Abstract
Purpose
This study examined the effects of photobiomodulation (PBM) associated with endurance running training on oxidative stress (OS) markers in untrained men.
Methods
Thirty untrained men distributed into a placebo group (PLA) or PBM group (PBMG) performed 8 weeks of running training, in which the PBMG had the LED application before all training sessions. The PBM was performed by LED application using an equipment with 56 diodes of red light (660 nm) and 48 diodes of infrared light (850 nm) with an energy dose of 60 J on each of the 5 points. The application was done in two regions of the quadriceps muscle, two regions of the biceps femoris, and one region of the gastrocnemius muscle in both legs. The assessments were performed at pre- and post-training, and the OS markers analyzed were malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH) and oxidized (GSSG).
Results
PBM associated with running training did not significantly affect the OS markers CAT, SOD, MDA, GSH, and reduced GSH/GSSG responses after training. GSSG levels decreased in the PBMG and increased in the PLA, with significant difference between the % change values (− 13.5 ± 30.3% vs. 56.3 ± 83.4%; P = 0.043).
Conclusions
Therefore, PBM associated with endurance running training did not significantly affect CAT, SOD, MDA, reduced GSH, and reduced GSH/GSSG ratio post-training; however, the GSSG increased in PLA group and decreased in PBMG, indicating a positive effect of the PBM in this marker of OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photobiomodulation (PBM), comprising the application of light-emitting diodes (LEDs) and as low-level lasers (LLL), combined with exercise is effective for improving muscular performance and reducing the signs of fatigue by accelerating the recovery process after exercise [1,2,3,4]. The main PBM effects were related to the increasing mitochondrial activity and ATP synthesis, improvement of tissue repair, decreasing levels of inflammatory markers and pain (e.g., delayed muscle soreness), increasing microcirculation, and positive effects on reducing oxidative stress (OS) [1,2,3,4].
Oxidative stress is related to high levels of reactive oxygen species (ROS) that exceed the capacity of the antioxidant defense system, leading to cell damage [5, 6]. It is well established that acute exercise is a factor that induces the increase of OS [7, 8]. Concerning the longitudinal effects after a period of exercise training, the majority of studies have demonstrated that training induces beneficial adaptations related to the reduction of lipid peroxidation markers and enhancement of the antioxidant defense system [5, 9, 10]; additionally, a recent systematic review and meta-analysis conducted by de Sousa et al. [11] investigated the effect of exercise on OS parameters and concluded that, regardless of the intensity, volume, or type of exercise, the antioxidant indicators tended to increase and pro-oxidant indicators tended to decrease after training. However, it is important to note that some studies have not demonstrated similar changes in OS markers, or reported any OS alterations post-training [12,13,14].
Furthermore, the OS response to exercise could be attenuated by PBM [15, 16]. Studies have shown that this intervention reported potential effects on counting the increase of ROS and malondialdehyde (MDA) and improving the antioxidant defense by increasing the superoxide dismutase (SOD) and catalase (CAT) activities [15, 16]. For the association of PBM with exercise training, one study with animal models showed that the group that performed swimming training combined with PBM applied before training sessions showed better results with respect to decreased OS than the other control groups [15]. In this context, the use of PBM associated with endurance running training would attenuate OS, and consequently optimize the physiological adaptations. However, it is important to take into account that there is no consensus on some parameters related to PBM application, and the energy dose applied is one such parameter, for which a biphasic dose–response pattern has been suggested for tissue stimulation through PBM [17, 18].
Therefore, because the effect of PBM associated with endurance running training on OS-related parameters in humans is unknown, we aimed to examine the effects of PBM associated with endurance running training on OS markers in untrained men. We hypothesized that the PBM group would report greater results in attenuating OS than the placebo group.
Methods
Participants
The sample size was calculated from a priori analysis for a group by time interaction comparison (F test; Anova: repeated measures, within-between interaction) according to an effect size of 0.52 (obtained from a pilot study), power of 80% and significance level of 5%. We used the software Gpower 3.1 (Düsseldorf, Germany) for the calculation. The a priori power analysis revealed a minimal sample of ten participants per group (n = 20).
Thus, 30 healthy, young, and untrained men volunteered to participate in this study and were randomized in 2 groups. Volunteers were excluded if they use regular pharmacological agents or nutritional supplements; if they were smoker, diabetic, hypertensive, asthmatic and/or present any cardiovascular disorder; present body mass index ≥ 30 kg m−2; and were engaged in other regular systematic physical training. Characteristics of the participants (mean ± SD) were age (years): PLA = 27.3 ± 5.2 and PBMG = 27.4 ± 3.7; height (m): PLA = 1.8 ± 0.1 and PBMG = 1.8 ± 1.0; body mass (kg): PLA = 80.2 ± 10.3 and PBMG = 79.2 ± 7.0; body mass index (kg m−2): PLA = 25.7 ± 2.9 and PBMG = 25.3 ± 2.5; body fat (%): PLA = 17.2 ± 5.4, PBMG = 17.7 ± 5.7. Prior to testing, written informed consent was obtained from all the participants, and all procedures and test protocols were explained individually to each participant. The protocol was approved by the Local Human Research Ethics Committee (#623.581/2014) and the experiments reported in the manuscript were performed in accordance with the ethical standards of the Helsinki Declaration.
Design and procedures
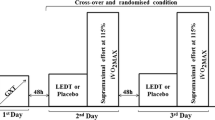
The present study was a randomized, double-blinded placebo-controlled experimental trial. The participants undertook four visits for baseline assessments (pre-training; week 1): 1—familiarization with the procedures and anthropometric measurements; 2—blood collection for OS markers; 3—incremental exercise test to determine peak treadmill speed, and 4—constant load test to determine time limit (tlim) at peak treadmill speed. These two running tests were used for training prescription. The visits were performed under laboratory conditions and the tests were performed on a motorized treadmill (Super ATL; Inbrasport, Porto Alegre, Brazil). After the baseline assessments, the participants were randomly distributed in two experimental groups: placebo group (PLA) or PBM group (PBMG). Both groups performed the same running training program, in which the only difference between the groups was that the PBMG received the LED application before each training session. The running tests were repeated after 4 weeks of running training at week 6 to re-prescribe training intensities during weeks 7–10 of training, and the blood collection was repeated at post-training (week 11).
Measures
Blood collection and biochemical analysis of oxidative stress (OS) markers
For the analysis of antioxidant and OS markers, blood samples (e.g., 6 mL) were collected in tubes containing heparin from an antecubital vein for each participant between 7:00 and 9:00 a.m. at baseline (pre-training) and at the end of the training period (post-training). Blood sampling was performed after 20 min of rest. Participants were in a seated position and had fasted overnight for 10 h. In addition, they abstained from alcohol and caffeine consumption for at least 24 h, and did not perform any exercise for the last 72 h before the collection [5, 9]. For the diet standardization before the blood samples collection, it was applied a 24 h food recall during the 2 days preceding the baseline collection. With this information, a nutritionist prescribed a diet with equivalent nutritional energetic value for the participants to replicate in the other 2 days before the post-training collection [9].
After the blood collection, samples were centrifuged at 3000 rpm, 4 °C for 10 min. The plasma samples were separated for the lipid peroxidation analysis for the determination of MDA concentrations, and the erythrocytes were used for the measurements of hemoglobin (Hb) concentrations, and for the antioxidant status determination obtained from enzyme activities of CAT and SOD and dosages of glutathione reduced (reduced GSH) and oxidized (GSSG). The plasma samples were analyzed readily or at a maximum of 2 days after collection. Erythrocytes were processed and stored at − 80 °C until analysis.
The assay for determining the CAT activity was based on the spectrophotometric method described by Adamo et al. [19], while the SOD activity was based on the spectrophotometric method described by Ewing and Janero [20]. The activity values were expressed as CAT or SOD units per milligram of hemoglobin (U/mg Hb). Glutathione (GSH) concentrations were analyzed by high-performance liquid chromatography (HPLC) through isocratic elution and fluorescence detection developed by Pfeiffer et al. [21] and subsequently modified by Da Silva et al. [22]. The GSH concentrations were expressed as micromol/g Hb (µmol/g Hb) and it was calculated as the reduced GSH/GSSG ratio. The thiobarbituric acid reactive substances (TBARS) assay to evaluate the MDA levels was based on the spectrophotometric method described by Ohkawa et al. [23] and was expressed as nanomol of MDA/mL (nmol MDA/mL). Concerning the glutathione samples, we had some problems with the equipment during the analysis and the data of eight participants for each group were presented in the results.
Endurance running training program
Both groups performed all training sessions on a 400 m outdoor track during the afternoon and evening on Mondays, Wednesdays, and Fridays. All training sessions were monitored by session rating of perceived exertion (session-RPE) that was derived by asking each participant “How intense was your session?” according to the 10-point scale (CR-10) 30 min after each training session. Training load was quantified by multiplying the whole RPE using the 10-point scale (CR-10) by its duration; training load was quantified by multiplying the whole session-RPE by the training session duration (min) [24]. There were two types of training: moderate-intensity continuous training (MICT) (i.e., 75 ± 4% of Vpeak) and high-intensity interval training (HIIT) (i.e., 100 ± 2% of Vpeak). The MICT and HIIT were prescribed on the basis of peak treadmill speed (Vpeak) and time limit (tlim) at Vpeak determined at pre-training and week 6 [25, 26]. Table 1 shows the endurance running program performed by the participants. The Vpeak was determined during an incremental test with warm-up at 6 km h−1 for 3 min and the test started at 8 km h−1 and increased by 1 km h−1 each 3 min [27, 28]; the Vpeak was calculated as the velocity of the last complete stage added to the completed fraction of the incomplete stage [29]. The test to determine the tlim at Vpeak had a prior warm-up of 15-min at 60% of Vpeak, and then the treadmill velocity was quickly increased to the individual Vpeak [30]; the time of permanency in this intensity was considered the tlim at Vpeak.
All training sessions were preceded by a 15 min warm-up, with 5 min of low self-selected intensity running, 5 min of stretching exercises, and 5 min of running at 60% of Vpeak. In total, both groups performed 24 training sessions (12 MICT sessions and 12 HIIT sessions), in non-consecutive days, during 8 weeks.
Photobiomodulation (PBM)
PBM was performed by LED application with a double blind control, in which neither the participant nor the principal researcher knew about who received or not the PBM application. Therefore, a second researcher who was only responsible for LED application manipulated the LED device and applied the PBM. During the application, the participants of both groups remained standing using a headset with music and blindfolded to avoid identification of the experimental group by audible and visual signal from the LED device and a second researcher controlled the groups, turning on (PBMG) or not (PLA) the LED equipment. PBM was performed immediately before all training sessions [16] in both legs simultaneously and had a total duration of two and a half minutes. For the LED application, the method used was a direct contact of the equipment on the site to be irradiated at an angle of 90° to the skin surface [16].
The application was done on two regions of the quadriceps muscle, two regions of the biceps femoris, and one region of the gastrocnemius muscle, along the axis of muscle fibers distribution in both legs, and for 30 s each application point [24] LED was applied using the equipment LED multidiode with 56 red diodes and 48 infrared diodes (THOR® DD2 control unit). The LED parameters are presented in Table 2.
Statistical analysis
Data were presented as mean ± standard deviation (SD) and were analyzed using the Statistical Package for the Social Sciences 17.0 software. The analysis was made using mixed ANOVA for repeated measures followed by Bonferroni as a post hoc test for multiple comparisons. The sphericity assumption was checked by Mauchly’s test and, where violations occurred, degrees of freedom were corrected using Greenhouse–Geisser. Additionally, it was calculated the percentage of change (% change from pre to post-training) for each variable; this value was calculated as the mean ± SD obtained from the values of percentage of change for each participant. The Shapiro–Wilk test was used to check the normality of the data distribution for % change values. Group comparisons between % change values were performed using t test for independent samples or Mann–Whitney U test for normal and non-normal data, respectively. Statistical significance was set at P < 0.05.
Results
A total of 30 participants (15 each group) completed the study. There was no age difference between groups for all variables evaluated at pre-training (P > 0.05). Concerning the training loads obtained from the RPE-session, the results were similar for both groups for all MICT sessions (PLA = 304.7 ± 94.1 arbitrary unit (AU); PBMG = 317.9 ± 94.4 AU; P = 0.705) and for all HIIT sessions (PLA = 381.0 ± 94.7 AU; PBMG = 373.7 ± 93.7 AU; P = 0.834). In addition, Vpeak significantly increased post-training in both groups (PLA = 13.4 ± 1.1 km h−1 to 14.4 km h−1 ± 1.0; PBMG = 13.4 ± 1.2 km h−1 to 14.6 ± 1.0 km h−1, P < 0.05) and the tlim did not change (P > 0.05) post-training for PLA (6.6 ± 0.7 min to 6.9 ± 1.1 min) and so did PBMG (6.6 ± 1.1 min to 6.8 ± 1.2 min).
Tables 3 and 4 show the antioxidant and OS markers evaluated at the pre-training and post-training moments. The mixed ANOVA for repeated measures did not demonstrate significant main effects of the group and time on all markers analyzed (P > 0.05). It was found a significant group–time interaction for GSSG (P = 0.048); however, multiple comparisons indicated no significant differences. For the GSSG, the responses after training were inverse in PLA and PBMG groups, in other words, increased in one group and decreased in other, that is reinforced by the significant difference comparing the % changes values of GSSG (P = 0.043).
Discussion
The present study examined the effects of PBM associated with endurance running training on OS markers in untrained men. The main findings were that there was no significant effect of PBM associated with endurance running training on modifying the responses of the OS markers, CAT, SOD, reduced GSH, and reduced GSH/GSSG ratio; however, the levels of GSSG increased in the PLA group and decreased in the PBMG, indicating a positive effect of PBM on this OS marker.
It is important to note that PBM is a potential intervention that could reduce OS by counting the increase of ROS and decreasing the levels of markers, such as MDA, and improving the antioxidant defense (e.g., CAT, SOD) after acute aerobic exercise in humans [2, 16], or after a period of endurance training in rats [15]. However, it is important to note that this was the first study to have demonstrated the effects of PBM associated with running training on OS markers in humans.
Our CAT and SOD results did not suggest significant effect of PBM. Contrary to our findings, Guaraldo et al. [15] who used animal models, and investigated the effects of a 6-week swimming aerobic training in conjunction with the low-level lasers (LLL) application before all training sessions, reported greater and significant improvements in CAT and SOD levels in the training groups that received the PBM intervention. However, with respect to % change values, the levels of these markers increased in the PBM group and decreased in the control group (CG) post-training. Guaraldo et al. [15] showed an increase of 78.6% in CAT activity in the LLL/training group compared to the CG and an increase of 70.9% in the training group without LLL application compared to the CG; regarding the SOD activity, an increase of 73.2% was noted in the LLL/training group compared to the CG, and an increase of 59.4% was noted in the training group compared to the CG.
Ferraresi et al. [31] and Ferraresi et al. [32] found positive effects of PBM associated with resistance training in studies with humans and animal models, respectively; in the first study, the authors reported that the SOD gene expression in the skeletal muscle increased on PBM application compared to that in the control after training (1.4 fold vs. 1.0 fold); and Ferraresi et al. [32] found higher CAT activity in mice groups subjected to PBM application compared to the controls after six strength training sessions (≥ 4.22 nmol/min/ml vs. 2.11 nmol/min/ml).
Furthermore, both groups presented similar unchanged responses mean ± SD MDA concentrations, post-training. Two other studies evaluated the changes in MDA levels, and while Ferraresi et al. [26] demonstrated that the MDA concentrations were similar and not significantly different among all the groups after the training program (PBM application and controls), Guaraldo et al. [15] reported that PBM attenuated the increase in MDA concentrations after training with lower values in the LLL/training group than in the training group (7.3 ± 1.8 vs. 11.1 ± 1.6 nmol/MDA/mg). However, no previous study has reported the effects of PBM on OS markers such as MDA in humans, after a period of endurance training.
To the best of our knowledge, this is the first study to examine the glutathione concentrations as an OS marker after PBM exposure in humans. It is important to mention that glutathione binds ROS efficiently, thus preventing the increased production of OS in a reaction wherein the reduced GSH is oxidized to GSSG via glutathione peroxidase [6, 33]. Thus, concerning the significant group–time interaction for GSSG wherein an increase was noted in the PLA group and a decrease in the PBMG, and that it was found a significant difference comparing the % changes values of GSSG, our results indicate a beneficial effect of PBM on attenuating OS evaluated by this marker. Similarly, Ferraresi et al. [32] found that the total GSH content of the muscles was higher in the CG than in the groups with animals treated with PBM after six strength training sessions, indicating OS.
Some factors related to PBM application can be suggested to explain our results concerning the absence of PBM effects on the majority of OS markers. It is important to mention that many factors could influence the magnitude of PBM effects (i.e., biphasic dose–response pattern), such as, for example, the dose applied in each site [2]. Among the factors that influence the PBM effects, the optimal dose to be applied prior to aerobic exercise is an important parameter that was investigated in a few studies [17, 18]. For example, Dellagrana et al. [18] verified the effects of PBM with different energy doses (15, 30, and 60 J per site) on physiological and performance parameters during running tests and found that all PBM doses positively affected the physiological and performance parameter. Moreover, it is important to emphasize that this assumption has not been completely proven in the available literature, and further investigations testing different doses during endurance training running and analyzing the effects on markers related to OS, muscle soreness, inflammation, and oxidative metabolism are warranted.
Concerning the OS responses in both groups, only one measurement (e.g., % change of GSSG) demonstrated a significant different response between groups after the training period in our study. These responses were different from the majority of studies [5, 9,10,11] that showed a positive effect of exercise on OS markers. Contrary to our study, some studies have not reported similar changes in OS markers post-training [12, 13]; they have also not reported any alterations in OS post-training [14]. Miyazaki et al. [12] found that the SOD and glutathione peroxidase activities increased in an untrained man after 12 weeks of running training, with no significant changes in the CAT activity. Spanidis et al. [13] examined the OS markers’ changes during a competitive season in basketball players, and found that only three markers exhibited a similar response between athletes (e.g., GSH); however, other markers (e.g., TBARS and CAT) exhibited marked variations between the athletes. Additionally, Spanidis et al. [13] did not found significant correlations of percentage changes (i.e., change between phase 1 and phase 2) between OS markers (e.g., GSH and CAT markers), reinforcing that the longitudinal changes between OS markers did not show good association.
Thus, it is important to emphasize that it is difficult to compare the responses of OS biomarkers between studies because there is a need for the standardization of some aspects related to the analysis of OS markers, such as the universal criteria for the classification of oxidative stress, experimental techniques, laboratory analyses, and measurement units used, so that better conclusions can be reached, based on the results obtained [34].
Despite the important findings concerning PBM on OS, the present study had some limitations. The glutathione results must be examined with caution because these data were obtained from a partial sample of 16 participants (8 from each group), which was smaller compared to the data of other biomarkers; we used a different sample for these variables, because we had some problems with the equipment during the analysis. Another limitation was the absence of another control group with the PBM application only without endurance running training. Future studies could test different PBM doses during endurance training running to analyze other biomarkers related to inflammation, for example.
Conclusions
Therefore, we concluded that PBM associated with endurance running training did not significantly affect CAT, SOD, MDA, reduced GSH, and reduced GSH/GSSG ratio post-training. However, a significant positive PBM effect was demonstrated on GSSG levels, indicated by the decrease of this marker in the PBMG and an increase in the PLA group post-training.
References
Borsa PA, Larkin KA, True JM (2013) Does phototherapy enhance skeletal muscle contractile function and postexercise recovery? A systematic review. J Athl Train 48:57–67. https://doi.org/10.4085/1062-6050-48.1.12
Ferraresi C, Huang YY, Hamblin MR (2016) Photobiomodulation in human muscle tissue: an advantage in sports performance? J Biophotonics 9:1273–1299. https://doi.org/10.1002/jbio.201600176
Leal Junior ECP, Vanin AV, Miranda EF, Carvalho PTC, Corso SD, Bjordal JM (2015) Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci 30:925–939. https://doi.org/10.1007/s10103-013-1465-4
Vanin AV, Verhagen E, Barboza SD, Costa LOP, Leal-Junior ECP (2018) Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: a systematic review and meta-analysis. Lasers Med Sci 33:181–214. https://doi.org/10.1007/s10103-017-2368-6
Azizbeigi K, Stannard SR, Atashak S, Haghighi M (2014) Antioxidant enzymes and oxidative stress adaptation to exercise training: comparison of endurance, resistance, and concurrent training in untrained males. J Exer Sci Fitness 12:1–6. https://doi.org/10.1016/j.jesf.2013.12.001
Finaud J, Lac G, Fialire E (2006) Oxidative stress: relationship with exercise and training. Sports Med 36:327–358. https://doi.org/10.2165/00007256-200636040-00004
Lewis NA, Howatson G, Morton K, Hill J, Pedlar CR (2015) Alterations in redox homeostasis in the elite endurance athlete. Sports Med 45:379–409. https://doi.org/10.1007/s40279-014-0276-5
Powers SK, Radak Z, Ji LL (2016) Exercise-induced oxidative stress: past, present and future. J Physiol 594:5081–5092. https://doi.org/10.1113/jp270646
Bogdanis GC, Stavrinou P, Fatouros IG, Philippou A, Chatzinkolaou A, Draganidis D, Ermidis G, Maridaki M (2013) Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem Toxicol 61:171–177. https://doi.org/10.1016/j.fct.2013.05.046
Miyazaki H, Oh-Ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S, Haga S, Ji LL, Ohno H (2001) Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physio 84:1–6. https://doi.org/10.1007/s004210000342
de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simões HG (2017) The antioxidant effect of exercise: a systematic review and meta-analysis. Sports Med 47:277–293. https://doi.org/10.1007/s40279-016-0566-1
Vezzoli A, Pugliese L, Marzorati M, Serpiello GR, La Torre A, Porcelli S (2014) Time-course changes of oxidative stress response to high-intensity discontinuous training versus moderate intensity continuous training in masters runners. PLoS One 9(e87506):9. https://doi.org/10.1371/journal.pone.0087506
Spanidis Y, Goutzourelas N, Stagos D, Mpesios A, Priftis A, Bar-Or D, Spandidos DA, Tsatsakis AM, Leon G, Kouretas D (2016) Variations in oxidative stress markers in elite basketball players at the beginning and end of a season. Exp Ther Med 11:147–153. https://doi.org/10.3892/etm.2015.2843
Rahnama N, Gaeini AA, Hamedinia MR (2007) Oxidative stress responses in physical education students during 8 weeks aerobic training. J Sports Med Phys Fitness 47:119–123
Guaraldo SA, Serra AJ, Amadio EM, Antonio EL, Silva F, Portes LA, Tucci PJF, Leal-Junior ECP, Carvalho PTC (2016) The effect of low-level laser therapy on oxidative stress and functional fitness in aged rats subjected to swimming: an aerobic exercise. Lasers Med Sci 31:833–840. https://doi.org/10.1007/s10103-016-1882-2
De Marchi T, Leal Junior ECP, Bortol C, Tomazoni SS, Lopes-Martins RAB, Salvador M (2012) Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci 27:231–236. https://doi.org/10.1007/s10103-011-0955-5
Vanin AV, De Marchi T, Tomazoni SS, Tairova O, Casalechi HL, de Carvalho PTC, Bjordal JM, Leal-Junior EC (2016) Pre-exercise infrared low-level laser therapy (810 nm) in skeletal muscle performance and postexercise recovery in humans, what is the optimal dose? A randomized, double-blind, placebo-controlled clinical trial. Photomed Laser Surg 34:473–482. https://doi.org/10.1089/pho.2015.3992
Dellagrana RA, Rossato M, Sakugawa RL, Baroni BM, Diefenthaeler F (2018) Photobiomodulation therapy on physiological and performance parameters during running tests: dose-response effects. J Strength Cond Res 32:2807–2815. https://doi.org/10.1519/JSC.0000000000002488
Adamo AM, Llesuy LF, Pasquini JM, Boveris A (1989) Brain chemiluminescence and oxidative stress in hyperthyroid rats. Biochem J 263:273–277. https://doi.org/10.1042/bj2630273
Ewing JF, Janero DR (1995) Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem 232:243–248. https://doi.org/10.1006/abio.1995.0014
Pfeiffer CM, Huff DL, Gunter EW (1999) Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 45:290–292
Da Silva VC, Fernandes L, Haseyama EJ, Agamme AL, Shinohara EMG, Muniz MTC, D’Almeida V (2014) Effect of vitamin B deprivation during pregnancy and lactation on homocysteine metabolism and related metabolites in brain and plasma of mice offspring. PLoS One 9:e92683. https://doi.org/10.1371/journal.pone.0092683
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Foster C (1998) Monitoring training in athletes with reference to overtraining syndrome. Med Sci Sports Exer 30:1164–1668. https://doi.org/10.1097/00005768-199807000-00023
Manoel FA, da Silva DF, Lima JRP, Machado FA (2017) Peak velocity and its time limit are as good as the velocity associated with VO2max for training prescription in runners. Sports Med Int Open 1:E8–E15. https://doi.org/10.1055/s-0042-119951
da Silva DF, Ferraro ZM, Adamo KB, Machado FA (2019) Endurance running training individually guided by HRV in untrained women. J Strength Cond Res 33:736–746. https://doi.org/10.1519/JSC.0000000000002001
Machado FA, Kravchychyn AC, Peserico CS, da Silva DF, Mezzaroba PV (2013) Incremental test design, peak ‘aerobic’ running speed and endurance performance in runners. J Sci Med Sport 16:577–582. https://doi.org/10.1016/j.jsams.2012.12.009
Peserico CS, Zagatto AM, Machado FA (2015) Evaluation of the best-designed graded exercise test to assess peak treadmill speed. Int J Sports Med 36:729–734. https://doi.org/10.1055/s-0035-1547225
Kuipers H, Rietjens G, Verstappen F, Schoenmakers H, Hofman G (2003) Effects of stage duration in incremental running tests on physiological variables. Int J Sports Med 24:486–491. https://doi.org/10.1055/s-2003-42020
Billat VL, Hill DW, Pinoteau J, Petit B, Koralsztein JP (1996) Effect of protocol on determination of velocity at VO2max and on its time to exhaustion. Arch Physiol Biochem 104:313–321. https://doi.org/10.1076/apab.104.3.313.12908
Ferraresi C, Bertucci D, Schiavinato J, Reiff R, Araújo A, Panepucci R, Matheucci E, Cunha AF, Arakelian VM, Hamblin MR, Parizotto N, Bagnato V (2016) Effects of light-emitting diode therapy on muscle hypertrophy, gene expression, performance, damage, and delayed-onset muscle soreness: case-control Study with a pair of identical twins. Am J Phy Med Rehabil 95:746–757. https://doi.org/10.1097/PHM.0000000000000490
Ferraresi C, Parizotto NA, Sousa MVP, Kaippert B, Huang YY, Koiso T, Bagnato VS, Hamblin MR (2015) Light-emitting diode therapy in exercise-trained mice increases muscle performance, cytochrome c oxidase activity, ATP and cell proliferation. J Biophotonics 8:740–745. https://doi.org/10.1002/jbio.201400087
Fisher-Wellman K, Bloomer RJ (2009) Acute exercise and oxidative stress: a 30 year history. Dyn Med 13:1–25. https://doi.org/10.1186/1476-5918-8-1
Pinchuk I, Shoval H, Dotam Y, Lichtenberg D (2012) Evaluation of antioxidants: scope, limitations and relevance of assays. Chem Phys Lipids 165:638–647. https://doi.org/10.1016/j.chemphyslip.2012.05.003
Acknowledgements
The authors would like to acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (#487312/2013-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and the Helsinki Declaration.
Informed consent
All of the subjects signed the consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peserico, C.S., D’Almeida, V., Cavalcante-Silva, V. et al. Effects of photobiomodulation associated with endurance running training on oxidative stress in untrained men. Sport Sci Health 16, 129–136 (2020). https://doi.org/10.1007/s11332-019-00588-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-019-00588-4