Abstract
Purpose
Obstructive sleep apnea (OSA) is a prevalent sleep disorder associated with increased risk of cardiovascular disease. Several studies have demonstrated elevated oxidative stress in patients with OSA. This oxidative stress is a direct inducer of lipid peroxidation. Malondialdehyde (MDA), a robust marker of lipid peroxidation, has been evaluated in patients with OSA but results have been inconsistent. The present systematic review and meta-analysis was performed to quantify the circulating levels of MDA in patients with OSA compared to controls.
Methods
Search was performed in data bases of PubMed, Scopus, EMBASE, Web of Science, and Cochrane library, to find out those studies that measured MDA in patients with OSA compared to controls.
Results
The search produced 563 records and after removing duplicates, 383 records remained. Screening by title and abstract and the evaluation of the full text resulted in the selection of 14 articles, which were included in the meta-analysis. Pooled analysis demonstrated higher levels of MDA in the patients compared to the controls (SMD (95% CI): 1.18 (0.68, 1.68), p < 0.0001).
Conclusion
The results of this meta-analysis demonstrated considerable elevation of MDA in patients with OSA compared to controls. The meta-analysis also indicated a positive association of MDA levels with the degree of severity of OSA. These results suggest a state of increased lipid peroxidation in patients with OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a highly prevalent type of sleep disorder associated with increased risk of cardiovascular disease (CVD) [1, 2]. Patients with OSA have intermittent hypoxia as a result of the recurrent pharyngeal collapse, which stops breathing during sleep [3, 4]. Recurrent hypoxia/oxygenation leads to mitochondrial dysfunction and leak of an electron from the respiratory chain of mitochondrial metabolism, which produces reactive oxygen species (ROS) [3, 5]. The increased ROS production in the body causes oxidative damage to many biomolecules including nucleic acids, proteins, and lipids [5, 6]. This condition leads to increased oxidative stress, and increased oxidative stress and ROS lead to the production of lipid peroxides. These conditions in patients with OSA are risk factors for developing other pathological conditions such as insulin resistance and inflammation [7].
A considerable number of studies have measured oxidative stress and anti-oxidant markers in venous blood in order to assess the oxidant/anti-oxidant balance in the body [8, 9]. For instance, Li et al. in a systematic review and meta-analysis established that homocysteine, as an important risk factor for CVD, is elevated in patients with OSA compared to controls [10]. However, we have shown that paraoxonase activity, as an atheroprotective enzyme, indicated no difference between patients with OSA and controls [11]. Malondialdehyde (MDA) is considered to be a robust marker of lipid peroxidation and oxidative stress. MDA is an end-product produced by the degradation of polyunsaturated fatty acids through enzymatic or nonenzymatic reactions [12]. Circulating levels of MDA have an independent association with risk of cardiovascular diseases [13, 14]. Accordingly, in several studies, MDA has been measured in patients with OSA though results were equivocal [15,16,17,18,19,20,21]. For instance, Lu et al. reported no significant difference between patients with OSA and controls in terms of MDA levels [21], while several other studies have reported elevated levels of MDA in patients with OSA [16, 22, 23]. Therefore, we performed a systematic review and meta-analysis to reassess the circulating levels of MDA in patients with OSA compared to controls.
Methods
Search
The search was performed in the following databases: PubMed, Scopus, EMBASE, Web of Science, and Cochran library from 1990 to August 2019. Several studies that measured MDA levels in the patients with OSA and controls were searched using the following keywords: (1) terms specific to the disease: obstructive sleep apnea, obstructive sleep apnoea OR OSA OR OSAS OR apnoea OR apnea OR hypopnea OR hypopnoea OR sleep-related breathing disorder, and (2) the terms specific to outcome: malonyldialdehyde OR MDA OR malonaldehyde OR thiobarbituric acid reactive substances OR TBARS. Furthermore, gray literature and reference lists were searched to find other possibly relevant articles. Two authors independently performed screening and disagreement was resolved by discussion. Screening for pertinence was performed on the title and abstract of these studies.
PICO format for formulating the question of the study is given in Table 1. All records were transferred to EndNote software. Duplicate articles, editorials, non-English articles, letters, reviews, and case reports were discarded.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) using polysomnography to diagnose OSA in patients and controls, (2) using the cut-off of AHI ≥ 5 for diagnosing OSA and AHI < 5 for selecting controls, (3) all participants were adult (aged over 18 years old), (4) measuring MDA as an outcome, and (5) providing adequate data for performing meta-analysis.
Data collection
Full texts of the articles were evaluated for collecting the following data: first author, year of publication, sample size (patients/controls), age, BMI, and MDA levels. Furthermore, we requested additional data from the corresponding author of the articles, if the required data were not found in the article.
Risk of bias
Two authors independently assessed the quality of the studies using Newcastle-Ottawa Quality Assessment Scale (NOS). Also, the following factors were checked using the NOS including the quality of the study population selection, comparability, exposure, and outcome. The maximum scores for the quality of a study were 9 points and disagreement on scoring was dissolved by discussion.
Statistical analysis
The circulating levels of MDA were presented using mean and standard deviation (SD) in patients with OSA and controls. The differences of MDA levels between OSA and control groups were evaluated using standardized mean difference (SMD). SMD measurement was performed using the Der Simoniane Laird random-effects model and the heterogeneity was measured using I2. Also, in the case of reporting SEM, SD was estimated using the following formula: SD = SEM × sqrt (n), where n is the number of subjects. Afterward, subgroup analysis was conducted according to AHI and NOS score cutoff and matching in terms of age and BMI. Begg’s and Egger’s tests were used to determine publication bias. In order to evaluate the influence of each study on the overall effect size, a sensitivity analysis was conducted using the leave-one-out method, i.e., removing one study each time and repeating the analysis. Random-effects meta-regression was performed using the unrestricted maximum likelihood method to evaluate the association between the calculated effect sizes of the MDA levels in patients with OSA. We used the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline to report the study [19]. All the analyses were conducted using STATA (version 14.2) and the Comprehensive Meta-Analysis (CMA) V2 (Biostat, NJ) software.
Results
Search
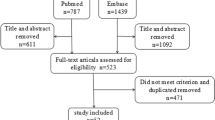
The search diagram is illustrated in Fig. 1. Search resulted in the selection of 563 records and after removing duplicate records, 383 records remained. After removing non-English articles, editorials, and reviews, 217 articles remained. Screening was performed on the studies’ titles and abstracts and 65 articles were included, which were then evaluated by full text. Twenty-four articles had no control groups, 15 articles performed no PSG for the selection of the control group, 5 articles had inadequate data for analysis, and 7 articles had unmet criteria. Finally, 14 studies met our criteria and were then included in the meta-analysis.
Characteristics of studies
Fourteen studies were included in the meta-analysis, comprising 429 controls with AHI < 5 and 867 patients with OSA defined by AHI ≥ 5. The mean age of the patients ranged from 39.6 to 65.8 years old and AHI in the patient group ranged from 8.6 to 69.2. Details of the eligible studies are given in Table 2. Six studies had subgroups and each subgroup was separately included in the meta-analysis. Wang et al. measured MDA in two groups (elderly and non-elderly) of OSA compared to the controls [16]. Moreover, Chen et al. divided patients with OSA into mild and moderate, and Vatansever et al. divided the patients into mild and severe [22, 25]. Furthermore, Li et al., Yildrim-Akaydin et al., Cofta et al., Ekind et al., and Ye et al. measured MDA in three subgroups of patients with OSA (mild, moderate, and severe) [18, 23, 24, 29, 30].
Pooled analysis
The heterogeneity test indicated significant heterogeneity in eligible studies (I2 = 92.5, p < 0.0001); therefore, the random model was used for meta-analysis. Pooled analysis showed that circulating levels of MDA considerably increased in the patients with OSA compared to the controls (1.18 (0.68, 1.68), p < 0.0001) (Fig. 2). Furthermore, a sensitivity analysis using the sequential exclusion of each study showed no significant changes in the estimated pooled results (Fig. 3).
Publication bias
The results of Begg’s rank correlation (two-tailed p value = 0.870) and Egger’s linear regression (two-tailed p value = 0.213) test were not statistically significant. The funnel plot of the study precision (inverse standard error) by the effect size SMD was symmetric, which suggested no publication bias in reporting the levels of MDA in OSA patients (Fig. 4).
Subgroup analyses
Subgroup analyses were performed using the subgroups of the eligible studies according to AHI cutoff, and matching in terms of age and BMI, and methods for measuring MDA and the results are given in Table 3. Subgroup analysis according to the mean of AHI showed that both subgroups had higher MDA levels compared to the controls (Supplementary Fig. 1). Moreover, it was found that MDA levels elevated in the patients with OSA compared to the controls in both matched and unmatched subgroups in terms of age (Supplementary Fig. 2). Similarly, MDA has increased in both subgroups of matched and unmatched BMI (Supplementary Fig. 3). In the next subgroup analysis, studies were categorized into 2 subgroups according to matching in terms of age and BMI simultaneously (Supplementary Fig. 4). The results showed that, in both subgroups, MDA significantly increased in OSA patients compared to the controls. Finally, studies divided into 2 subgroups according to NOS score (5 < and ≥ 5), and the results indicated that MDA elevated in both subgroups (Supplementary Fig. 5). Moreover, three different methods (thiobarbituric acid-reacting substances (TBARS), N-methyl-2-phenylindole (NMPI), and high-performance liquid chromatography (HPLC)) used for measuring MDA, and the results showed that circulating levels of MDA increased in OSA patients in all three subgroups (Supplementary Fig. 6).
Meta-regression
Meta-regression showed that AHI, age, and BMI were not possible sources of heterogeneity; the results of meta-regression are shown in Table 4.
Discussion
In the present study the results demonstrated higher circulating levels of MDA in patients with OSA compared to controls. Fourteen studies with 429 controls and 867 patients with OSA were included in the meta-analysis. In addition, a sensitivity analysis was utilized and the overall results were not affected. Furthermore, categorizing studies according to NOS score showed elevated MDA levels in both high- and low-quality studies. Hence, the outcome of the analysis may be considered with a high degree of certainty.
Repetitive hypoxia/oxygenation events in patients with OSA are believed to be the main cause of increased oxidative stress and ROS, and lipid peroxidation is one of the direct consequences of oxidative stress. MDA is a degradation product of lipid peroxidation, which has been suggested as an important risk factor for CVD [31]. MDA is produced as an end-product of polyunsaturated fatty acids degradation and it demonstrated a high capability to the formation of adducts with multiple biomolecules such as DNA or proteins, and studies have shown a significant relation of MDA with cardiovascular diseases, diabetes, and cancer [12]. There are several inconsistent reports on the circulating levels of MDA in patients with OSA [16, 19, 21,22,23,24,25]. Also, three studies by Wysocka et al., Araujo Lda et al., Lu et al., and Ntalapascha et al. reported no significant differences between patients with OSA and controls in terms of MDA [19, 21, 26, 27]. However, there are several studies that reported the increased levels of MDA in patients with OSA compared to the controls [16, 18, 23, 25, 28]. The present study demonstrated higher concentration of MDA in patients with OSA using the robust method of systematic review and meta-analysis.
To further understand whether or not age, AHI, and BMI affected the relationship between MDA and OSA, we performed subgroup analyses. It was demonstrated that increased levels of MDA in patients with OSA are independent of the confounding factors (age and BMI), and meta-regression showed no effect of these factors on the circulating levels of MDA. In addition, subgroup analysis according to AHI cutoff showed that MDA significantly increased in both patients with high and low AHI; however, the patients with higher AHI (> 30) demonstrated greater SMD. Therefore, it seems likely that the severity of OSA may be a factor participating in exacerbating lipid peroxidation in the body. The results of the present study may suggest the utility of considering strategies for reducing oxidative stress in patients with OSA.
It is worthy to note that the included studies had relatively small sample sizes. Studies with larger samples would allow calculation of a more price effect size. Another limitation of the present study was exclusion of non-English articles.
In conclusion, the present study demonstrates that MDA is significantly increased in patients with OSA and the levels of MDA show a positive relationship with disease severity. These findings support the potential role of OSA therapy especially in severe cases in order to reduce lipid peroxidation.
References
Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL (2008) Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proceedings of the American Thoracic Society 5(2):185–192
Khazaie H, Najafi F, Rezaie L, Tahmasian M, Sepehry AA, Herth FJ (2011) Prevalence of symptoms and risk of obstructive sleep apnea syndrome in the general population. Arch Iran Med 14(5):335–338
Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, Lavie L, Pépin JL (2015) Obstructive sleep apnoea syndrome. Nature Reviews Disease Primers 1(1):15015
Lavie L (2015) Oxidative stress in obstructive sleep apnea and intermittent hypoxia – revisited – the bad ugly and good: Implications to the heart and brain. Sleep Medicine Reviews 20:27–45
Lavie L (2003) Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Medicine Reviews 7(1):35–51
Pizzino G et al (2017) Oxidative stress: harms and benefits for human health. Oxidative medicine and cellular longevity 2017:8416763–8416763
Hurrle S, Hsu WH (2017) The etiology of oxidative stress in insulin resistance. Biomedical journal 40(5):257–262
Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA (2013) Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biology 1(1):483–491
Fadaei R, Safari-Faramani R, Rezaei M, Ahmadi R, Rostampour M, Moradi N, Khazaie H (2020) Circulating levels of oxidized low-density lipoprotein in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep and Breathing 24:809–815
Li K et al (2017) Association between serum homocysteine level and obstructive sleep apnea: a meta-analysis. Biomed Res Int 2017:7234528
Fadaei R, Safari-Faramani R, Rezaei M, Ahmadi R, Rostampour M, Moradi N, Khazaie H (2020) Paraoxonase activity in patients with obstructive sleep apnea: a systematic review and meta-analysis. SN Comprehensive Clinical Medicine 2(1):25–31
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity 2014:360438
Ayala A, Munoz MF, Arguelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438
Lee R, Margaritis M, M. Channon K, Antoniades C (2012) Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Current medicinal chemistry 19(16):2504–2520
Barcelo A et al (2000) Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J 16(4):644–647
Wang L et al (2010) Association between serum homocysteine and oxidative stress in elderly patients with obstructive sleep apnea/hypopnea syndrome. Biomed Environ Sci 23(1):42–47
Jurado-Gamez B, Cabrera CB, Ballesteros LC, Hinojosa CM, Cabrera LM, Perez-Jimenez F, Lopez-Miranda J (2012) Association of cellular adhesion molecules and oxidative stress with endothelial function in obstructive sleep apnea. Intern Med 51(4):363–368
Yardim-Akaydin S, Caliskan-Can E, Gökalp F, Firat H, Ardiç S, Simsek B (2013) Lipid peroxidation and DNA damage in apnea patients with or without metabolic syndrome. Sleep and Biological Rhythms 11(2):116–124
Ntalapascha M, Makris D, Kyparos A, Tsilioni I, Kostikas K, Gourgoulianis K, Kouretas D, Zakynthinos E (2013) Oxidative stress in patients with obstructive sleep apnea syndrome. Sleep Breath 17(2):549–555
Jurado-Gamez B, Fernandez-Marin MC, Gomez-Chaparro JL, Munoz-Cabrera L, Lopez-Barea J, Perez-Jimenez F, Lopez-Miranda J (2011) Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. Eur Respir J 37(4):873–879
Lu CH, Lin HC, Huang CC, Lin WC, Chen HL, Chang HW, Friedman M, Chen CT, Tsai NW, Wang HC, Kung CT, Su YJ, Cheng BC (2015) Increased circulating endothelial progenitor cells and anti-oxidant capacity in obstructive sleep apnea after surgical treatment. Clinica Chimica Acta 448:1–7
Chen PC et al (2013) Blood trace minerals concentrations and oxidative stress in patients with obstructive sleep apnea. J Nutr Health Aging 17(8):639–644
Li J et al (2018) Homocysteine level in patients with obstructive sleep apnea/hypopnea syndrome and the impact of continuous positive airway pressure treatment. Advances in Clinical and Experimental Medicine 27(11):1549–1554
Ye L, Ma GH, Chen L, Li M, Liu JL, Yang K, Li QY, Li N, Wan HY (2010) Quantification of circulating cell-free DNA in the serum of patients with obstructive sleep apnea-hypopnea syndrome. Lung 188(6):469–474
Vatansever E, Surmen-Gur E, Ursavas A, Karadag M (2011) Obstructive sleep apnea causes oxidative damage to plasma lipids and proteins and decreases adiponectin levels. Sleep Breath 15(3):275–282
Wysocka E et al (2013) Blood antioxidant status, dysglycemia and obstructive sleep apnea. Adv Exp Med Biol 756:121–129
Araujo Lda S et al (2015) Obstructive sleep apnea is independently associated with inflammation and insulin resistance, but not with blood pressure, plasma catecholamines, and endothelial function in obese subjects. Nutrition 31(11-12):1351–1357
Asker S et al (2015) Oxidative stress parameters and their correlation with clinical, metabolic and polysomnographic parameters in severe obstructive sleep apnea syndrome. Int J Clin Exp Med 8(7):11449–11455
Cofta S et al (2019) Oxidative stress markers and severity of obstructive sleep apnea. Adv Exp Med Biol 1222:27–35
Ekin S, Yildiz H, Alp HH (2020) NOX4, MDA, IMA and oxidative DNA damage: can these parameters be used to estimate the presence and severity of OSA? Sleep Breath
Strobel NA, Fassett RG, Marsh SA, Coombes JS (2011) Oxidative stress biomarkers as predictors of cardiovascular disease. International Journal of Cardiology 147(2):191–201
Funding
This work was supported by a grant (980499) from Kermanshah University of Medical Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent is needed for a systematic review.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Fig. 1
Subgroup analysis according to AHI cut off (< 30 and ≥ 30) (DOCX 20 kb)
Supplementary Fig. 2
Subgroup analysis according to matching in term of BMI (matched and unmatched) (DOCX 20 kb)
Supplementary Fig. 3
Subgroup analysis according to matching in term of age (matched and unmatched) (DOCX 19 kb)
Supplementary Fig. 4
Subgroup analysis according to matching in terms of BMI and age simultaneously (matched and unmatched) (DOCX 20 kb)
Supplementary Fig. 5
Subgroup analysis according to NOS score (≤ 5 and > 5) (DOCX 20 kb)
Supplementary Fig. 6
Subgroup analysis according to method of measuring MDA (thiobarbituric acid-reacting substances (TBARS), N-methyl-2-phenylindole (NMPI), and high-performance liquid chromatography (HPLC)) (DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Fadaei, R., Safari-Faramani, R., Hosseini, H. et al. Increased the circulating levels of malondialdehyde in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath 25, 1753–1760 (2021). https://doi.org/10.1007/s11325-021-02293-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-021-02293-4