Abstract
The growth response of the marine alga Dunaliella tertiolecta to different concentrations of lead and aluminum was investigated. Both metals had a stimulatory effect at low concentration and an inhibitory effect at high concentration (hormesis). The IC25 values of lead are 8.43, 7.29, and 6.74 mg L−1 for 24, 48, and 72 h, respectively. The corresponding values for aluminum are 30.54, 22.42, and 18.16 mg L−1. Although it seems that the two metals are not directly toxic to the alga at the concentrations found in the environment, as implied by the IC25 values and the environmental concentrations of the metals, low concentrations of both metals, alone and in combination, affected the ultrastructure. The growth of batch-grown cells exposed to 0.5 mg L−1 lead and aluminum, alone and combined, during the 24-h exponential phase was investigated. The same cells were also examined under an electron microscope to determine the biological effects of the two metals on the ultrastructure. The most obvious effects of lead were disrupted thylakoidal membranes, accumulated polyphosphate bodies and vacuoles, and lead precipitates on the cell surface. These ultrastructural alterations were partially present in aluminum-treated and lead–aluminum-treated cells. In joint exposure, the most important change was the lysis of the cell membrane. Aluminum and lead seem to act synergistically on the cell membrane leading to cell membrane lysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metallic elements are found in all living organisms. Some are essential, as they serve specific biological functions. Others are toxic even at low doses. Among the toxic metals, lead (Pb) and aluminum (Al) are widespread in our environment as a result of industrial activity, acid rain, and domestic use. Although the average concentration of Pb in the Earth’s crust is around 13 μg L−1, the use of Pb as a fuel additive and in various industrial processes, combined with the effects of mining and the combustion of coal, has led to the bioaccumulation of this metal in topsoils and aquatic ecosystems. The reported total Pb concentration in stagnant surface water ranges from 6 to 1,410 μg L−1 [1]. The toxic effects of Pb have been widely documented for organisms including algae and the environment [2, 3].
Although Al is not considered to be a heavy metal, it is among the most abundant elements in the environment as a result of the use of Al salts (alum) as coagulants for the treatment of water and in industrial process [4, 5]. The reported mean value of Al in drinking water (tap water) is 213.7 μg L−1 [6], which is higher than the threshold value set by the World Health Organization in 1993. The contribution of the use of Al (in the form of Al sulfate) as flocculant in the treatment of drinking water to this high Al concentration was reported [6]. Al was generally described as an innocuous element to many aquatic organisms under circum-neutral conditions. However, there was a great interspecies variability. Not only the free Al+3 species but also polynuclear Al species that occur at neutral pH [5] and the highly reactive hydroxo-complexes are considered to be toxic to algae [7].
Exposure of phytoplankton to pollutants may have various influences on aquatic ecosystems, either by initiating a chain of bioaccumulation [8–10] or by inhibiting the flow of energy into food webs. Microorganisms, microalgae in particular, are the first organisms affected by heavy-metal discharges in aquatic environments because they are directly in contact with the medium; they are separated only by the cytoplasmic membrane and the cell wall. Studies have demonstrated that some metals, such as Al, Pb, Cd, Cr, Cu, Zn, Hg, and Ag, and various organic toxic agents inhibited algal growth and had harmful effects on the structure of different algal species [11–14]. Electron microscope observations reveal that the chloroplasts, pyrenoids, mitochondria, nuclei, polyphosphate bodies, lipids, vacuoles, and cell membranes in algal cells are the target structures for metals and toxic organic compounds [13].
The marine green algae Dunaliella tertiolecta are widely used as test organisms in toxicity assays to evaluate the effects of industrial effluents because of their advantageous characteristics like abundant population, minimal culture requirements, and ease of use. However, there is no information about the effect of Pb and Al on the ultrastructural features of this microalga. The objectives of this study were (1) to determine the IC25 values (the concentration of the tested substance that decreases the growth by 25%) of Pb and Al for D. tertiolecta and (2) to investigate the biological effects of Pb and Al, alone and combined, on D. tertiolecta both at the population level (effect on growth) and at the subcellular level (effect on ultrastructure).
Material and Methods
Toxicity and Morphological Assays
This study used the unicellular green alga D. tertiolecta (Butcher) provided by the Marmara Scientific Research Center (Turkey). D. tertiolecta stock cultures were kept in 500-ml Erlenmeyer flasks containing natural seawater filtered through GF/C glass fiber filter (Whatman) and enriched with modified f/2 medium [15]. The tests were carried out in triplicate at 21 ± 2°C under continuous illumination with two warm-light fluorescent lamps (2 × 40 W) in a growth chamber (LEEC). The pH of the medium was 8.2 ± 0.1. Metal solutions of Pb(NO3)2 (Merck) and Al2(NO3)2·9H2O (Merck) were prepared by dissolving reagent-grade metal compounds in deionized water. For toxicity experiments, cultures of D. tertiolecta in the log phase were exposed to four concentrations of Pb (2, 4, 6, and 8 mg L−1) and four concentrations of Al (4, 8, 16, and 32 mg L−1) for 72 h. The growth response of D. tertiolecta exposed to each of the chemicals studied was determined by daily measurements of in vivo chlorophyll fluorescence with a RF-4000 Model Shimadzu spectrofluorophotometer. The IC25 values were determined by using linear interpolation combined with bootstrapping [16]. The SC20 value (stimulatory concentration) describing the concentration that increases growth by 20% above that of the control population was used for growth stimulation. The area under the growth curve was calculated by using the equation of LeBlond and Duffy [17]. The percent inhibition/stimulation, I g (%), was calculated from the equation used by Saçan and Balcıoğlu [18]. To obtain a confidence interval for the SC20 value, each I g value was plotted against each concentration.
To determine the growth response of the algae and the ultrastructural changes at lower concentrations, algal cells were exposed to 0.5 mg L−1 Pb and 0.5 mg L−1 Al for 24 h alone and in combination on the eighth day of inoculation. For morphological analysis, algal cells were collected by centrifugation (3,000 rpm, 20 min) and were fixed in 3.5% glutaraldehyde and in 1% OsO4 in sodium cacodylate buffer (0.1 M, pH 7.4) for 1 h at room temperature. The fixation technique used was a modification of that proposed by Hayat [19]. Subsequently, the specimens were dehydrated by changes of increasing concentrations of acetone. Infiltration was carried out with increasing concentrations of Spur followed by dehydration. The specimens were transferred to pure Spur in silicon molds, which allowed easy orientation. The samples were polymerized overnight at 60°C. Blocks were sectioned by using an ultramicrotome (Reichert Om U3), and ultrathin sections stained with uranyl acetate/lead citrate were examined under an electron microscope (JEOL-1200 Ex-II).
Statistical Analysis
Curve-fitting analysis for SC20 values was carried out directly on the data by using the SPSS 11.0 software. Fisher statistic (F) and p values were taken into consideration in testing the quality of the curve fitting. The results were considered significantly different at the level p ≤ 0.05. The paired t test was used to determine significant differences among the IC25 and SC20 values.
Results
Effect of the Metals at the Population Level
The areas under the growth curve and the growth stimulation/inhibition (I g) values appear in Table 1 together with the coefficients of variation for the two metals. As it can be seen from Table 1, both metals had a stimulatory effect at low concentrations and an inhibitory effect at higher concentrations on the growth of algae. In other words, a hormetic response (stimulation at low concentration, inhibition at high concentration) was observed for both metals.
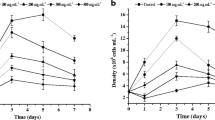
The 24-h SC20 values for Pb and Al were 1.27–7.5 and 2.23–24.5 mg L−1, respectively. By increasing the exposure time from 24 to 72 h, the average IC25 values decreased significantly from 8.43 to 6.74 mg L−1 for Pb and from 30.54 to 18.16 mg L−1 for Al.
Because the average IC25 values for both metals are above the environmentally measured concentrations [1, 6] and knowing that clear changes in the morphological shape for tolerant species exposed to pollutants may occur as reported by Shehata et al. [14], we used exposure concentrations of 0.5 mg L−1 for the two metals alone and combined. Figure 1 represents the growth curves of algae exposed to 0.5 mg L−1 Pb, 0.5 mg L−1 Al and 0.5 mg L−1 Pb and 0.5 mg L−1 Al. A stimulatory effect was observed for the two metals added alone and in combination for the concentration studied.
Effect of the Metals at the Subcellular Level
Figure 2a shows a cross-section through the control cell of D. tertiolecta. There is a large chloroplast occupying the anterolateral region and almost completely enclosing the central region of the cytoplasmic matrix. The chloroplast in the cell contains tightly bound thylakoids typically situated parallel to the longitudinal axis of the chloroplast. Two types of starch grains are clearly distinguished in the chloroplast, firstly those situated between thylakoids (stromal starch grains) and secondly those connected with the pyrenoid. In short, the appearance of the control cells matches the ultrastructural description of this organism given by Hoshaw and Maluf [20].
Electron micrographs of D. tertiolecta. a Control cell. b Cell exposed to 0.5 mg L−1 Pb. c Cell exposed to 0.5 mg L−1 Al. d Cell exposed to 0.5 mg L−1 Pb and 0.5 mg L−1Al. Legend for all figures: (→) metal precipitates and thickening of cell membranes, (H) breakdown of the cell membrane, (Δ) polyphosphate body, (*) starch plates, (c) chloroplast, (n) nucleus, (p) pyrenoid, (s) starch grain, (t) disruption and loosening of the thylakoidal membranes, (v) vacuole; 16,000×
Cell ultrastructure was visibly affected by exposure to 0.5 mg L−1 Pb (see Fig. 2b). The most conspicuous change in cells of D. tertiolecta exposed to Pb was the loosely packed and disrupted appearance of the thylakoid membranes. Other alterations were a thickening of and/or Pb precipitation on the cell membrane, the disruption in the peripheral cytoplasm, extensive vacuolization and polyphosphate bodies, and the presence of membranous structures in the vacuoles and over-accumulation of starch.
In cells treated with 0.5 mg L−1 Al, the cell membrane exhibited an undulant appearance and contained a lot of precipitation on its surface (see Fig. 2c). The loosening of some thylakoidal membranes and numerous starch grains were observed, although these changes were fewer than those observed for Pb exposure. In addition, numerous small vacuoles were noticed in the cytoplasm.
Simultaneous exposure to Pb and Al also revealed an undulated, partly thick and disintegrated cell membrane (see Fig. 2d). The thylakoidal membranes were disrupted in some places. In joint exposure, another alteration was an increase in the number of starch grains in chloroplast stroma (visual observation). Extensive vacuolization in the cytoplasm relative to the controls and a large buildup of polyphosphate bodies often occupying almost the whole vacuole were observed. Dilatation of some cristae in some of the mitochondria and formation of myelin figures were also observed.
Discussion
Toxicity of the Metals at the Population Level
I g (%) values in Table 1 indicate that both metals may cause adverse effects on the aquatic environment by stimulation of algal growth at low concentrations and inhibition of algal growth at high concentrations (hormesis), as the entire aquatic ecosystem may be influenced by changes in algal populations. If the biomass of algae becomes too high or if certain species become abundant, water quality may be negatively affected: Decreased water transparency and oxygen consumption in bottom waters after settling are two principal consequences of algal overproductivity. Decreases in water transparency may affect growth and survival of higher order, vascular aquatic plants and cause changes in fish populations.
Hormetic-like biphasic dose–response is routinely observed by US Environmental Protection Agency scientists. Hormetic effects have also been reported in studies dealing with complex mixtures of petroleum [21, 22] with metals [23, 24] and with wastewater effluent [18, 25, 26].
The toxicities of Pb and Al, alone and combined, have never been assessed for D. tertiolecta; therefore, we could not compare our results with literature values. The decrease in the IC25 values as the exposure time increased from 24 to 72 h indicates that algae can adapt to the studied metals. Although adaptation may be of high ecological significance in providing a level of protection from higher concentration of effluents, replacement of sensitive species may occur, and this may affect the structure and the functioning of the whole aquatic ecosystem [27].
The amount of 0.5 mg L−1 of Pb, Al, and Pb–Al each stimulated the growth of algae (see Fig. 1). The stimulatory effect of Al is higher than that of Pb. Although environmental factors such as pH and suspended sediments may enhance or decrease the growth response of algae at low concentrations, the release of Pb or Al to the environment separately or in combination can lead to uncontrolled algal growth and consequently exacerbate the eutrophication problem.
Toxicity of the Metals at the Subcellular Level
It has been known that the primary organelle affected by high metal concentration is the chloroplast. The disruption of the thylakoidal membranes and enlargement of interthylakoidal spaces in the chloroplast of the alga Chlorella fusca and Chaetomorpha brachygona exposed to water having high concentrations of metals such as Cu, Ni, Pb, Zn, Fe, Mn, Al, As, Cd, and Cr were reported [12, 13]. The same effects have also been detected in D. tertiolecta exposed to triphenyltin [28]. Similarly, the results represented here showed that the chloroplasts of all experimental groups were disrupted after Pb and Al treatment. However, the most striking effect on chloroplasts was observed in cells exposed to Pb. The decrease in these structural alterations in algal cells exposed to the combination of Pb and Al indicated that Al acts antagonistically on Pb in relation to this parameter. The disruption causing the thylakoidal membrane to spread from its usual compact arrangement of lamella may be the result of the uncoupling of the protein bonds causing them to stretch as stated by Rachlin et al. [29]. Accordingly, in the present study, it is likely that Pb rather than Al acts as an uncoupling agent; this may explain the strong influence of Pb on the thylakoidal membranes.
The increase in vacuolization and in the number of vacuolar inclusions like membranous structures within the cytoplasm in all experimental groups, particularly in Pb-treated cells, may be due to a cellular detoxification mechanism. Cytoplasmic vacuolization was also reported for Skeletonema costatum after sublethal cation exposure [30] and for Chlamydomonas bullosa cells following 0.78 μM Cu treatment [11]. Sicko-Goad and Stoermer [31] observed the development of membranous structures within vacuoles in Diatoma tenue cells exposed to Pb. The exact role of vacuoles in heavy-metal detoxification is not yet clear, but vacuolization could contribute to compartmentalization of toxic metals [32]. These vacuoles may represent the breakdown site of accumulated byproducts [33]. In Al- and Pb–Al-treated cells (Figs. 2c and d, respectively), the scattered appearance of diverse sized electron-dense deposits in vacuoles can be attributed to the deposition of Al as stated by Sauvant et al. [34]. They identified various electron-dense granules with dots inside numerous vacuoles by X-ray microanalysis as Al precipitates in cells of Tetrahymena pyriformis exposed to Al.
Several investigators [8–10, 29, 31, 35] clearly demonstrated that polyphosphate bodies could incorporate cations into their internal structure and thereby act as cellular detoxification centers. The decrease or increase in the number of polyphosphate bodies depends on the type and concentration of metals and algal species. A decrease in their number after exposure to either copper or cadmium has been shown for Diatoma tenue, Anabena flosaquae, and C. bullosa [11, 31, 35, 36], while a significant increase in the relative volume and number of polyphosphate bodies of A. variabilis exposed to Zn has been reported [33]. In our study, Pb treatment led to an increase in the number of polyphosphate bodies to a greater extent than the other treatments. On the other hand, Pb–Al treatment led to the formation of a large buildup of polyphosphate bodies. In the other words, Pb was not effective in increasing the number of polyphosphate bodies in the presence of Al.
In contrast to the shrinkage of the cell wall of acid-tolerant freshwater algae exposed to a combination of Al and Cu [37], in our study, an undulant appearance of the cell membrane was observed in Dunaliella cells exposed to Al and the combination of Pb and Al. This may lead to an increase in the surface area and hence an increase in the removal of heavy metals from the aqueous phase. In all experimental groups, the thickening of the cell membrane in D. tertiolecta can be attributed to the adsorption and/or binding of metals to the algal surface, as phytoplankton cells contain various functional groups, such as carboxyl, amino, thio, hydroxy, and hydroxy-carboxyl, which can interact with metal ions. However, in Al-treated and Pb–Al-treated cells, precipitations on the cell membrane were not as extensive as those in cells treated with Pb. This can be related to the competition between metal ions for binding to the cell membrane. Accordingly, D. tertiolecta cells can accumulate the two metals on the same surface components. Additionally, Al has a higher affinity for binding the algal surface relative to Pb, and the adsorption of Al reduces charge density, thereby making the remaining sites less effective for the adsorption of Pb. Similarly, Santana-Casiana et al. [38] reported that the addition of appreciable amounts of Zn, Al, and Cd to the algal solution competitively inhibits Fe uptake in D. tertiolecta.
It is of interest to consider the different species of Al in water, as free Al3+ ions are reported to be the most toxic ones at acidic pH [37], and insoluble hydroxides of Al favored in the aqueous solution at alkaline pH lead to a significant decrease of Al uptake by phytoplanktons. However, in our study, ultrastructural changes that appeared in the Al-treated cells at alkaline pH (8.2 ± 0.1) can be attributed to toxic effects of Al at the subcellular level. This can be explained by the high affinity of Al for being bound to organic acids and hydroxy and carboxyl groups present in the structure of the cell membrane [39]. These groups are able to mobilize free Al from Al–hydroxo complexes; hence, they are able to enhance the intracellular uptake of Al.
The present study demonstrated that the ultrastructural alterations in Dunaliella as a result of exposure to Al and Pb–Al are not as extensive as those caused by Pb exposure from the point of view of the cellular constituents. It is obvious that Al acted on Pb antagonistically to limit its toxic effects at the cellular level. Although D. tertiolecta is likely to develop detoxification mechanisms in response to exposure to a single metal, the outcome of joint exposure is more drastic, as lysis of the cell membrane will lead to the death of the cell and contribute to the altering of the structure of the microalgal community. Finally, this organism seems to be useful for the detoxification of metal-polluted water, as it has a great potential for the removal of significant amounts of metal from the aqueous phase. However, the degenerative alterations originating from the bioaccumulation of these metals on the algal structure constitute a risk of heavy-metal transfer to the other organisms and will affect the ecological balance.
It is important to realize that the results obtained in this study are only applicable to the conditions used in our study. Laboratory experiments should be carried to field studies to see their applications in real life, as pH, temperature, organic carbon content, and the presence of other elements are among the environmental factors that influence the toxicity of metals to marine organisms.
References
Patel KS, Shrivas K, Hoffmann P, Jakubowski N (2006) A survey of lead pollution in Chhattisgarh State, Central India. Environ Geochem Health 28:11–17
Jain SK, Vasudevan P, Jha NK (1989) Removal of some heavy metals from polluted water by aquatic plants: studies on duckweed and water velvet. Biol Wastes 28:115–126
Yap CK, Ismail A, Omar H, Tan SG (2004) Toxicities and tolerances of Cu, Cd, Pb and Zn in a primary producer (Isochrysis galbana) and in a primary consumer (Perna viridis). Environ Int 29:1097–1104
Simpson AM, Hatton W, Brockbank M (1988) Aluminum; its use and control in potable water. Environ Technol Lett 9:907–916
Sollars CJ, Bragg S, Simpson AM, Perry R (1989) Aluminum in European drinking water. Environ Technol Lett 10:131–150
Souad C, Farida Z, Nadra L, François B, Bougle D, Azeddine S (2006) Trace elements level in infant hair and diet, and in the local environment of the Moroccan city of Marrakech. Sci Total Environ 370:337–342
Lindemann J, Holtkamp E, Herrmann R (1990) The impact of Al on green algae isolated from two hydrochemically different headwater streams, Bavaria, Germany. Environ Pollut 67:61–77
Jensen TE, Baxter M, Rachlin JW, Jani V (1982) Uptake of heavy metals by Plectonema boryanum (Cyanophyceae) into cellular components, especially polyphosphate bodies: An X-ray energy dispersive study. Environ Pollut A 27:119–127
Jensen TE, Rachlin JW, Jani V, Warkentine B (1982) An X-ray energy dispersive study of cellular compartmentalization of lead and zinc in Chlorella saccharophila (Chloropyhta), Navicula insertia and Nitzschia closterium (Bacillariophyta). Environ Exp Bot 22:319–328
Rai LC, Gaur JP, Kumar HD (1981) Phycology and heavy metal pollution. Biol Rev 56:99–151
Visiviki I, Rachlin JW (1994) Acute and chronic exposure of Dunaliella salina and Chlamydomonas bullosa to copper and cadmium: effects on ultrastructure. Arch Environ Contam Toxicol 26:154–162
Chan K, Wong SLL (1987) Ultrastructural changes of Chaetomorpha brachygona growing in metal environment. Cytologia 52:97–105
Wong SL, Nakamoto L, Wainwright JF (1994) Identification of toxic metals in affected algal cells in assays of wastewaters. J Appl Phycol 6:405–414
Shehata SA, Lasheen MR, Kobbia IA, Ali GH (1999) Toxic effects of certain metal mixtures on some physiological and morphological characteristics of freshwater algae. Water Air Soil Pollut 100:119–135
Okay O, Gaines A (1996) Toxicity of 2, 4- D acid to phytoplankton. Wat Res 30:688–696
USEPA (1993) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms in Environmental Monitoring Systems Laboratory. EPA-600/4-90/027F. USEPA, Cincinnati, OH, p 293
Leblond JB, Duffy, LK (2001) Toxicity assessment of total dissolved solids in effluent of Alaska mines using 22-h chronic microtox and Selenastrum capricornitium assay. Sci Total Environ 271:49–59
Saçan MT, Balcıoğlu IA (2006) A case study on algal response to raw and treated effluents from an aluminum plating plant and a pharmaceutical plant. Ecotoxicol Environ Saf 64:234–243
Hayat MA (1972) Basic electron microscopy techniques. Van Nostrand Reinhold, New York, p 119
Hoshaw RW, Maluf LY (1981) Ultrastructure of the green flagellate, Dunaliella tertiolecta (Chloropyhceae, Volvocales) with comparative notes on three other species. Phycologia 20:199–206
Dunstan WM (1975) Stimulation and inhibition of phytoplankton growth by low molecular weight hydrocarbons. Mar Biol 31:305–310
Delistrary D (1986) Growth and photosynthetic responses of a freshwater alga, Selenastrum capricornitum, to oil shale by-product water. Bull Environ Contam Toxicol 36:114–121
Sarabia R, Torreblanca A, Del Ramo JJ, Diaz-Mayans J (1998) Review: effect of low mercury concentration on hatching in the Artemia strain La Mata parthenogenetic diploid. Comp Biochem Physiol Part A Mol Integr Physiol 120:93–97
Sauve S, Brousseau P, Pellerin J, Morin Y, Senecal L, Goudreau P, Fournier M (2002) Phagocytic activity of marine and freshwater bivalves: in vitro exposure of hemocytes to metals (Ag, Cd, Hg and Zn). Aquat Toxicol 58:189–200
Srivastava N, Sahai R (1987) Effects of distillery waste on the performance of Cicerarictinum L. Environ Pollut 43:91–102
Joy CM (1990) Toxicity testing with freshwater algae in River Periyar (India). Bull Environ Contam Toxicol 45:915–922
Schmitt-Jansen M, Altenburger R (2005) Toxic effects of isoproturon on peripyhton communities—a microcosm study. Estuar Coast Shelf Sci 62:539–545
Mooney HM, Patching JM (1998) Electron microscopy of the marine microalga Dunaliella tertiolecta exposed to triphenyltin. J Ind Microbiol Biotech 20:200–204
Rachlin JW, Jensen TE, Baxter M, Jani V (1982) Utilization of morphometric analysis in evaluating response of Plectonema boryanum (Cyanophyceae) to exposure to eight heavy metals. Arch Environ Contam Toxicol 11:323–333
Smith MA (1983) The effect of heavy metals on the cytoplasmic fine structure of Skeletonema costatum (Bacillariophyta). Protoplasma 116:24–33
Sicko-Goad L, Stoermer EF (1979) A morphometric study of lead and copper effects on Diatoma tenue var elongatum (Bacillariophyta). J Phycol 15:316–321
Nishikawa K, Yamakoshi Y, Uemura I, Tominaga N (2003) Ultrastructural changes in Chlamydomonas acidophila (Chlorophyta) induced by heavy metals and polyphosphate metabolism. FEMS Microbiol Ecol 44:253–259
Rachlin JW, Jensen TE, Warkentina BE (1985) Morphometric analysis of response of Ananaena flos-aquae and Anabaena variabilis (Cyanophyceae) to selected concentrations of zinc. Arch Environ Contam Toxicol 14:395–402
Sauvant MP, Pepin D, Bohatier J, Groliere CA (2000) Effect of chelators on the acute toxicity and bioavailability of aluminum to Tetrahymena pyriformis. Aquat Toxicol 47:259–275
Rachlin JW, Jensen TE, Warkentine B (1984) The toxicological response of the alga Anabena flos-aquae (Cyanophyceae) to cadmium. Arch Environ Contam Toxicol 13:143–151
Rai LC, Jensen TE, Rachlin JW (1990) A morphometric and X-ray energy dispersive approach to monitoring pH-altered cadmium toxicity in Anabena flos-aquae. Arch Environ Contam Toxicol 19:479–487
Folsom BR, Popescu NA, Wood JM (1986) Comparative study of aluminum and copper transport and toxicity in an acid-tolerant freshwater green alga. Environ Sci Technol 20:616–620
Santana-Casiana JM, Gonzales-Davila M, Laglera LM, Perez-Pena J, Brand L, Millero FJ (1997) The influence of zinc, aluminum and cadmium on the uptake kinetics of iron by algae. Mar Chem 59:95–111
Schnitzer M, Kenndorf H (1981) Reactions of fulvic acids with metal ions. Water Air Soil Pollut 15:97–108
Acknowledgment
The authors would like to thank the technicians of the Electron Microscopy Unit of Marmara University for the use of the JEM-1200 Ex-II electron microscope and for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saçan, M.T., Oztay, F. & Bolkent, S. Exposure of Dunaliella tertiolecta to Lead and Aluminum: Toxicity and Effects on Ultrastructure. Biol Trace Elem Res 120, 264–272 (2007). https://doi.org/10.1007/s12011-007-8016-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-007-8016-4