capacity and antioxidant enzyme activity in cucumber seedling leaves but also caused chlorophyll degradation or reduced the synthesis of chlorophyll. Moreover, DBP treatment could also enhance the accumulation of ROS which induced membrane lipid peroxidation. DBP might induce damage by dropping their antioxidant systems and ROS-scavenging capabilities in cucumber seedlings. Thus, DBP could be harmful in terms of its effects on the growth of seedlings, especially at high concentrations and long-time treatment conditions.
References
Ahammed GJ, Yuan HL, Ogweno JO, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ (2012) Brassinosteroid alleviates phenanthrene and pyrene phytotoxicity by increasing detoxification activity and photosynthesis in tomato. Chemosphere 86(5):546–555
Anjum NA, Ahmad I, Mohmood I, Pacheco M, Duarte AC, Pereira E, Umar S, Ahamad A, Khan NA, Iqbal M, Prasad MNV (2012) Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—a review. Environ Exp Bot 75:307–324
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Api AM (2001) Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chem Toxicol 39(2):97–108
Barhoumi Z, Djebali W, Chaıbi W, Abdelly C, Smaoui A (2007) Salt impact on photosynthesis and leaf ultrastructure of Aeluropus littoralis. J Plant Res 120:529–537
Chen JA, Liu H, Qiu Z, Shu W (2008) Analysis of di-n-butyl phthalate and other organic pollutants in Chongqing women undergoing parturition. Environ Pollut 156(3):849–853
Colacino JA, Harris TR, Schecter A (2010) Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Perspect 118(7):998
Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ (2006) Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am J Physiol Endocrinol Metab 291(1):E182–E189
Dai AH, Nie YX, Yu B, Li Q, Lu LY, Bai JG (2012) Cinnamic acid pretreatment enhances heat tolerance of cucumber leaves through modulating antioxidant enzyme activity. Environ Exp Bot 79:1–10
Deng YS, Kong FY, Zhou B, Zhang S, Yue MM, Meng QW (2014) Heterology expression of the tomato LeLhcb2 gene confers elevated tolerance to chilling stress in transgenic tobacco. Plant Physiol Biochem 80:318–327
Dobra J, Motyka V, Dobrev P, Malbeck J, Prasil IT, Haisel D, Gaudinova A, Havlova M, Gubis J, Vankova R (2010) Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J Plant Physiol 167:1360–1370
dos Santos RW, Schmidt ÉC, Bouzon ZL (2013) Changes in ultrastructure and cytochemistry of the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales) treated with cadmium. Protoplasma 250:297–305
Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS (2010) Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect 118(4):565–571
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155(1):2–18
Foyer CH, Theodoulou FL, Delrot S (2001) The functions of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci 6(10):486–492
Fromme H, Gruber L, Schlummer M, Wolz G, Böhmer S, Angerer J, Mayer R, Liebl B, Bolte G (2007) Intake of phthalates and di (2-ethylhexyl) adipate: results of the integrated exposure assessment survey based on duplicate diet samples and biomonitoring data. Environ Int 33(8):1012–1020
Ge Y, Wang T, Wang N, Wang Z, Liang C, Ramchiary N, Choi SR, Limd YP, Piao ZY (2012) Genetic mapping and localization of quantitative trait loci for chlorophyll content in Chinese cabbage (Brassica rapa ssp. pekinensis). Sci Hortic 147:42–48
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Gomes MP, Duarte DM, Carneiro MM, Barreto LC, Carvalho M, Soares AM, Guilherme LR, Garcia QS (2013) Zinc tolerance modulation in Myracrodruon urundeuva plants. Plant Physiol Biochem 67:1–6
Gray LE, Ostby J, Furr J, Price M, Veeramachaneni DR, Parks L (2000) Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58(2):350–365
Guo Y, Kannan K (2011) Comparative assessment of human exposure to phthalate esters from house dust in China and the United States. Environ Sci Technol 45(8):3788–3794
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141(2):312–322
Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE (2008) A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague–Dawley rat in a cumulative, dose-additive manner. Toxicol Sci 105(1):153–165
Jebara S, Jebara M, Fe L, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162:929–936
Kondo T, Shono T, Suita S (2006) Age-specific effect of phthalate ester on testicular development in rats. J Pediat Surg 41(7):1290–1293
Kong S, Ji Y, Liu L, Chen L, Zhao X, Wang J, Bai ZP, Sun Z (2012) Diversities of phthalate esters in suburban agricultural soils and wasteland soil appeared with urbanization in China. Environ Pollut 170:161–168
Kumar S, Gupta D, Nayyar H (2012) Comparative response of maize and rice genotypes to heat stress: status of oxidative stress and antioxidants. Acta Phys Plant 34(1):75–86
Latini G (2005) Monitoring phthalate exposure in humans. Clin Chim Acta 361(1):20–29
Li X, Yang Y, Jia L, Chen H, Wei X (2013) Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol Environ Saf 89:150–157
Lovekamp-Swan T, Davis BJ (2003) Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 111(2):139
Lyche JL, Gutleb AC, Bergman Å, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU (2009) Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health, Part B 12(4):225–249
Ma TT, Christie P, Teng Y, Luo Y (2013) Rape (Brassica chinensis L.) seed germination, seedling growth, and physiology in soil polluted with di-n-butyl phthalate and bis (2-ethylhexyl) phthalate. Environ Sci Pollut Res 20(8):5289–5298
Matés JM, Pérez-Gómez C, de Castro IN, Asenjo M, Márquez J (2002) Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol 34(5):439–458
Matsumoto M, Hirata-Koizumi M, Ema M (2008) Potential adverse effects of phthalic acid esters on human health: a review of recent studies on reproduction. Regul Toxicol Pharm 50(1):37–49
Miguel AS, Faure M, Ravanel P, Raveton M (2012) Biological responses of maize (Zea mays) plants exposed to chlorobenzenes. Case study of monochloro-, 1,4-dichloro- and 1,2,4-trichloro-benzenes. Ecotoxicology 21(2):315–324
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mo CH, Cai QY, Li YH, Zeng QY (2008) Occurrence of priority organic pollutants in the fertilizers, China. J Hazard Mater 152(3):1208–1213
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160(2):291–299
Nehnevajova E, Lyubenova L, Herzig R, Schröder P, Schwitzguébel JP, Schmülling T (2012) Metal accumulation and response of antioxidant enzymes in seedlings and adult sunflower mutants with improved metal removal traits on a metal-contaminated soil. Environ Exp Bot 76:39–48
Nishikawa K, Yamakoshi Y, Uemura I, Tominaga N (2003) Ultrastructural changes in Chlamydomonas acidophila (Chlorophyta) induced by heavy metals and polyphosphate metabolism. FEMS Microbiol Ecol 44:253–259
Oguntimehin I, Eissa F, Sakugawa H (2010) Negative effects of fluoranthene on the ecophysiology of tomato plants (Lycopersicon esculentum Mill): fluoranthene mists negatively affected tomato plants. Chemosphere 78(7):877–884
Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Wang P, Zhang S, Yoshimura M, Hanaoka T, Takahashi K (2006) Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect 114(11):1643
Pichrtová M, Remias D, Lewis LA, Holzinger A (2013) Changes in phenolic compounds and cellular ultrastructure of Arctic and Antarctic strains of Zygnema (Zygnematophyceae, Streptophyta) after exposure to experimentally enhanced UV to PAR ratio. Microb Ecol 65:68–83
Quartacci MF, Pinzino C, Sgherri CLM, Dalla VF, Navari-Izzo F (2000) Growth in excess copper induces changes in the lipid composition and fluidity of PSII-enriched membranes in wheat. Physiol Plant 108:87–93
Rai V, Vajpayee P, Singh SN, Mehrotra S (2004) Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci 167(5):1159–1169
Rao KM, Raghavendra AS, Reddy KJ (2006) Physiology and molecular biology of stress tolerance in plants. Springer, Amsterdam
Rausch T, Gromes R, Liedschulte V, Müller I, Bogs J, Galovic V, Wachter A (2007) Novel insight into the regulation of GSH biosynthesis in higher plants. Plant Biol 9(5):565–572
Sanchez-Pardo B, Ferna’ndez-Pascual M, Zornoza P (2014) Copper microlocalisation and changes in leaf morphology, chloroplast ultrastructure and antioxidative response in white lupin and soybean grown in copper excess. J Plant Res 127:119–129
Saçan MT, Oztay F, Bolkent S (2007) Exposure of Dunaliella tertiolecta to lead and aluminum: toxicity and effects on ultrastructure. Biol Trace Elem Res 120:264–272
Schettler T (2006) Human exposure to phthalates via consumer products. Int J Androl 29(1):134–139
Seth CS, Remans T, Keunen E, Jozefczak M, Gielen H, Opdenakker K, Weyens N, Cuypers A (2012) Phytoextraction of toxic metals: a central role for glutathione. Plant Cell Environ 35(2):334–346
Shah K, Nahakpamb S (2012) Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol Biochem 57:106–113
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exper Bot 57(4):711–726
Sultan C, Balaguer P, Terouanne B, Georget V, Paris F, Jeandel C, Lumbroso S, Nicolas JC (2001) Environmental xenoestrogens, antiandrogens and disorders of male sexual differentiation. Mol Cell Endocrinol 178(1):99–105
Sun ZP, Li TL, Liu YL (2011) Effects of elevated CO2 applied to potato roots on the anatomy and ultrastructure of leaves. Biol Plant 55(4):675–680
Tijen D, Ìsmail T (2005) Comparative lipid peroxidation, antioxidant defense systems and praline content in roots of two rice cultivars differing in salt tolerance. Environ and Exp Bot 53:247–257
Vella NGF, Joss TV, Roberts TH (2012) Chilling-induced ultrastructural changes to mesophyll cells of Arabidopsis grown under short days are almost completely reversible by plant re-warming. Protoplasma 249:1137–1149
Wan S, Kang Y, Wang D, Liu SP (2010) Effect of saline water on cucumber (Cucumis sativus L.) yield and water use under drip irrigation in North China. Agr Water Manag 98(1):105–113
Wang SG, Lin XG, Yin R, Hou YL (2003) Effects of di-n-butyl phthalate on mycorrhizal and non-mycorrhizal cowpea plants. Biol Plant 47(4):637–639
Wang X, Lin Q, Wang J, Lu X, Wang G (2013) Effect of wetland reclamation and tillage conversion on accumulation and distribution of phthalate esters residues in soils. Ecol Eng 51:10–15
Wei AX, Xu XB (2005) Research on the pollution of phthalate esters compounds in the environment. Tech and Equip for Environ Pollut Control 6:89–93
Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K (2006) What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal 26(3):803–824
Yana M, Korshinb GV, Changc HS (2014) Examination of disinfection by-product (DBP) formation in source waters: a study using log-transformed differential spectra. Water Res 50:179–188
Zhang YX, Li XL (2003) Toxicity of three organophosphorus pesticides on Hordeum vulgare seedling. J Agro-Environ Sci 22:754–757
Zhang QF, Li YY, Pang CH, Lu CM, Wang BS (2005) NaCl enhances thylakoid-bound SOD activity in the leaves of C3 halophyte Suaeda salsa L. Plant Sci 168:423–430
Zhang Y, Wang L, Du N, Ma GP, Yang AM, Zhang H, Wang ZG, Song QX (2014) Effects of diethylphthalate and di-(2-ethyl) hexylphthalate on the physiology and ultrastructure of cucumber seedlings. Environ Sci Pollut Res 21(2):1020–1028
Zhou B, Deng YS, Kong FY, Li B, Meng QW (2013) Overexpression of a tomato carotenoid-hydroxylase gene alleviates sensitivity to chilling stress in transgenic tobacco. Plant Physiol Biochem 70:235–245
Acknowledgments
This work was financially supported by the National High Technology Research and 863 Development Program of China, project 2012AA101405.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zhang, Y., Du, N., Wang, L. et al. Physical and chemical indices of cucumber seedling leaves under dibutyl phthalate stress. Environ Sci Pollut Res 22, 3477–3488 (2015). https://doi.org/10.1007/s11356-014-3524-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3524-1