Abstract
Purpose
This study explored the interactive effects between polymorphonuclear neutrophils (PMNs) and vascular endothelial cells under intermittent hypoxia (IH) and investigated the mechanisms underlying these effects.
Methods
Endothelial cells were co-cultured with PMNs isolated from rats exposed to normoxia or IH. The PMN apoptotic rate was determined using flow cytometry. Expression of apoptosis-related proteins in the endothelial cells were evaluated using Western blotting, and the levels of intercellular adhesion molecules in the co-culture supernatants were measured using enzyme-linked immunosorbent assay.
Results
The PMN apoptotic rate in the IH-exposed rat group was significantly lower than that of the normoxia control group. There was a positive relationship between the PMN apoptotic rate and IH exposure time. In endothelial cells co-cultured with PMNs isolated from IH-exposed rats, a significant increase in the protein expression levels of Bax, Bcl-2, and caspase-3 and a significant decrease in the Bcl-2/Bax ratio were observed. Furthermore, the intercellular cell adhesion molecule-1(ICAM-1) and E-select element (E-S) levels were elevated significantly in the co-cultured supernatants of endothelial cells and PMNs from IH-exposed rats compared to that from controls. The above IH-induced alterations were partially restored by tempol pretreatment.
Conclusions
The apoptotic rate was low in PMNs from IH-exposed rats, which consequently increased the apoptotic signals in endothelial cells in vitro. This may be associated with the increased levels of intercellular adhesion molecules. Further, tempol partially attenuates the PMN-mediated pro-apoptotic effects on endothelial cells under IH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common sleep-breathing disorder. Approximately one billion adults worldwide suffer from OSA, with half of this population suffering from moderate-to-severe OSA, which poses a major threat to human health [1]. Intermittent hypoxia (IH) during sleep, resulting from repeated intermittent apnea and/or hypopnea, is a landmark pathophysiological characteristic of OSA [2], and is considered to be the key intermediary mechanism associated with OSA-related cardiovascular complications[3, 4].
In patients with untreated OSA, long-term exposure to IH causes endothelial injury and dysfunction, which directly affects the function of the corresponding organs and tissues, thus contributing to cardiac and vascular diseases [5]. IH can promote excessive oxidative stress and inflammatory immune responses mostly derived from circulating blood cells, which are considered the underlying mechanisms of atherosclerosis [6]. Our research team has previously demonstrated that lymphocytes from IH-exposed rats could promote apoptosis of endothelial cells via oxidative and inflammation injury in vitro [7]. Polymorphonuclear neutrophil (PMN) is another classic inflammatory cell, which forms an essential component of non-specific immunity. Specifically, they are crucially involved in the body’s resistance to microbial invasion, as well as the inflammatory promotion, development, and regression [8]. However, uncontrolled release of their toxic substances may cause damage to the surrounding tissues [9]. Normally, neutrophils limit their activity through apoptosis. PMN apoptosis is critically involved in controlling the progression of oxidative stress and inflammation, as well as vascular injury. The survival, function, and metabolic status of PMNs directly affect the vascular endothelium. A large number of activated and infiltrated PMNs were found in lesions of acute coronary syndromes [10,11,12,13], which significantly increased the risk of future cardiovascular events [14]. In addition, PMNs were involved in the pathogenesis of lethal myocardial reperfusion injury [15], whereas their depletion reduced the myocardial infarct size [16], and protected the myocardium [17]. The interaction between PMNs and vascular endothelial cells under IH, which is termed as cross-talking, has been increasingly researched. Previous study has found that the apoptosis of PMNs in IH-exposed rats with heart failure were delayed, which contributed to the exacerbation of myocardial damage and progression of heart dysfunction [18]. Delayed PMN apoptosis has also been assessed in patients with OSA, and negatively correlated with OSA severity [9]. However, there are few studies on the direct relationship between PMN apoptosis and endothelial cells under IH. Therefore, this study aimed to explore the interactive effects between PMNs and vascular endothelial cells under IH and investigate the mechanisms involved.
Material and methods
Animals
This study was approved by the Animal Ethical and Welfare Committee of Tianjin Medical University. We used 64, 8-week-old, male Wistar rats (250–280 g) provided by the Model Animal Center of Hygiene and Environmental Medicine Research Institute, Chinese Academy of Medical Science (License No. SCXK-(Army) 2019–003). The rats were randomly allocated to the following eight groups (n = 8, each group): normal oxygen control for 4 (NC4), 6 (NC6), and 8 (NC8) successive weeks; 5% (v/v) IH exposure for 4 (IH4), 6 (IH6), and 8 (IH8) successive weeks; and 5% IH exposure pretreated with equal volume of normal saline or tempol for 6 successive weeks (IHN6 and IHT6, respectively).
IH exposure
The rats were placed in similar self-made sealed Plexiglas chambers (23 cm × 20 cm × 12 cm = 5520 cm3 ≈ 5.5 L). Pure nitrogen or clean air was infused into every chamber through timer-controlled solenoid valves controlled by respiratory simulation system 1.0 (Copyright: Feng Jing, Tianjin, China, 2005), based on the IH cycle. Each IH cycle lasted for 2 min and consisted of an initial 30-s hypoxia phase, when nitrogen was infused into the chamber until the minimum oxygen concentration reached 5%, and a final 90-s reoxygenation phase, when compressed air was infused into the chamber until the oxygen saturation gradually returned to 21%. Rats in the normal oxygen control group were exposed to alternating cycles of compressed room air in the chamber. Rats in the IHT6 group were treated via intraperitoneal injection of 10% tempol at 100 mg/kg/day for 6 successive weeks before 5% IH exposure. Contrastingly, rats in the IHN6 group received equal volumes of normal saline through the abdominal cavity for 6 successive weeks before 5% IH exposure. The exposure experiments were performed daily from 8:30 a.m. to 4:30 p.m. for 4, 6, or 8 weeks. All the rats were allowed food and water ad libitum, in addition to the exposure period.
PMN isolation
All animals were anesthetized through intraperitoneal injection of 3% pentobarbital (30 mg/kg) for euthanasia at the end of IH or normal oxygen exposure. To obtain whole blood samples, the abdominal skin was retracted to expose the abdominal aorta. PMNs were isolated as follows. Blood samples (5 mL) were layered on discontinuous gravity gradients, including 1.119 g/ml Histopaque-1119 and 1.083 g/ml Histopaque-1083 (Sigma-Aldrich, USA) at 25 °C. After centrifugation at 700 g at room temperature of 30 min, the liquid layers from top to bottom were as follows: the plasma layer, lymphocyte layer, Histopaque-1083 layer, polymorphonuclear leukocyte layer, Histopaque-1119 layer, and red blood cell layer. Therefore, PMNs were extracted at the interphase between the Histopaque solutions. Subsequently, they were washed twice using phosphate buffer saline (PBS; pH 7.4) and re-suspended in serum-free 1640 medium. The cells were assessed for viability (98%) and purity (96%) using the trypan blue exclusion [19] and Wright-Giemsa stain (Accustain; Sigma-Aldrich) assay, respectively. For subsequent experiments, PMNs were adjusted to 106 cells/mL.
Assessment of PMN apoptosis
A viability dye containing annexin V and 7-aminoactinomycin D (eBioscience, USA) was used to differentiate the late-stage apoptotic and necrotic cells from the early-stage apoptotic cells [20]. After re-suspending neutrophils to 106 cells/mL in annexin V binding buffer, 5 μL of fluorochrome-conjugated annexin V was added to 100 μL of cell suspension and incubated for 15 min at room temperature. The cells were washed and re-suspended in 200-μL binding buffer; subsequently, we added 5 μL of 7-aminoactinomycin D viability staining solution. The final mixtures were stored in the dark at 4 °C and analyzed using a fluorescence-activated cell sorter/scanning flow cytometer (BD Biosciences FACS Calibur) within 4 h for apoptotic neutrophil detection. We measured 20,000 events per sample. The PMN apoptotic rate was determined based on the cell number in each quadrant, which was expressed as the percentage of the total cell count.
Preparation of rat aortic vascular endothelial cells
Cryopreserved primary rat aortic endothelial cells (Wuhan Pricells Biomedical Technology Co., Ltd., Wuhan, China) were initially thawed and resuscitated in a 37 °C water bath. Subsequently, they were added to a 50-mL flask containing RPMI-1640 medium with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 g/mL streptomycin; finally, they were cultured in a 5% CO2 and 37 °C incubator with saturated humidity. After two or three passages, the cells in the logarithmic growth phase were collected, washed in PBS, and digested using 2.5 g/L trypsin and 2 g/L EDTA (Gibco, USA). The cells were arranged into a single cell suspension using a pipette. Subsequently, the cell concentration was adjusted to 105 cells/mL using RPMI-1640 medium containing 10% FBS. The cell suspension was added to a 12-well plate at 1 mL/well followed by incubation for 48 h at 37 °C in a 5% CO2 incubator with saturated humidity. After the cells adhered to the flask wall, they were rinsed using a serum-free medium (pH 7.4), and the medium was changed to serum-free medium for subsequent use.
Co-culture of PMNs and endothelial cells
Purified PMNs were added to an endothelial cell culture plate at 1 mL/well, with the ratio of PMNs to endothelial cells being 10:1 [21]. The co-cultured cells were divided into the following groups: NC6E group, endothelial cells co-cultured with PMNs isolated from rats in the NC6 group; IH6E group, co-cultured with PMNs isolated from rats in the IH6 group; IHN6E group, co-cultured with PMNs isolated from rats in the IHN6 group; and IHT6E group, co-cultured with PMNs isolated from rats in the IHT6 group. The co-cultured cells were placed in a 5% CO2 and 37 °C incubator with saturated humidity for 4 h. We then collected the supernatant and adherent endothelial cells. The supernatant and endothelial cells were frozen at − 80 °C for further experiments.
Enzyme-linked immunosorbent assays of cellular adhesion molecules in co-cultured supernatants
Enzyme-linked immunosorbent assays were used to determine the concentration of cellular adhesion molecules, including intercellular cell adhesion molecule-1 (ICAM-1; Biosource, CA, USA) and E-select element (E-S; Biosource, CA, USA). The procedure was performed following the manufacturer’s instructions and the absorbance was measured at 450 nm in a microplate reader (Labsystems Multiskan, USA).
Western blotting
After endothelial cell lysis, the proteins were loaded to sodium dodecyl sulfate (SDS)-polyacrylamide gradient gel, separated using electrophoresis, and transferred to nitrocellulose membranes. These membranes were blocked using 5% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h and then incubated with primary antibodies against GAPDH (1:1000), Bcl-2 (1:300), Bax (1:300), and caspase-3 (1:400) (Santa Cruz Biotechnology, CA, USA) overnight at 4 °C. The membranes were washed using TBST and incubated with secondary antibody (1:5000) at room temperature for 1 h. Films with Bcl-2, Bax, caspase-3, and GAPDH bands were scanned; furthermore, the protein band intensity on the scanned images was quantified using ImageJ software (NIH, USA). Bcl-2, Bax, and caspase-3 accumulation were expressed as the ratio of the respective protein intensity to GAPDH intensity.
Statistical analysis
All statistical analyses were performed using software version SPSS 19.0 (SPSS Inc., Chicago, IL), and the results are expressed as mean ± standard deviation. Comparisons among the groups were performed using one-way analysis of variance, while those between the two groups were performed using Student’s t test. P < 0.05 was considered statistically significant.
Results
Comparison of PMN apoptotic rate in normoxia and IH
Flow cytometric assays revealed PMN distribution in NC4, NC6, NC8, IH4, IH6, and IH8 groups, which distinguished apoptotic cells from healthy and dead cells. The PMN apoptotic rate increased with time passage in all groups. Moreover, for similar exposure times, the PMN apoptotic rate in the IH group was significantly lower than that in the normal oxygen control group. In the normoxia group, only the PMN apoptotic rate at 4 weeks was significantly different from that at 8 weeks. In the IH group, the PMN apoptotic rate at 8 weeks was significantly higher than that at 4 or 6 weeks (Fig. 1). These results indicated that IH exposure could result in delay in PMN apoptosis.
Flow cytometry assay shows polymorphonuclear neutrophil (PMN) distribution in the NC4, NC6, NC8, IH4, IH6, and IH8 groups (n = 8). The left lower quadrant (Q3) contains healthy neutrophils (annexin V− and 7-AAD−). Early apoptotic neutrophils (annexin V+ and 7-ADD−) are displayed in the lower right quadrant (Q4). The upper right quadrant (Q2) contains late apoptotic and necrotic neutrophils (annexin V+ and 7-ADD+). The bar diagram shows the apoptotic rate of PMNs isolated from rats exposed to NC or IH for different groups. a P < 0.05 indicates comparison with the NC4 group; b P < 0.05 indicates comparison with the IH4 group; c P < 0.05 indicates comparison with the IH6 group; d P < 0.01 indicates comparison with the corresponding group under normoxic condition. NC, normal oxygen control; IH, intermittent hypoxia; PMNs, polymorphonuclear neutrophils; NC4, NC6, NC8, normal oxygen control for 4, 6, and 8 successive weeks, respectively; IH4, IH6, IH8, 5% (v/v) IH exposure for 4, 6, and 8 successive weeks, respectively
Comparison of apoptosis protein expression levels in endothelial cells
We performed Western blot assay to explore the pro-apoptotic effect of PMNs, obtained from differently treated rats, on endothelial cells. Compared with the normal oxygen co-cultured group (NC6E), the IH co-cultured group (IH6E) showed a significant increase in Bcl-2, Bax, and Caspase-3 levels in the endothelial cells. However, the Bcl-2/Bax ratio was remarkably decreased in the IH6E group than that in the NC6E group. Compared with the IH6E group, tempol pretreatment significantly prevented these aforementioned alterations; however, normal saline pretreatment did not (Fig. 2). These results demonstrated that PMNs obtained from IH rats showed elevated cytotoxicity by accelerating endothelial apoptosis via alterations in apoptosis-related proteins. Further, the pro-apoptotic effect was partly attenuated by pretreatment with tempol.
Representative western blot analysis for Bcl-2, Bax, caspase-3, and GAPDH expression in endothelial cells co-cultured with PMNs from rats exposed to different treatments. The bar diagram shows densitometric analysis of western blot results for Bcl-2, Bax, Bcl-2/Bax ratio, and caspase-3 in each group. a P < 0.05 versus the NC6E group, b P < 0.05 versus the IH6E group. NC6E, endothelial cells co-cultured with PMNs obtained from normal oxygen control rats; IH6E, endothelial cells co-cultured with PMNs obtained from IH-exposed rats; IHN6E, endothelial cells co-cultured with PMNs obtained from IH-exposed rats pretreated with normal saline; IHT6E, endothelial cells co-cultured with PMNs obtained from IH-exposed rats pretreated with tempol; PMNs, polymorphonuclear neutrophils
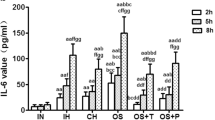
Comparison of cellular adhesion molecules in the supernatant
ELISA was used to detect the ICAM-1 and E-S levels in the co-cultured supernatants of PMNs and endothelial cells. As demonstrated in Fig. 3, ICAM-1 and E-S levels were significantly higher in the IH6E group than in the NC6E group. Moreover, tempol pre-intervention resulted in a significant downregulation in ICAM-1 and E-S levels.
ICAM-1 and E-S levels in the co-cultured supernatants. a P < 0.05 versus the NC6E group; b P < 0.05 versus the IH6E group. NC6E, endothelial cells co-cultured with PMNs obtained from normal oxygen control rats; IH6E, endothelial cells co-cultured with PMNs obtained from IH-exposed rats; IHN6E, endothelial cells co-cultured with PMNs obtained from IH-exposed rats pretreated with normal saline; IHT6E, endothelial cells co-cultured with PMNs obtained from IH-exposed rats pretreated with tempol; PMNs, polymorphonuclear neutrophils; ICAM-1, intercellular cell adhesion molecule-1; E-S, E-select element
Discussion
In this study, we explored the effect of PMNs, from IH-exposed rats, on apoptotic signals in endothelial cells and the interventional role of tempol, and investigated the mechanisms involved. The main findings are as follows: (1) IH exposure resulted in PMN apoptosis delay, and the PMN apoptosis rate was positively associated with IH exposure time. (2) PMNs isolated from IH-exposed rats increased endothelial cell apoptosis in vitro—this may be associated with an enhanced adhesion effect—and tempol pretreatment partially weakened the PMN-mediated pro-apoptotic effect on endothelial cells under IH.
PMNs are a key factor in the immune system and are crucially involved in homeostasis and clearing the inflammatory response [22]. Under normal physiological conditions, the body inhibits the inflammatory response through the normal process of PMN apoptosis, with apoptotic PMNs being cleared by macrophages [23]. The present study simulated the conditions of IH in severe OSA. We found the apoptotic rate of PMNs from IH-exposed rats was lower than that of PMNs obtained from rats in the normoxia group. This suggested that IH could induce delay in the PMN apoptosis. Our results are consistent with previous studies. Both in vitro and in vivo studies have reported a delay in the PMN apoptotic rate under IH [9, 18, 24,25,26]. Moreover, IH exposure time was positively associated with the PMN apoptosis rate, which could involve a partial adaptation mechanism for reducing the delay in PMN apoptosis and protecting the vascular endothelial cells. This is consistent with a previous study on IH inflammation, where the inflammatory response peaked following 6 weeks of hypoxia exposure and subsequently showed a downward trend [27].
Delayed PMN apoptosis can facilitate increased procoagulant activity, leukocyte plugging in the capillaries [28], and release of proinflammatory cytokines and reactive oxygen species [28,29,30], which exert a toxic effect on endothelial cells, and are directly or indirectly involved in the occurrence and development of cardiovascular diseases. Vascular endothelial cell apoptosis could be the initial step in the pathogenesis of atherosclerosis [31, 32] and is positively associated with the incidence of advanced and unstable atherosclerotic plaques in the cardiovascular system [33]. In our study, compared with endothelial cells co-cultured with PMNs obtained from normoxia-exposed rats, in cells co-cultured with PMNs isolated from IH-exposed rats, the expression of Bax, Bcl-2, and caspase-3 was significantly increased, and there was a decrease in the Bcl-2/Bax ratio. This showed that PMNs obtained from IH-exposed rats had an increased cytotoxicity impact on endothelial cells by increasing their degree of apoptosis.
The manifestation of vascular endothelial dysfunction includes the activation of intercellular adhesion molecules [34], such as ICAM-1 and E-S, which are classic endothelial dysfunction indicators [35,36,37]. In many chronic vascular diseases, PMNs adhere and aggregate to the vascular walls. Therefore, the adhesion of neutrophils to the vascular endothelium could be a key factor in endothelial injury. Under IH, PMNs are activated with increased expression of intercellular adhesion molecules, which promotes the PMN adhesion and transmigration to endothelial cells [9, 38]. Concomitantly, endothelial cells are activated to express proinflammatory factors, inflammatory chemokines, and adhesion molecules, which further improve the adhesion between PMNs and endothelial cells [39]. Nocturnal hypoxemia in patients with OSA reportedly increased the expression of adhesion molecules, including ICAM-1 and VCAM-1, in blood circulation [40]. The levels of ICAM-1 and VCAM-1, and apoptosis of circulating endothelial cells, which were seen as an ex vivo indicator of vascular injury, were higher in IH-exposed rats than in the normoxia control rats [41]. In this study, co-culturing of PMNs isolated from IH-exposed rats with endothelial cells increased the supernatant levels of ICAM-1 and E-S. It indicated that co-culturing endothelial cells and PMNs obtained from IH-exposed rats increased the apoptosis signal of endothelial cells; this could be associated with enhanced intercellular adhesion.
Tempol is a nitrogen oxide compound that can directly remove intracellular and extracellular reactive oxygen species. It has an antioxidant effect similar to that of superoxide dismutase. In this study, a significant increase of apoptosis-related proteins was found in endothelial cells co-cultured with PMNs isolated from the IH-exposed rats. Moreover, the ICAM-1 and E-S levels were raised under IH. Contrastingly, the endothelial cells co-cultured with PMNs isolated from IH-exposed rats with tempol intervention showed a significant alteration in the aforementioned values. These findings concluded that tempol may partially weaken the IH-induced, PMN-mediated pro-apoptotic effect on the endothelial cells by reducing the adhesion.
This study has some limitations. First, we demonstrated the pro-apoptotic effect of neutrophils on endothelial cells in IH at the cellular level, and did not observe the cardiovascular histopathological changes in IH-exposed rats. Second, we did not use anti-adhesion molecules, such as such as ICAM-1 and E-S monoclonal antibodies, to further demonstrate that adhesion molecules could play an important role in PMN-induced apoptosis of endothelial cells under IH. We will continue this study in the next step. Third, our study did not include patients with OSA. In the future, we will conduct such clinical studies. For example, specific serum biomarkers and ultrasound will be used to evaluate changes in vascular function and structure in patients with OSA, and to explore the relationship between PNM apoptosis and endothelial injury and dysfunction.
In summary, delayed apoptotic PMNs obtained from IH-exposed rats, as effector cells with potential for endothelial injury, increased the apoptosis signal in co-cultured endothelial cells, which was associated with increased cellular adhesion effect. Tempol partially attenuated the PMN-mediated pro-apoptotic effect on endothelial cells under IH.
References
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin J-L, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A (2019) Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Resp Med 7(8):687–698. https://doi.org/10.1016/s2213-2600(19)30198-5
Garvey JF, Taylor CT, McNicholas WT (2009) Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J 33(5):1195–1205. https://doi.org/10.1183/09031936.00111208
Arnaud C, Bochaton T, Pepin JL, Belaidi E (2020) Obstructive sleep apnoea and cardiovascular consequences: pathophysiological mechanisms. Arch Cardiovasc Dis 113(5):350–358. https://doi.org/10.1016/j.acvd.2020.01.003
Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK (2017) Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 69(7):841–858. https://doi.org/10.1016/j.jacc.2016.11.069
Badran M, Golbidi S, Devlin A, Ayas N, Laher I (2014) Chronic intermittent hypoxia causes endothelial dysfunction in a mouse model of diet-induced obesity. Sleep Med 15(5):596–602. https://doi.org/10.1016/j.sleep.2014.01.013
Levy P, Kohler M, McNicholas WT, Barbe F, McEvoy RD, Somers VK, Lavie L, Pepin JL (2015) Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 1:15015. https://doi.org/10.1038/nrdp.2015.15
Guo H, Cao J, Li J, Yang X, Jiang J, Feng J, Li S, Zhang J, Chen B (2015) Lymphocytes from intermittent hypoxia-exposed rats increase the apoptotic signals in endothelial cells via oxidative and inflammatory injury in vitro. Sleep Breath 19(3):969–976. https://doi.org/10.1007/s11325-015-1128-8
Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW (2011) Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol 32(10):461–469. https://doi.org/10.1016/j.it.2011.06.009
Dyugovskaya L, Polyakov A, Lavie P, Lavie L (2008) Delayed neutrophil apoptosis in patients with sleep apnea. Am J Respir Crit Care Med 177(5):544–554. https://doi.org/10.1164/rccm.200705-675OC
Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, Komatsu R, Ikura Y, Ogami M, Shimada Y, Ehara S, Yoshiyama M, Takeuchi K, Yoshikawa J, Becker AE (2002) Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation 106(23):2894–2900. https://doi.org/10.1161/01.cir.0000042674.89762.20
Zidar N, Jeruc J, Balazic J, Stajer D (2005) Neutrophils in human myocardial infarction with rupture of the free wall. Cardiovasc Pathol 14(5):247–250. https://doi.org/10.1016/j.carpath.2005.04.002
Biasucci LM, Liuzzo G, Giubilato S, Della Bona R, Leo M, Pinnelli M, Severino A, Gabriele M, Brugaletta S, Piro M, Crea F (2009) Delayed neutrophil apoptosis in patients with unstable angina: relation to C-reactive protein and recurrence of instability. Eur Heart J 30(18):2220–2225. https://doi.org/10.1093/eurheartj/ehp248
Garlichs CD, Eskafi S, Cicha I, Schmeisser A, Walzog B, Raaz D, Stumpf C, Yilmaz A, Bremer J, Ludwig J, Daniel WG (2004) Delay of neutrophil apoptosis in acute coronary syndromes. J Leukoc Biol 75(5):828–835. https://doi.org/10.1189/jlb.0703358
Haumer M, Amighi J, Exner M, Mlekusch W, Sabeti S, Schlager O, Schwarzinger I, Wagner O, Minar E, Schillinger M (2005) Association of neutrophils and future cardiovascular events in patients with peripheral artery disease. J Vasc Surg 41(4):610–617. https://doi.org/10.1016/j.jvs.2005.01.013
Vinten-Johansen J (2004) Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 61(3):481–497. https://doi.org/10.1016/j.cardiores.2003.10.011
Jolly SR, Kane WJ, Hook BG, Abrams GD, Kunkel SL, Lucchesi BR (1986) Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am Heart J 112(4):682–690. https://doi.org/10.1016/0002-8703(86)90461-8
Kin H, Wang NP, Halkos ME, Kerendi F, Guyton RA, Zhao ZQ (2006) Neutrophil depletion reduces myocardial apoptosis and attenuates NFkappaB activation/TNFalpha release after ischemia and reperfusion. J Surg Res 135(1):170–178. https://doi.org/10.1016/j.jss.2006.02.019
Li S, Feng J, Wei S, Qian X, Cao J, Chen B (2015) Delayed neutrophil apoptosis mediates intermittent hypoxia-induced progressive heart failure in pressure-overloaded rats. Sleep Breath 20(1):95–102. https://doi.org/10.1007/s11325-015-1190-2
Strober W (2015) Trypan Blue Exclusion Test of Cell Viability. Curr Protoc Immunol 111:A3.B.1-a3.B.3. https://doi.org/10.1002/0471142735.ima03bs111
Istvan V, Clemens H, Helga SN, R. C, (1995) A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184:39–51
Harbrecht BG, Billiar TR, Curran RD, Stadler J, Simmons RL (1993) Hepatocyte injury by activated neutrophils in vitro is mediated by proteases. Ann Surg 218:120–128
Dyugovskaya L, Polyakov A (2010) Neutrophil apoptosis and hypoxia. Fiziol Zh 56(5):115–124
Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PC, Salmon M, Matharu NM, Vohra RK, Nash GB, Rainger GE (2006) Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 79(2):303–311. https://doi.org/10.1189/jlb.0905496
Dyugovskaya L, Polyakov A, Ginsberg D, Lavie P, Lavie L (2011) Molecular pathways of spontaneous and TNF-{alpha}-mediated neutrophil apoptosis under intermittent hypoxia. Am J Respir Cell Mol Biol 45(1):154–162. https://doi.org/10.1165/rcmb.2010-0025OC
Larissa Dyugovskaya AP, Cohen-Kaplan V, Lavie P, Lavie L (2012) Bax/Mcl-1 balance affects neutrophil survival in intermittent hypoxia and obstructive sleep apnea: effects of p38MAPK and ERK1/2 signaling. J Transl Med 10:211–224
Lavie L (2015) Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: implications to the heart and brain. Sleep Med Rev 20:27–45. https://doi.org/10.1016/j.smrv.2014.07.003
Li S, Qian XH, Zhou W, Zhang Y, Feng J, Wan NS, Zhang Z, Guo R, Chen BY (2011) Time-dependent inflammatory factor production and NFkappaB activation in a rodent model of intermittent hypoxia. Swiss Med Wkly 141:w13309. https://doi.org/10.4414/smw.2011.13309
Chello M, Anselmi A, Spadaccio C, Patti G, Goffredo C, Di Sciascio G, Covino E (2007) Simvastatin increases neutrophil apoptosis and reduces inflammatory reaction after coronary surgery. Ann Thorac Surg 83(4):1374–1380. https://doi.org/10.1016/j.athoracsur.2006.10.065
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Foster GE, Poulin MJ, Hanly PJ (2007) Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol 92(1):51–65. https://doi.org/10.1113/expphysiol.2006.035204
Yamagata K (2020) Protective Effect of Epigallocatechin Gallate on Endothelial Disorders in Atherosclerosis. J Cardiovasc Pharmacol 75(4):292–298. https://doi.org/10.1097/FJC.0000000000000792
Perrotta I (2020) The microscopic anatomy of endothelial cells in human atherosclerosis: Focus on ER and mitochondria. J Anat 237(6):1015–1025. https://doi.org/10.1111/joa.13281
Cancel LM, Ebong EE, Mensah S, Hirschberg C, Tarbell JM (2016) Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis 252:136–146. https://doi.org/10.1016/j.atherosclerosis.2016.07.930
Hlubocka Z, Umnerova V, Heller S, Peleska J, Jindra A, Jachymova M, Kvasnicka J, Horky K, Aschermann M (2002) Circulating intercellular cell adhesion molecule-1, endothelin-1 and von Willebrand factor-markers of endothelial dysfunction in uncomplicated essential hypertension: the effect of treatment with ACE inhibitors. J Hum Hypertens 16(8):557–562. https://doi.org/10.1038/sj.jhh.1001403
Pilkauskaite G, Miliauskas S, Vitkauskiene A, Sakalauskas R (2014) Vascular adhesion molecules in men with obstructive sleep apnea: associations with obesity and metabolic syndrome. Sleep Breath 18(4):869–874. https://doi.org/10.1007/s11325-014-0958-0
Kaczmarek E, Bakker JP, Clarke DN, Csizmadia E, Kocher O, Veves A, Tecilazich F, O’Donnell CP, Ferran C, Malhotra A (2013) Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS ONE 8(7):e70559. https://doi.org/10.1371/journal.pone.0070559
Hung MW, Kravtsov GM, Lau CF, Poon AM, Tipoe GL, Fung ML (2013) Melatonin ameliorates endothelial dysfunction, vascular inflammation, and systemic hypertension in rats with chronic intermittent hypoxia. J Pineal Res 55(3):247–256. https://doi.org/10.1111/jpi.12067
Jiang Y, Jiang L-LI, Maimaitirexiati X-MZY, Zhang Y, Wu L (2015) Irbesartan attenuates TNF-α-induced ICAM-1, VCAM-1, and E-selectin expression through suppression of NF-κB pathway in HUVECs. Eur Rev Med Pharmacol Sc 19(17):3295–3302
Kent BD, Ryan S, McNicholas WT (2011) Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respir Physiol Neurobiol 178(3):475–481. https://doi.org/10.1016/j.resp.2011.03.015
Ramar K, Caples SM (2011) Vascular changes, cardiovascular disease and obstructive sleep apnea. Future Cardiol 7(2):241–249. https://doi.org/10.2217/fca.10.123
Zhao H, Zhao Y, Li X, Xu L, Jiang F, Hou W, Dong L, Cao J (2018) Effects of Antioxidant Tempol on Systematic Inflammation and Endothelial Apoptosis in Emphysematous Rats Exposed to Intermittent Hypoxia. Yonsei Med J 59(9):1079–1087. https://doi.org/10.3349/ymj.2018.59.9.1079
Funding
This work was supported by the National Natural Science Foundation of China (No. 81670084, 81970084).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Ethical and Welfare committee of Tianjin Medical University guidelines for the care and use of animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Wang, L., Hu, J. et al. Polymorphonuclear neutrophils promote endothelial apoptosis by enhancing adhesion upon stimulation by intermittent hypoxia. Sleep Breath 26, 1173–1180 (2022). https://doi.org/10.1007/s11325-021-02503-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-021-02503-z