Abstract
Background
Obstructive sleep apnea (OSA) is associated with an increased risk of developing atherosclerotic cardiovascular disease (ASCVD). Patients with OSA have increased levels of oxidative stress and several studies have shown higher levels of oxidative stress markers. Oxidized-LDL (Ox-LDL) is an important risk factor for ASCVD and a number of studies have measured its levels in patients with OSA, though results from these studies are conflicting. This meta-analysis aimed to reassess circulating levels of Ox-LDL in patients with OSA in comparison with controls.
Methods
Studies evaluating Ox-LDL levels in patients with OSA and controls were explored in databases of PubMed, EMBASE, Scopus, and Web of Science. Two authors independently performed the search from January 1990 to February 2019. Two authors independently screened the studies according to title, abstract, and full text. In addition, the Newcastle-Ottawa Quality Assessment Scale was utilized to evaluate the quality of the studies. The impact of OSA on Ox-LDL levels was determined using the random effects model.
Results
Of 195 articles retrieved, 98 were duplicates, 49 were excluded by title, 20 excluded by abstract, and 22 by full texts. Six eligible studies were included in the meta-analysis. Pooled analysis demonstrated that Ox-LDL increased in patients with OSA compared with controls. In addition, subgroup analysis revealed that studies matching age or BMI between OSA patients and controls showed no significant difference between patients with OSA and healthy controls, while unmatched studies had higher levels of Ox-LDL in patients with OSA in comparison with controls.
Conclusion
This study demonstrated higher circulating concentrations of Ox-LDL in patients with OSA. However, no significant difference was found in studies in which patients and controls were matched for age and BMI, suggesting the involvement of these two confounding factors as a cause for elevated concentrations of circulating Ox-LDL in patients with OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is one of the most prevalent sleep disorders influencing a considerable part of the world population [1, 2]. The prevalence of OSA is growing, which is linked to the increasing prevalence of obesity [3]. OSA increases the hazard of atherosclerosis-based cardiovascular disease (ASCVD) progression and development and it has been established that OSA independently increases the risk ASCVD [4]. The exact mechanism for this relationship is not clear, though several possible mechanisms such as oxidative stress, insulin resistance, inflammation, hypertension, and dyslipidemia have been suggested for this link [4]. Apnea and hypopnea lead to oxygen desaturation and re-oxygenation [5, 6]. This process leads to increase in the leakage of electron and reactive oxygen species (ROS) from the mitochondrial electron transport chain [5, 6]. This deleterious molecule can damage all types of biomolecules in the body such as proteins, nucleic acids, and lipids and impair antioxidant/oxidant balance [7]. Lipoprotein oxidation is one of these damages and it plays a central role in the progression and development of atherosclerosis; indeed, the oxidation of lipoproteins is the initial step in the inflammatory process of atherosclerosis [8]. Oxidized-low-density lipoprotein (Ox-LDL) is one of the most pro-atherosclerotic factors, which plays a pivotal role in atherosclerosis pathogenesis and foam cell formation in the arterial walls. The immune system recognizes Ox-LDL as a pathogen in the intima of arterial wall where macrophages uptake them with their scavenger receptors [8]. This uncontrolled uptake of Ox-LDL leads to foam cell formation and subsequent accumulation of cholesterol in the arterial wall, which is the hallmark of atherosclerosis. In addition, Ox-LDL triggers inflammation, endothelial dysfunction, and smooth muscle cell proliferation, which are the most important risk factors for atherosclerosis [8].
Several studies have suggested that Ox-LDL level is a predictor of ASCVD; its levels increase in patients with ASCVD [9]. Circulating levels of Ox-LDL were reported to be elevated in inflammatory diseases and metabolic syndrome [10,11,12]. In addition, numerous investigations have reported circulating levels of Ox-LDL in OSA patients [10, 13,14,15,16,17,18]. However, the results are conflicting. Therefore, we aimed the present systematic review and meta-analysis study to reassess circulating levels of Ox-LDL in these patients compared to controls.
Materials and methods
Search strategy
Electronic search was carried out in databases of Embase, Scopus, PubMed, Cochrane Library, and Web of Science. There was no systematic review on Ox-LDL levels in patients with OSA. In addition, reference checking was carried out on eligible studies. The following search strategy was used to identify eligible articles: [Oxidized low density lipoprotein OR Oxidized-low density lipoprotein OR Ox-LDL OR Oxidized-LDL OR Ox LDL OR Oxidized LDL OR] AND [Sleep Aponea OR Sleep Apnea OR OSA OR Sleep Apnea Syndrome OR OSAS OR Obstructive sleep apnea]. Moreover, wildcard terms were employed in the search, and reference checking was also performed.
PICO format for formulating the question of study was as follows: the participants included patients with OSA and controls, and the outcome was risk of OSA and circulating levels of Ox-LDL were considered as risk factor. The search was performed before February 2019.
Inclusion and exclusion criteria
There were four inclusion criteria: (1) Diagnosis of OSA and non-OSA according to polysomnography (PSG) test, (2) Apnea hypopnea index (AHI) < 5 for controls and AHI ≥ 5 for diagnosis of OSA, (3) all participants aged > 18 (adult), and (4) reporting circulating levels of Ox-LDL.
Study selection
Two authors carried out study selection independently. All records were transferred to Endnote program. Duplicate papers were removed; then, the title and abstract of the remaining articles were evaluated. Articles with irrelevant title or abstract were removed and after that, the full text of articles was checked. Finally, the articles that met the inclusion and exclusion criteria were included in the meta-analysis.
Risk of bias
Newcastle-Ottawa Quality Assessment Scale (NOS) was utilized to determine the quality of the eligible studies by two authors independently. Indeed, the quality of study population selection, exposure, comparability, and outcome were determined through NOS, where the maximum score for a study was 9 points. According to the scores, the studies were divided into two categories: (I) low quality with scoring ranging from 0 to 4 points and (II) high quality with scores of 5 to 9 points. Disagreement in scoring was dissolved through discussion.
Data collection
Two authors performed data extraction independently. The data included (1) the name of first author, (2) date of publication, (3) number of patients with OSA and controls, (4) AHI, (5) age, (6) body mass index (BMI), (7) circulating levels of Ox-LDL, (8) sample type for measuring Ox-LDL, (and 9) assay approach for measuring Ox-LDL.
Statistical analysis
Levels of Ox-LDL were presented by mean and standard deviation (SD) in OSA and control groups. The main summary measure for evaluating the differences of Ox-LDL levels between OSA and control groups was standardized mean difference (SMD). The DerSimonian Laird random-effects model was utilized for measurement of SMDs, while heterogeneity was measured using I2. Subgroups analysis was carried out according to AHI, age, and BMI. Begg’s and Egger’s tests were applied to determine publication bias and the guideline of Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) was followed to report the study [19]. STATA software (version 14.2) was applied for all analyses.
Results
Search results
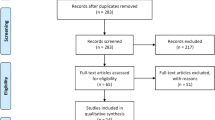
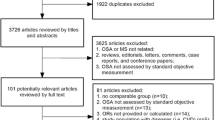
The search diagram is shown in Fig. 1. A total of 195 studies were obtained by the search; 98 duplicates were removed. Further, 49 and 20 articles were excluded because of their title and abstract, respectively. Then, 28 articles were read entirely; of them, 13 articles had no results on Ox-LDL, 5 studies had unmet diagnosis criteria and study design, and 2 studies had inadequate data for meta-analysis. Finally, the meta-analysis was performed on six studies.
Characteristics of eligible studies
Six studies with 232 controls and 391 patients with OSA were eligible for performing the meta-analysis. All of them measured circulating levels of Ox-LDL in patients with OSA and controls. Two studies categorized studied population into subgroups, where Lee et al. divided patients to mild to moderate (AHI of < 30/h and ≥ 5/h) and severe (AHI ≥ 30/h) [15], while Feres et al. had two patient groups with comorbidities (i.e., hypertension and dyslipidemia) and without comorbidities [10]. All subgroups were analyzed separately. Table 1 demonstrated the characteristics of the eligible studies; they included the name of first author, publication date (year), sample size of each group, mean of AHI, mean of age, mean of BMI, and circulating Ox-LDL levels. All studies had used ELISA technique for measuring Ox-LDL levels. The quality assessment results, according to NOS, are shown in Table 1.
Pooled analysis
Regarding the results of heterogeneity test (I2 = 94.1 and p < 0.0001), combined effect size was determined using random effect model. The meta-analysis verified higher circulating levels of Ox-LDL levels in patients suffering from OSA compared to controls (Fig. 2). A study by Jiang et al. revealed a high SMD (5.57), which was 5-fold higher than other studies. In the next model of meta-analysis, the study by Jiang et al. was removed and the results showed higher concentration of Ox-LDL in patients with OSA compared to controls (Fig. 3).
Comparison of Ox-LDL levels between OSA patients and controls after removing a study by Jiang et al. [13]
Subgroup analysis
Subgroup analysis is given in Table 2. Subgroup analysis according to AHI (subgroup I < 30 and subgroup II ≥ 30) showed higher Ox-LDL levels in subgroup I compared to their control groups. However, subgroup II indicated no significant difference compared to their controls. In addition, subgroup analysis was performed according to BMI (subgroup I matched BMI and subgroup II unmatched BMI). The results indicated higher concentrations of Ox-LDL in unmatched subgroup compared to controls. However, the matched group showed no significant difference. The final subgroup analysis was conducted according to age (subgroup I matched age and subgroup II unmatched age). The unmatched group showed higher Ox-LDL in OSA patients compared to controls, while the matched group revealed no significant difference.
Publication bias
The Begg’s (p < 0.711) and Egger’s (p < 0.161) tests showed low risk of publication bias.
Discussion
Studies have shown that OSA independently associated with the risk of ASCVD [1, 9]. Meanwhile, Ox-LDL plays a central role in developing ASCVD [8]. Increased levels of Ox-LDL could be a possible factor that links OSA to ASCVD. Oxidative stress and reactive substance are two deleterious factors that produce oxidized-lipoproteins, such as Ox-LDL, by damaging the lipoproteins [2]. This condition in intima media of arteries leads to initiation of inflammatory response, macrophage recruitment, foam cell formation, and development of atherosclerotic plaque [8, 20]. Ox-LDL is an important risk factor for ASCVD and several studies have evaluated this factor in patients with OSA, but the results are conflicting [10, 13, 14, 16,17,18]. In the present study, we reassessed the circulating Ox-LDL in six eligible studies.
This systematic review and meta-analysis confirmed that circulating concentration of Ox-LDL increased in OSA patients. Patients suffering from OSA had increased concentrations of several oxidative stress markers [21]. It has been shown that intermittent hypoxia caused impairment in mitochondrial electron transfer chain and resulted in uncontrolled production of ROS. This condition can contribute to an elevation in oxidative stress. Increased oxidative stress plays the main role in oxidative damage to vital biomolecules such as proteins, DNA, and lipids. Ox-LDL is a product of oxidative stress and is a risk factor for ASCVD [5, 6]. Studies have suggested that increased Ox-LDL is associated with ASCVD events [22]. According to subgroup analysis, OSA patients with AHI ≥ 30 showed no significant difference with controls where patients with AHI < 30 had higher Ox-LDL. These results suggested an intensified compensatory response to elevated oxidative stress in patients suffering from severe OSA leading to a decrease in Ox-LDL levels. However, because of a small number of studies in the subgroup analysis, reaching a definite conclusion was impossible and more studies are needed regarding this issue.
On the other hand, BMI has a close relationship with OSA, with subgroup analysis showing that studies matching patients and controls according to BMI demonstrated no considerable difference between patients with OSA and controls [14,15,16], while between unmatched studies, patients with OSA demonstrated higher circulating levels of Ox-LDL in comparison to controls [10, 17]. Note that BMI indicated a higher value in patients in comparison to controls in unmatched studies offering the role of BMI in increasing Ox-LDL levels in OSA patients, which is consistent with previous studies [12, 23]. In addition, studies that matched in terms of age demonstrated no considerable difference in Ox-LDL circulating levels [14,15,16], while in unmatched studies, Ox-LDL indicated higher concentration in the patients [10, 17]. In the unmatched studies, age was higher in OSA patients indicating a possible role for age to affect the circulating levels of Ox-LDL in patients suffering from OSA.
In conclusion, the results of the present systematic review and meta-analysis indicated higher Ox-LDL concentrations in patients with OSA. Nevertheless, regarding the effect of age and BMI on Ox-LDL levels, increased levels of Ox-LDL may be partially related to age and BMI.
References
Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers AK et al (2017) Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord 1(4):00019
Khazaie H, Najafi F, Rezaie L, Tahmasian M, Sepehry AA, Herth FJ (2011) Prevalence of symptoms and risk of obstructive sleep apnea syndrome in the general population. Arch Iran Med 14(5):335–338
Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK (2010) Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 137(3):711–719. https://doi.org/10.1378/chest.09-0360
Levy P, Pepin JL, Arnaud C, Baguet JP, Dematteis M, Mach F (2009) Obstructive sleep apnea and atherosclerosis. Prog Cardiovasc Dis 51(5):400–410. https://doi.org/10.1016/j.pcad.2008.03.001
Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP (2010) Pathophysiology of sleep apnea. Physiol Rev 90(1):47–112. https://doi.org/10.1152/physrev.00043.2008
Levy P, Kohler M, McNicholas WT, Barbe F, McEvoy RD, Somers VK et al (2015) Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 1:15015. https://doi.org/10.1038/nrdp.2015.15
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5(1):9–19. https://doi.org/10.1097/WOX.0b013e3182439613
Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN (2017) Mechanisms of foam cell formation in atherosclerosis. J Mol Med (Berl) 95(11):1153–1165. https://doi.org/10.1007/s00109-017-1575-8
Drager LF, Polotsky VY, Lorenzi-Filho G (2011) Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 140(2):534–542. https://doi.org/10.1378/chest.10-2223
Feres MC, Fonseca FA, Cintra FD, Mello-Fujita L, de Souza AL, De Martino MC et al (2015) An assessment of oxidized LDL in the lipid profiles of patients with obstructive sleep apnea and its association with both hypertension and dyslipidemia, and the impact of treatment with CPAP. Atherosclerosis 241(2):342–349. https://doi.org/10.1016/j.atherosclerosis.2015.05.008
Holvoet P, De Keyzer D, Jacobs DR Jr (2008) Oxidized LDL and the metabolic syndrome. Futur Lipidol 3(6):637–649. https://doi.org/10.2217/17460875.3.6.637
Njajou OT, Kanaya AM, Holvoet P, Connelly S, Strotmeyer ES, Harris TB, Cummings SR, Hsueh WC, Health ABC Study (2009) Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev 25(8):733–739. https://doi.org/10.1002/dmrr.1011
Yong-qian Jiang J-SX, Xu J, Zhou Z-x, Ji Y-l (2016) Influence of CPAP therapy on the levels of advanced glycation end products and oxidated low density lipoprotein in patients with OSAHS. Int J Clin Exp Med 9(7):12825–12831
Kizawa T, Nakamura Y, Takahashi S, Sakurai S, Yamauchi K, Inoue H (2009) Pathogenic role of angiotensin II and oxidised LDL in obstructive sleep apnoea. Eur Respir J 34(6):1390–1398. https://doi.org/10.1183/09031936.00009709
Lee SD, Ju G, Choi JA, Kim JW, Yoon IY (2012) The association of oxidative stress with central obesity in obstructive sleep apnea. Sleep Breath 16(2):511–517. https://doi.org/10.1007/s11325-011-0536-7
Svatikova A, Wolk R, Lerman LO, Juncos LA, Greene EL, McConnell JP et al (2005) Oxidative stress in obstructive sleep apnoea. Eur Heart J 26(22):2435–2439. https://doi.org/10.1093/eurheartj/ehi440
Tan KC, Chow WS, Lam JC, Lam B, Wong WK, Tam S et al (2006) HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 184(2):377–382. https://doi.org/10.1016/j.atherosclerosis.2005.04.024
Tauman R, Lavie L, Greenfeld M, Sivan Y (2014) Oxidative stress in children with obstructive sleep apnea syndrome. J Clin Sleep Med 10(6):677–681. https://doi.org/10.5664/jcsm.3800
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Yang X, Li Y, Li Y, Ren X, Zhang X, Hu D, Gao Y, Xing Y, Shang H (2017) Oxidative stress-mediated atherosclerosis: mechanisms and therapies. Front Physiol 8:600. https://doi.org/10.3389/fphys.2017.00600
Eisele H-J, Markart P, Schulz R (2015) Obstructive sleep apnea, oxidative stress, and cardiovascular disease: evidence from human studies. J Oxid Med Cell Longev 2015:9. https://doi.org/10.1155/2015/608438
Gao S, Liu J (2017) Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. Chronic Dis Transl Med 3(2):89–94. https://doi.org/10.1016/j.cdtm.2017.02.008
Hurtado-Roca Y, Bueno H, Fernandez-Ortiz A, Ordovas JM, Ibañez B, Fuster V et al (2017) Oxidized LDL is associated with metabolic syndrome traits independently of central obesity and insulin resistance. Diabetes 66(2):474–482. https://doi.org/10.2337/db16-0933
Funding
This work was supported by a grant (980499) from Kermanshah University of Medical Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent is needed for a systematic review.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fadaei, R., Safari-Faramani, R., Rezaei, M. et al. Circulating levels of oxidized low-density lipoprotein in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath 24, 809–815 (2020). https://doi.org/10.1007/s11325-020-02089-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-020-02089-y