Abstract
Purpose
High-intensity long-interval training (long-HIIT; interval ≥ 1 min; intensity 85–100% of maximal oxygen uptake [V̇O2max]) is often applied for cardiorespiratory adaptations; however, long-HIIT can also challenge the anaerobic and neuromuscular systems. Therefore, this study aimed to investigate the effects of 4-week long-HIIT (11 sessions) on anaerobic capacity, repeated sprint ability (RSA), and neuromuscular function.
Methods
Twenty active men (V̇O2max: 44.8 ± 5.3 mL.kg−1.min−1) performed an incremental running test (TINC), a supramaximal test consisting in running until the task failure at 115% of maximum velocity achieved in TINC (VINC) for anaerobic capacity determination, and an RSA test (2 × 6 × 35-m all-out sprints) pre- and post-HIIT. Before and after RSA, the neuromuscular function was assessed with counter movement jumps (CMJ) and knee extensors maximal isometric voluntary contractions (MVC) with femoral nerve electrical stimulation. Long-HIIT consisted of 10 × 1-min runs at 90% of VINC with 1-min recovery.
Results
Long-HIIT induced significant increase in V̇O2max (P = 0.0001). Although anaerobic capacity did not change significantly, 60% of the participants improved above the smallest worthwhile change (0.2 × standard deviation of pre-HIIT). The changes in sprint performance over RSA was significantly less post-HIIT than pre (P = 0.01). RSA induced significant drop of MVC, high frequency doublet, voluntary activation and CMJ performance at pre- and post-HIIT (P < 0.01); however, the percentage of reduction from rest to fatigued conditions were not significantly altered at post-HIIT compared to Pre.
Conclusions
11 sessions of long-HIIT over 4-week improved maximal aerobic power but not anaerobic capacity, and neuromuscular function. Yet, neuromuscular fatigue was similar despite greater speeds reached during RSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-intensity interval training (HIIT) is characterized by intermittent efforts of vigorous exercise, interspersed by periods of rest or low intensity exercise [1]. HIIT has been proposed as a time-efficient alternative to traditional continuous/endurance training, promoting similar adaptations in several cardiorespiratory, physiological, and health-related markers in healthy and diseased populations [1,2,3]. In addition, the acute and chronic physiological effects of HIIT programs are directly related to the precise manipulation of several variables (i.e., intensity, interval duration, relief duration, etc.) that compose the different HIIT set ups [3].
Buchheit and Laursen [3] characterized the physiological strain/adaptations induced by each type of HIIT. Although the enhancement of cardiorespiratory system is often assumed as the primary objective of a HIIT program [1, 3], short interval HIIT (short-HIIT—intervals < 1 min, intensity 100–120% maximal oxygen uptake [V̇O2max] and work:rest ratio = 1:1) and especially sprint interval training (SIT—intervals 10–30 s, intensity between 150% V̇O2max—all out and work:rest ratio = 1:4–1:8) can substantially challenge/enhance the non-oxidative energy pathways (i.e., glycolytic and phosphagen pathways) [3, 4] and neuromuscular system [5, 6]. The anaerobic and neuromuscular adaptations following long interval HIIT (long-HIIT—interval ≥ 1 min and intensity 85–100% V̇O2max), however, have been under investigated.
Panissa et al. [7] measured the contribution of the anaerobic pathways during a long-HIIT session composed of 10 × 1-min runs at 100% of V̇O2max, interspaced by 1 min of passive recovery, and demonstrated that anaerobic contribution was 32 ± 5% of total energy expenditure when considering the exercise efforts plus recovery, and 46 ± 5% when considering only efforts, confirming a relevant contribution of anaerobic pathways to the total energy expenditure during a long-HIIT session. This may explain why Little et al. [2] showed an 11% improvement in an anaerobic cycling time-trial (time needed to reach 50 kJ, ~ 120 s at mean power of 436 ± 22 W) after only six sessions of long-HIIT.
Regarding neuromuscular adaptations induced by long-HIIT, data are even scarcer [8]. Martinez-Valdes et al. [8] investigated the effect of long-HIIT on neuromuscular function and showed a ~ 7% increase in knee extensors maximal torque after six sessions of long-HIIT. Electromyographic activity of vastus medialis, and vastus lateralis muscles also increased by ~ 17% and ~ 14%, respectively, suggesting neural adaptations.
Based on the previously established anaerobic and neuromuscular demand of long-HIIT, this type of training could be considered as an interesting protocol to enhance the anaerobic system and neuromuscular function and must be investigated for a more comprehensive understand. Similarly, the ability to perform sprints repeatedly (RSA) with short recovery periods could also benefit, since RSA has a high energetic contribution from anaerobic pathways [9, 10], and induces significant neuromuscular fatigue at the peripheral and central levels [11, 12]. Finally, the utilization of long-HIIT, as opposed to short-HIIT and SIT, may be a safer and more appealing strategy, especially for non-trained individuals (i.e., sedentary and physically active), who may not tolerate the extreme intensity of short-HIIT and SIT [1].
Therefore, the aim of the present study was to investigate the effect of long-HIIT program on the anaerobic energy systems, RSA performance and neuromuscular function. Since the present investigation is the first to perform a comprehensive analysis of the effects of a long-HIIT program on anaerobic capacity and neuromuscular function, we hypothesized that, besides aerobic gains, long-HIIT will enhance anaerobic capacity and, consequently, improve RSA performance. We also hypothesized that the greater amount of work done during RSA will increase the neuromuscular fatigue after long-HIIT.
Methods
Participants
Twenty healthy men, physically active (age: 25 ± 5 years; height: 1.74 ± 0.07 m; weight: 73.5 ± 8.4 kg; V̇O2max: 44.8 ± 5.3 mL.kg−1.min−1) participated in the study. Participants were free of any musculoskeletal disorders and were requested to abstain from strenuous activities and caffeine for 24 h and consume a light meal 2 h before each session. The study was approved by the Local Ethics Committee and was conducted according to the Declaration of Helsinki. All participants were informed about the procedures, benefits and risks of the investigation before signing an informed consent form prior to beginning the study.
Experimental design
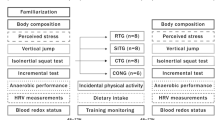
The participants attended two familiarization sessions and were evaluated pre- and post-HIIT at the same time of the day (± 3 p.m.). Each evaluation consisted of three sessions carried out on separate days with 48 h recovery between them (Fig. 1). On day 1, the participants performed an incremental running test (TINC) until task failure. A supramaximal running test (TSUPRA) to the task failure and the RSA test were performed on days 2 and 3. Before (at rest) and after (fatigued state) RSA, neuromuscular assessments were performed consisting of three counter movement jumps (CMJ) and three maximal voluntary isometric contractions (MVC) with electrically evoked contractions. During TINC and TSUPRA, oxygen uptake (V̇O2) was measured breath-by-breath using an ergospirometer (Quark PFT, Cosmed, Rome, Italy) and heart rate was measured using a transmitter belt coupled to the gas analyzer (Wireless HR 138 monitor; Cosmed, Rome, Italy). The blood lactate concentration was analyzed in an electrochemical analyzer YSI 2300 STAT (Yellow Spring Instruments, Yellow Spring, OH, USA). The physiological variables analyze described in detail in Milioni et al. [13]
Schematic representation of the experimental protocol. Numbers between brackets are recovery time. VINC Maximal velocity achieved in incremental treadmill-running test; CMJ Counter movement jump; MVC Maximal isometric voluntary contractions of knee extension; NMA Neuromuscular function assessment; TINC Incremental treadmill-running test; TSUPRA Supramaximal running test; RSA Repeated sprint ability test
Approximately 72–96 h following the RSA test, the participants started the long-HIIT program, which lasted 4 weeks. The post-HIIT evaluations were performed 72–96 h after the last training session (Fig. 1).
Incremental running test (TINC)
After 5-min warm up at 8 km.h−1, participants performed the TINC on a stationary treadmill (ATL, Inbramed, Porto Alegre, Brazil), which started at 8 km.h−1 with increments of 1.5 km.h−1 every 2 min until task failure [13]. The V̇O2max, maximal heart rate (HRmax), maximal velocity achieved in the TINC (VINC) and peak blood lactate concentration ([La]peak) were determined according Milioni et al. [13] procedures.
Supramaximal running test (TSUPRA) and anaerobic capacity (AC[La+PCr]) determination
The participants remained seated for 10 min to determine baseline V̇O2 (average of the last 2 min) and blood lactate concentration. After the baseline measurements, the participants performed a warm-up at 8 km.h−1 for 5 min followed by a supramaximal running at 115% of VINC until the task failure [14, 15]. Determination of AC[La+PCr] was the primary aim of the TSUPRA; since the intensity in which the test is carried out is protocol-dependent [14, 15], the intensity of TSUPRA carried out at post-HIIT was properly adjusted according the post-HIIT TINC performance. After the task failure, VO2 measurements continued for ~ 10 min (until the VO2 stabilization at 2–3 mL.kg−1.min−1 above baseline) to determine the fast phase of the excess post-oxygen consumption (EPOCfast). Blood samples were collected 3, 5, and 7 min after the task failure for determination of the peak blood lactate concentration [14, 15].
The sum of the oxygen equivalents from the glycolytic (ELa) and phosphagen (EPCr) energy systems was considered as a representative index of the anaerobic capacity (AC[La+PCr]) [14, 15] and was estimated according Zagatto et al. [14] and Miyagy et al. [15]. ELa was estimated by the net blood lactate concentration, after subtracting the baseline values from the peak blood lactate concentration after TSUPRA, and assuming a correction factor of 3 mL.kg−1 of oxygen for each 1 mmol.L−1 of net blood lactate accumulation [14, 15]. EPCr was calculated as the product between the amplitude 1 and time constant 1 from EPOCfast after a biexponential mathematical fit (OriginPro 8.0 software, OriginLab Corp, Northampton, MA, USA) [14, 15].
Repeated sprint ability test (RSA)
After a 5 min warm-up at 8 km.h−1, the participants performed the running-based anaerobic sprint test (RAST) twice [10, 16], interspaced by 4-min of passive recovery. Briefly, the RAST consisted of six 35-m all-out efforts with 10-s passive recovery between sprints. The variables extracted from the RSA were total time (sum of 35-m sprint times), best time (best 35-m sprint time), performance decrement [100 × (total time / (best time × 12)–100] and peak blood lactate concentration. The procedures of RSA data collection and analysis are fully described in Milioni et al. [13].
Neuromuscular function assessments
Pre- and post-HIIT, neuromuscular function was assessed by CMJ and MVC with electrically evoked contractions, at rest (before RSA) and at fatigued state (after RSA).
Vertical jumps. A set of three CMJ (Jump test, CEFISE, Nova Odessa, SP, Brazil) with a 1-min rest between jumps was performed, and the highest jump attempt (cm) of each participant was measured [13].
Force measurements and femoral nerve electrical stimulations. After the jumps, the participants performed three MVCs with a 1-min rest, and the highest MVC (N) of each participant was used to subsequent analysis. The hips and knees were firmly fixed in a chair at 90° flexion with straps crossed on the chest, hip, and thigh. To measure the isometric force of the knee extension, the ankle of the dominant leg was attached to a load cell (SDK200, Miotec, Porto Alegre, RS, Brazil). The force signal was acquired at 2000 Hz. Supramaximal, square wave, electrical pulses were delivered on the femoral nerve by a constant current electrical stimulator (Bioestimulador, Insigth, Ribeirão Preto, SP, Brazil) (400 V max). Electrodes with conductive gel (5 × 5 cm) were placed in the femoral triangle (cathode) and the gluteal fold (anode). The optimal intensity of stimulation was determined by the application of consecutive and incremental doublet pulses (100 Hz; Db100) to the relaxed muscle until reaching the twitch force plateau [17]. Supramaximal stimulation was ensured by increasing the stimulation intensity by 20%. The following sequence of femoral nerve electrical stimulations was applied: Db100 superimposed to MVC (Db100sup) and potentiated Db100 on relaxed muscle 5 s after MVC termination (Fig. 1).
The data analyses and variables extraction (MVC peak force, Db100 and voluntary activation [VA]) were carried out using specific MatLab algorithms (The Math Works Inc, Natick, MA) according to Milioni et al. [13].
High intensity interval training (HIIT)
The long-HIIT program was derived from Little et al. [2]. After a 5-min warm-up at 8 km.h−1, the HIIT sessions consisted of 10 × 1-min runs at 90% of VINC with 1 min of passive recovery. Participants were required to achieve at least 90% of HRmax in the last 5 runs, and if this was not achieved, the intensity was increased by ~ 3% of VINC in the subsequent HIIT session. HR (Polar RS400, Kempele, Finland) was measured in each run. All training sessions were performed on a treadmill (ATL, Inbramed, Porto Alegre, RS, Brazil), and participants were required to attend 11 training sessions over 4 weeks (3 sessions in the first 3 weeks and 2 sessions in the last week) with 36 h–72 h rest between sessions.
Statistical analysis
All data are presented as mean ± standard deviation (SD) and effect size (i.e., Cohen’s d for t test (ES) and partial eta square for ANOVA [η2]). Initially, the Shapiro–Wilk test was performed to confirm normality of the data. A paired student t test was used to compare variables from the TINC, TSUPRA, and RSA (pre- vs. post-HIIT).
The neuromuscular function (CMJ, MVC, and evoked stimulations) was analyzed by a two-way ANOVA (RSA effect [rest and fatigued] × time [pre- and post-HIIT]) for repeated measures. The Mauchly’s sphericity test was applied and corrected by Greenhouse–Geisser when the sphericity was violated. The Sidak post hoc was applied when significant differences were demonstrated by the ANOVA analyses. For all cases, the significance level was determined as 5% (P < 0.05).
In addition, the TSUPRA and RSA outcomes were qualitatively analyzed. The smallest worthwhile change (SWC) was calculated (pre-HIIT SD multiplied by 0.2) [18] and the individual pre- to post-HIIT variation (Δ) of variables was plotted. The number of cases among the 20 participants in which the Δ were greater than the SWC were counted.
Results
HIIT
All participants successfully completed the training intervention. The intensity of the first training session was 12.4 ± 1.5 km.h−1 (90 ± 0.0% VINC), while the final session was performed at 13.6 ± 1.7 km.h−1 (99.0 ± 2.6% VINC). The percentage of HRmax reached in the last five runs (89 ± 2%) over the 4 weeks of training was not statistically different [F(1, 19) = 2.2; P = 0.06) between HIIT sessions.
Incremental running test
V̇O2max, VINC, and peak blood lactate concentration increased significantly after 4 weeks of HIIT, whereas HRmax was not significantly different between pre- and post-HIIT (Table 1).
Supramaximal running test
No significant differences were found between pre- and post-HIIT for AC[La+PCr], ELa, EPCr and peak blood lactate concentration. Time-to-task failure during TSUPRA was significantly decreased at post-HIIT (Table 1).
V̇O2max maximal oxygen uptake; VINC highest velocity achieved during TINC; [La]peak peak of blood lactate concentration; HRmax maximal heart rate; AC[La+PCr] anaerobic capacity; ELa oxygen equivalents from the glycolytic energy systems; EPCr oxygen equivalents from the phosphagen energy systems; TTF time-to-task failure during TSUPRA.
Repeated sprint ability test
The sprint times increased significantly throughout the RSA test [F(1, 19) = 79.4; P < 0.001; η2: 0.81]. There was a significant sprint × time interaction [F(1, 19) = 7.2; P < 0.001; η2: 0.28], with slower velocity during sprint #2 at post-HIIT compared to pre-HIIT (P = 0.009), and faster velocities during #10, #11 and #12 sprints at post-HIIT compared to pre-HIIT (P < 0.043) (Fig. 2). There were no significant changes from pre- to post-HIIT for total time or best time. However, performance decrement and peak blood lactate were significantly decreased at post-HIIT (Table 2).
%Dec performance decrement; [La]peak peak of blood lactate concentration.
Smallest worthwhile change of AC[La+PCr] and RSA variables
Absolute pre- to post-HIIT change above the SWC for AC[La+PCr], ELa and EPCr was 60% (12 out of 20), 45% (9 out of 20) and 60% (12 out of 20) of the participants, whereas 35% of the participants (7 out of 20) improved the total time and 25% (5 out 20) improved the best time above the SWC (Fig. 3).
Neuromuscular function assessments
RSA induced a significant decrement in MVC [F(1, 19) = 32.7; P = 0.001; η2: 0.53], Db100 [F(1,19) = 12.5; P = 0.01; η2: 0.32], VA [F(1,19) = 11.7; P = 0.001; η2: 0.30] and CMJ performance [F(1,19) = 112.3; P = 0.001; η2: 0.79], at pre- and post-HIIT. There was no significant RSA effect × time interaction for any variable [F(1,19) < 1.2; P > 0.28; η2: < 0.14]. The percentage of reduction induced by RSA for MVC (P = 0.44; ES: 0.13), Db100 (P = 0.93; ES: − 0.02), VA (P = 0.98; ES: 0.01) and CMJ performance (P = 0.14; ES: 0.29) were not statistically different between pre- and post-HIIT (Fig. 4).
Left panels: mean ± SD outcomes of neuromuscular function assessment before (at rest) and after (fatigued state) RSA at pre- and post-HIIT. Right panels: percentage of reduction induced by RSA at pre- and post-HIIT. *P < 0.05 comparison between rest and fatigue of the same time; **P < 0.01 comparison between rest and fatigue of the same time
Discussion
The present study investigated the effects of 11 long-HIIT training sessions covered in 4 weeks on anaerobic capacity, neuromuscular function and RSA performance. In addition to the expected aerobic gains, the main findings were the non-significant changes in AC[La+PCr] or RSA performance. Although changes in AC[La+PCr] and EPCr presented a high rate of responsiveness (60% of the participants) that was not directly transfer into RSA total time and best time performance. Neuromuscular fatigue after 4 weeks of long-HIIT was similar despite greater speeds reached during RSA.
Anaerobic capacity adaptations after long-HIIT
The ~ 5% improvement in V̇O2max as well as the significant increase in VINC, corroborate many previous studies indicating that long-HIIT is effective for inducing positive adaptations in the cardiorespiratory and oxidative systems [19,20], because it allows individuals to spend a long time in the so-called red zone, i.e., above 90% of V̇O2max [3,21]. Lee et al. [22] and Campos et al. [23] reported RSA improvements along with an impressive V̇O2max enhancement after HIIT program (+ 18.4%; 51.9 ± 9.2 mL.kg−1.min−1 to 61.4 ± 12.2 mL.kg−1.min−1 and ~ + 6%; 56.5 ± 5.2 mL.kg−1.min−1 to 59.9 ± 4.1 mL.kg−1.min−1, respectively), contrary to McGinley and Bishop [24] who did not show any alteration in V̇O2max (~ + 1.7%; 47.7 ± 5.5 mL.kg−1.min−1 to 48.5 ± 5.1 mL.kg−1.min−1). The enhancement of V̇O2max shown in the present study is more modest than Lee et al. [22], similar to Campos et al. [23], but higher than McGinley and Bishop [24] and may play an important role in performance maintenance during short sprints (≤ 10 s). Indeed, Milioni et al. [10] found a significant increase in the oxidative system contribution after the third sprint during 6 × 35-m all-out sprints with 10 s of passive recovery, as well as a significant association with total time, best time, worst time, and mean time. Reinforcing these findings, McGawley and Bishop [25] found strong correlations (r = 0.81–0.93, P < 0.01) between the oxidative contribution during the fifth, sixth, and tenth 6-s all-out.
We hypothesized that 4 weeks of long-HIIT would enhance AC[La+PCr], however, that was not confirmed. The present study qualitatively analyzed results based on absolute variation between pre- and post-HIIT and the smallest worthwhile change (SWC), since sometimes “null hypothesis” statistics is not sensitive enough to detect small important changes induced by a training intervention [26]. In the present study, 60% of the participants improved their AC[La+PCr] above the meaningful threshold of SWC, and among the twelve participants that increased the AC[La+PCr], nine of them also increased their EPCr above the SWC.
The 1-min passive recovery between bouts may allow significant replenishment of the muscle PCr storage [9], reloading this energy system for the subsequent effort, which may induce positive adaptations to the phosphagen system as suggested by Bishop et al. [27]. In addition, the readiness of the phosphagen system and the 1-min work interval at submaximal intensity (i.e., 90% of VINC) may demand less from the glycolytic system [5], not being enough to enhance this energy system. In fact, when longer work intervals are used (i.e., 4 min instead 1 min), significant increase in peak lactate concentration was verified [28]. During supramaximal efforts (i.e., 115% of V̇O2max intensity), the glycolytic contribution is close to 60% of the total anaerobic energy expenditure [14, 15]; therefore, this non-significant improvement in glycolytic system may explain the lack of AC[La+PCr] enhancement.
Still, the key factor to improve anaerobic capacity might be the exercise intensity and, consequently, the high demand of the non-mitochondrial energy pathways, since Tabata et al. [29] and Ravier et al. [30] found significant improvement of maximal accumulated oxygen deficit (28–10.3%) after 2–4 training sessions per week during 6–7 weeks, composed of 6–9 bouts of 20 s of cycling/running at 140–170% of intensity attained at V̇O2max with 10–15 s of passive recovery.
The decrease in TTF at post-HIIT is difficult to explain. A recent study from our research group has investigated this particular topic and tested supramaximal efforts at 150% of respiratory compensation point (RCP) physiologically matched for the physical fitness status verified at pre- and post-5 weeks of SIT. The glycolytic pathway (content and activity of several glycolytic enzymes), buffering capacity and glycogen content were significantly increased after SIT. However, the TTF was not altered after training at matched intensity, nor was the capacity to access the glycogen content. In spite of the different HIIT set ups investigated in both studies (long-HIIT vs. SIT), these results may be related to a possible decrement of the sensitivity of catecholamine secretion due an over-stimulation of sympathetic system by the high intensities of the training sessions, leading to a decrease of glycogenolysis activity and consequently affecting the supramaximal exercise performance [31].
Repeated sprint ability after long-HIIT
Total time and best time for the RSA were not modified following training and there was a low responsiveness of the participants (35% were above the SWC for total time and 25% for best time). The absence of an improvement in best time was expected and was likely related to the long-HIIT set up, since shorter intervals with higher intensity are closely linked to sprint speed development [3, 5]. In contrast, the performance in sprints #10, #11 and #12 were significantly faster at post-HIIT compared with pre-HIIT, which is in line with the improvement of performance decrement which, once again, may be explained by the augmented V̇O2max verified post-HIIT.
This particular topic (i.e., RSA enhancement by long-HIIT) is not deeply investigated in literature, and the different long-HIIT set ups may be an important confounding factor and generate divergent results. For instance, after long-HIIT, Lee et al. [22] found significant improvements in peak power output, mean power output and performance decrement during 6 × 10-s all-out cycling sprints with 1-min active recovery at 50 W, Campos et al. [23] found improvement only in a mean time of a specific RSA test and McGinley and Bishop [24] did not find any significant change in total work and performance decrement during 5 × 6-s all-out cycling sprints with 24-s passive recovery.
Neuromuscular function assessment
As expected, all-out repeated sprints (i.e., RSA) induced both central and peripheral fatigue [12, 13, 32] at pre- and post-HIIT. In addition, the long-HIIT program did not change neuromuscular function at rest or in a fatigued state, as well as did not alter neuromuscular fatigue resistance either, since the percentage of reduction induced by RSA at pre- and post-HIIT was not statistically different for any neuromuscular variable. Yet, the same neuromuscular fatigue was observed with more power produced during the sprints #10, #11 and #12.
The same level of neuromuscular fatigue between pre- vs. post-HIIT after RSA may be explained by the difference in the muscle mass involved during the training interventions (i.e., entire lower limbs) and that assessed for fatigue by the neuromuscular evaluations (i.e., only quadriceps), which may not reflect the overall fatigue derived from exercise [33]. In addition, the time-window to carried out the neuromuscular assessments after sprints (~ 3 min), added to an improved oxidative system, may allowed the washout of the metabolites produced by RSA and a faster recovery and performance [17, 32].
More intense HIIT set ups, especially SIT, have shown effective results in terms of neuromuscular function, inducing enhancement of muscle activation, maximal force, explosive force, and reducing the co-activation of agonist muscle [6, 34]. The higher intensities and shorter intervals induce more accelerations, decelerations and re-accelerations, generating higher neuromuscular loads [5].
Regarding the CMJ performance, which reflects muscle activation and muscle contractile properties [5], the present study presented a negligible + 0.1% improvement (at rest) after 11 sessions of long-HIIT. To the best of our knowledge, only two works have investigated the effect of long-HIIT on CMJ performance. Whereas Viaño-Santasmarinas et al. [35] showed non-significant ~ + 1.6% increase after 12 sessions, Campos et al. [23] found ~ + 8.6% after 8 sessions. Both studies [23, 35] were conducted with intermittent sports athletes (handball and futsal), who remain in their daily-based training routine, including strength training and technical–tactical sport-related sessions with jumps and change of direction, which may explain the discrepant results among three studies.
Limitations
The absence of a control group and/or a group performing another type of HIIT is a negative point in our investigation. In addition, the fact that the subjects in the present study were not specifically trained may generate different results compared to the same intervention (i.e., 4-week long-HIIT) applied to highly trained subjects or sprint-trained subjects. Finally, the qualitative approach provide in the present study for anaerobic capacity and RSA performance outcomes may be read with caution. This is an attempt to provide to the reader the small influence of the long-HIIT on anaerobic capacity, which may be not sensitive enough to be detected by null hypothesis statistics, yet may be important for coaches and practitioners.
Conclusion
The findings of the present study provide a comprehensive overview of the effects of a short-term (4 weeks) long-HIIT program. It confirmed the significantly increases V̇O2max and, more importantly, adds new knowledge on the effects of this type of training. No significant differences of anaerobic capacity were verified, the capacity to maintain RSA performance was also improved and the enhanced oxidative system may have played an important role in this outcome. The long-HIIT did not alter neuromuscular function at rest or in a fatigue state, or affect neuromuscular fatigue resistance. Most likely, the training intensity is the key factor to anerobic and neuromuscular adaptations, as induced by short-HIIT and SIT [3, 5].
Practical perspectives
The present investigation is highly applicable in a practical context, since data regarding the anaerobic and neuromuscular adaptations induced by long-HIIT interventions are scarce and the present work provides evidence that may help athletes and coaches to accurately plan the adaptations promoted by a long-HIIT program. The participants who improved their anaerobic capacity above the meaningful threshold of SWC after this specific long-HIIT model (60% of the participants) may be due the discrete enhancement in the phosphagen pathway contribution. Future studies should (i) investigate the effects of different periods of training interventions (i.e., 2–8 weeks) and (ii) test different populations such as highly trained athletes.
Data availability
All the statistical data used to support the findings are included in the article.
References
Gibala MJ, Little JP, MacDonald MJ, Hawley JA (2012) Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 590(5):1077–1084
Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ (2010) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588(6):1011–1022
Buchheit M, Laursen PB (2013) High-intensity interval training, solutions to the programming puzzle: Part I: Cardiopulmonary emphasis. Sports Med 43(5):313–338
Buckley S, Knapp K, Lackie A, Lewry C, Horvey K, Benko C et al (2015) Multimodal high-intensity interval training increases muscle function and metabolic performance in females. Appl Physiol Nutr Metab 40(11):1157–1162
Buchheit M, Laursen PB (2013) High-intensity interval training, solutions to the programming puzzle: Part II: Anaerobic energy, neuromuscular load and practical applications. Sports Med 43(10):927–954
Creer AR, Ricard MD, Conlee RK, Hoyt GL, Parcell AC (2004) Neural, metabolic, and performance adaptations to four weeks of high intensity sprint-interval training in trained cyclists. Int J Sports Med 25(2):92–98
Panissa VLG, Fukuda DH, Caldeira RS, Gerosa-Neto J, Lira FS, Zagatto AM et al (2018) Is oxygen uptake measurement enough to estimate energy expenditure during high-intensity intermittent exercise? Quantification of anaerobic contribution by different methods. Front Physiol 9:1–8
Martinez-Valdes E, Falla D, Negro F, Mayer F, Farina D (2017) Differential motor unit changes after endurance or high-intensity interval training. Med Sci Sports Exerc 49(6):1126–1136
Gaitanos GC, Williams C, Boobis LH, Brooks S (1993) Human muscle metabolism during intermittent maximal exercise. J Appl Physiol 75(2):712–719
Milioni F, Zagatto AM, Barbieri RA, Andrade VL, Dos Santos JW, Gobatto CA et al (2017) Energy systems contribution in the running-based anaerobic sprint test. Int J Sports Med 38(3):226–232
Girard O, Bishop DJ, Racinais S (2013) Neuromuscular adjustments of the quadriceps muscle after repeated cycling sprints. PLoS ONE 8(5):1–9
Tomazin K, Morin JB, Millet GY (2017) Etiology of neuromuscular fatigue after repeated sprints depends on exercise modality. Int J Sports Physiol Perform 12(7):878–885
Milioni F, De Poli RAB, Saunders B, Gualano B, Da Rocha AL, Da Silva ASR et al (2019) Effect of β-alanine supplementation during high-intensity interval training on repeated sprint ability performance and neuromuscular fatigue. J Appl Physiol 127(6):1599–1610
Zagatto AM, Bertuzzi R, Miyagi WE, Padulo J, Papoti M (2016) MAOD determined in a single supramaximal test: A study on the reliability and effects of supramaximal intensities. Int J Sports Med 37(9):700–707
Miyagi WE, de Poli RA, Papoti M, Bertuzzi R, Zagatto AM (2017) Anaerobic capacity estimated in a single supramaximal test in cycling: Validity and reliability analysis. Sci Rep 7:42485
Zagatto AM, Beck WR, Gobatto CA (2009) Validity of the running anaerobic sprint test for assessing anerobic power and predicting short-distance performances. J Strength Cond Res 23(6):1820–1927
Milioni F, Vieira LHP, Barbieri RA, Zagatto AM, Nordsborg NB, Barbieri FA et al (2016) Futsal match-related fatigue affects running performance and neuromuscular parameters but not finishing kick speed or accuracy. Front Physiol 7:1–10
Cohen J (1988) Statistical power analysis for the behavioral sciences. Vol. 2nd, Statistical Power Analysis for the Behavioral Sciences. LawrenceErlbaum, Hillsdale
Perry CGR, Heigenhauser GJF, Bonen A, Spriet LL (2008) High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl Physiol Nutr Metab 33(6):1112–1123
Perry CGR, Talanian JL, Heigenhauser GJF, Spriet LL (2006) The effects of training in hyperoxia vs normoxia on skeletal muscle enzyme activities and exercise performance. J Appl Physiol 102(3):1022–1027
Millet GP, Candau R, Fattori P, Bignet F, Varray A (2003) Responses to different intermittent runs at velocity associated with. Can J Appl Physiol 28(3):410–423
Lee CL, Hsu WC, Cheng CF (2017) Physiological adaptations to sprint interval training with matched exercise volume. Med Sci Sports Exerc 49(1):86–95
Campos FS, Borszcz FK, Flores LJF, Barazetti LK, Teixeira AS, Hartmann Nunes RF et al (2021) HIIT models in addition to training load and heart rate variability are related with physiological and performance adaptations after 10-weeks of training in young futsal players. Front Psychol 12:636123
McGinley C, Bishop DJ (2016) Influence of training intensity on adaptations in acid/base transport proteins, muscle buffer capacity, and repeated-sprint ability in active men. J Appl Physiol 121(6):1290–1305
McGawley K, Bishop DJ (2015) Oxygen uptake during repeated-sprint exercise. J Sci Med Sport 18(2):214–218
Buchheit M (2016) The numbers will love you back in return — I promise. Int J Sports Physiol Perform 11(4):551–554
Bishop D, Edge J, Thomas C, Mercier J (2008) Effects of high-intensity training on muscle lactate transporters and postexercise recovery of muscle lactate and hydrogen ions in women. AJP: Regulatory, Integrative and Comparative Physiology. 295(6):1991–1998
Stöggl TL, Björklund G (2017) High intensity interval training leads to greater improvements in acute heart rate recovery and anaerobic power as high volume low intensity training. Front Physiol 8:1–8
Tabata I, Nishimura K, Kouzaki M, Hirai Y, Ogita F, Miyachi M et al (1996) Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and VO2max. Med Sci Sports Exerc 28(10):1327–1330
Ravier G, Dugué B, Grappe F, Rouillon JD (2009) Impressive anaerobic adaptations in elite karate athletes due to few intensive intermittent sessions added to regular karate training. Scand J Med Sci Sports 19(5):687–694
Poli RAB, Murias JM, Marinari G, Dutra YM, Milioni F, Zagatto AM (2024) Five weeks of SIT improve muscle glycolytic content and activity but not time to task failure in severe intensity exercise. Med Sci Sports Exerc 25:0. https://doi.org/10.1249/MSS.0000000000003425
Milioni F, Azevedo RA, Zagatto AM, Millet GY (2021) Time course of recovery after cycling repeated sprints. Med Sci Sports Exerc 53(2):413–420
Millet GY, Martin V, Martin A, Vergès S (2011) Electrical stimulation for testing neuromuscular function: From sport to pathology. Eur J Appl Physiol 111(10):2489–2500
Kinnunen JV, Piitulainen H, Piirainen JM (2017) Neuromuscular adaptations to short-term high-intensity interval training in female ice hockey players. J Strength Cond Res 33(2):479–485
Viaño-Santasmarinas J, Rey E, Carballeira S, Padrón-Cabo A (2018) Effects of high-intensity interval training with different interval durations on physical performance in handball players. J Strength Cond Res 32(12):3389–3397
Acknowledgements
The authors wish to thank participants for their enthusiastic participation and Dr Bryan Saunders for English proofread. This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP—protocol nº 2016/11076-6). FM, RABP, GMPB and ESM scholarships are supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP—protocols nº 2016/02683-6, 2016/17836-2, 2017/03660-2 and 2017/21724-8).
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo, protocol nº 2016/02683-6, protocol nº 2016/17836-2, protocol nº 2017/03660-2, protocol nº 2017/21724-8, protocol nº 2016/11076-6
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Fabio Milioni, Guillaume Y Millet, Rodrigo de Araújo Bonetti de Poli, Gabriel Motta Pinheiro Brisola, Elvis de Souza Malta, Paulo Eduardo Redkva, Fabio Augusto Barbieri and Alessandro Moura Zagatto. The first draft of the manuscript was written by Fabio Milioni, Guillaume Y Millet and Alessandro Moura Zagatto and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. Alessandro Moura Zagatto is currently Section Editor of Sport Sciences for Health.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committee (Ethics Committee, São Paulo State University, protocol No. 1.461.011/2016) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
All participants provided informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Milioni, F., Millet, G.Y., de Poli, R.A.B. et al. Effects of 4-week high intensity interval training on anaerobic capacity, repeated-sprints performance and neuromuscular function. Sport Sci Health 20, 1109–1118 (2024). https://doi.org/10.1007/s11332-024-01214-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-024-01214-8