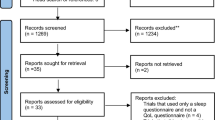
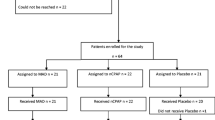
Abstract
Purpose
To examine the craniofacial and airway morphology as well as the quality of life before and after passive myofunctional therapy (PMFT) for 1 year in children with obstructive sleep apnea (OSA).
Methods
Forty children with OSA wearing an oral device nightly (treatment group) and seventeen without the device (control group) were followed up for 1 year. Lateral cephalometric radiography, polysomnography (without participants wearing the oral device), and quality of life survey (OSA-18) were performed before and after the study period.
Results
The apnea-hypopnea index (AHI) during sleep, REM AHI, hypopnea count, and desaturation count in the treatment group dropped significantly, compared with the control group. The craniofacial linear measurements increased significantly in both groups, while the length of mandible (Co-Gn) and anterior facial height (N-Me) became significantly larger in the treatment group. For the airway morphology, the intergroup comparison showed that OPha-Ophp (distance between anterior and posterior sides of oropharynx) increased significantly in the treatment group. For quality of life, the intergroup comparison found statistically significant improvements in the following in the treatment group, based on the OSA-18 survey: loud snoring, dysphagia, mood swings, discipline problems, difficulty awakening, total score for the emotional distress portion, and total survey score.
Conclusions
Preliminary evidence is substantiated for the benefits of 1-year PMFT using an oral device with a built-in tongue bead, including improvements in nasal breathing during sleep, mandible linear growth (Co-Gn and N-Me), airway morphology (OPha-Ophp), and patients’ quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric obstructive sleep apnea syndrome (OSAS) can negatively impact children’s development and systemic health [1,2,3,4], leading to metabolic, cardiovascular, and neurocognitive morbidities. In animal studies, increased nasal resistance dramatically affects maxillary and mandibular bones, halting growth [5, 6] and causing adaptive changes to soft tissues of the tongue as well as deviations in the jaw posture [5, 7, 8]. For example, obstruction of nasal airflow in newborn rhesus monkeys was found to negatively affect the maxilla and limit the development of the upper jaw and the nose, and the displacement of the mandible thus resulted triggered mouth breathing [5, 8]. Increased nasal resistance led to oral breathing and mouth opening during both waking and sleep hours. The craniofacial bone also became narrower because of these changes [5,6,7,8,9,10]. When obstruction of the monkey’s nose was removed during the active growth phase, these changes were reversed [5]. Systematic reviews and meta-analysis [11, 12] have also found retrusive chin, steep mandibular plane, long facial growth, a tendency toward Class II malocclusion, and reduced upper airway width in patients with pediatric obstructive sleep apnea (OSA) due to adenoid hypertrophy.
The pathophysiology of pediatric OSA is multifaceted. Recent research suggests that OSAS may be due to a combination of abnormalities [4]. Conditions such as craniofacial anomalies, obesity, and adenotonsillar hypertrophy could narrow the anatomic airway. Abnormal neuromotor tone in the upper airway—such as abnormal neuromuscular activation, ventilatory control, and arousal threshold—could cause airway collapse during sleep.
The first-choice treatment for pediatric OSA has been adenotonsillectomy (T&A), although recurrence of OSA after T&A has also been reported [13]. On the other hand, rapid maxillary expansion (RME) can produce an orthopedic force to open the mid-palatal suture, resulting in a lateral expansion of the maxillary bones without tipping the teeth [5, 14]. Maxillary widening also appears to independently affect the mandibular growth [5, 14].
Myofunctional therapy (MFT), another treatment for OSA, can improve breathing during sleep and may decrease the apnea-hypopnea index (AHI) by approximately 50% in adults and 62% in children [15, 16]. Given the common suboptimal compliance of MFT in children with OSA as reported in our previous study [17], a passive MFT appliance (an oral device with a built-in tongue bead) was then introduced. Despite the demonstrated improvement of clinical respiratory symptoms during sleep (AHI dropping from 5.4 ± 5.9 to 1.9 ± 1.5/h) in children with OSA after 6 months of treatment using a passive MFT appliance [17, 18], the long-term outcome—such as changes in craniofacial, airway morphology and quality of life—and side effects after passive MFT warrant further investigation. Therefore, the present study aims to evaluate the treatment effect of passive MFT based on a neutral mandibular advancement appliance with a built-in tongue bead placed near the palate, by comparing the craniofacial and airway morphology as well as the quality of life in children with OSA before and after they wear the oral appliance for 1 year.
Methods
The study protocol was approved by the Institutional Review Broad of the Human Investigation Committee of the Chang Gung Memorial Hospital (104-9308A3). Informed consent from the legal guardian of each participant was obtained prior to the investigation.
Participants
Children suspected of pediatric OSA and those with a confirmed diagnosis based on nocturnal polysomnography according to the International Classification of Sleep Disorders Third Edition were recruited for the study, ranging in age from 4 to 14 years. The final diagnosis was based on children’s complaints/clinical symptoms as well as the results of PSG conducted at the Sleep Center of the Chang Gung Memorial Hospital. The following demographic and clinical information was also collected during children’s initial visit to the sleep laboratory: age, sex, body mass index (BMI), body weight and height, gestational age, and birth body weight.
Inclusion and exclusion criteria
The inclusion criteria were clinical symptoms of OSA, and apnea-hypopnea index (AHI) ≥ 1 event/h or respiratory disturbance index (RDI) > 5 as measured by the PSG. The exclusion criteria were as follows: epilepsy, head injury, severe developmental delay and mental retardation, autism, schizophrenia, severe depression, craniofacial abnormality, severe cardiac or medical disease, or inability to cooperate for the measurement of PSG. Patients with severe hypertrophic tonsil or adenoid tissues were also excluded.
Pediatric OSA is different from the adult type as most pediatric cases are associated with a low AHI. Twenty-eight of the study participants (18 in the treatment group and 10 in the control group) had undergone adenoidectomy, tonsillectomy, or adenotonsillectomy (depending on the pattern of their hypertrophic, adenoid and tonsil tissues) before the study. They had a residual AHI meeting the enrollment threshold and were eligible to participate. The rest of participants did not suffer severe adenotonsillar hypertrophy and were thus included in the study.
Treatment and control groups
Age-matched children either were treated with a custom-designed oral appliance with a built-in tongue bead [17, 18] during sleep (passive MFT) for 1 year or received no further treatment while agreeing to be followed up for 1 year. Participants were assigned to the control group, if they met one of the following criteria: (1) they were unwilling to wear the oral device, (2) wearing the oral device was against the parent’s wish, (3) they had too few teeth for the retention of the oral device, or (4) they were unable to cooperate for the fabrication of oral devices. There were more hypertrophic adenoid (70.6% vs 50.0%) and tonsil (52.9% vs 32.5%) tissues in the control group than the treatment group. All participants underwent the PSG and lateral cephalometric X-ray before and after the study period.
Tools
PSG during sleep
A Neurovirtual BWIII PSG Plus sleep system™ (Fort Lauderdale, FL, USA) was used. The following variables were recorded: electroencephalogram (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1), electrooculogram (EOG), chin and leg electromyogram (EMG), and electrocardiogram (ECG) (modified V2 lead). Additional instruments used during the study included the following: body-position sensor, nasal cannula/pressure transducer, mouth thermistor, thoracic and abdominal plethysmography bands, neck microphone, and finger pulse oximeter. The scoring was based on the AASM Manual (2014) and performed by an individual who was not involved in the study and blinded to the study design.
Quality of life survey (the Chinese version of OSA-18)
Caregivers of all study subjects were asked to complete the quality of life questionnaire which consists of 18 items. The items are grouped into 5 domains (sleep disturbance, physical suffering, emotional distress, daytime problems and caregiver concerns). Each item was graded on a 7-point scale ranging from 1 (none of the time) to 7 (all of the time) to evaluate the severity of the problem. The total score, domain score, and item score were recorded. The OSA-18 quality of life survey was performed before and after the study period.
Cephalometric radiography
To determine participants’ lateral craniofacial and airway morphology, digital lateral cephalometric radiographs were obtained for all participants before and after the study period, using the same cephalostat (read by the same specialist) and following a standard guideline. Participants kept their heads in the natural position and their teeth in centric occlusion while holding their breath at the end-expiration phase.
Investigators blinded to the timing of the imaging test traced the digital cephalometric radiographs with a digital image software package (GE Medical Systems Inc. Released 2006. Centricity Enterprise, Version 3.0., USA: GE Healthcare Inc.). An experienced pediatric dentist (the lead author of this study) verified all the cephalometric tracings. The landmarks used for the cephalometric analysis have been detailed in our earlier study [19] and included in Figs. 1, 2, and 3. The error of the method was assessed by tracing and measuring, under the same conditions, 20 randomly selected radiographs from the study at least 2 weeks after the initial measurement. The error of the method was calculated using the Dahlberg formula. The mean [SD] of the error of the method was 0.6 [0.5] mm (range, 0.0–1.9 mm) for all linear variables and 0.5 [0.4]° (range, 0.0–1.8°) for all angular variables.
Cephalometric landmarks. 7: SGo: posterior facial height; 8: Hy-C3: distance from hyoid bone to C3; 9: LSP: length of soft palate; 10: PMi-PNS-ANS: inferior angle of hard palate/soft palate; 11: PNS-NPhp: distance between PNS and posterior side of nasopharynx; 12: PMm-NPh: distance between soft palate and posterior side of nasopharynx; 13: Ar-Go-Gn: gonial angle
Cephalometric landmarks. 14: OPha-OPhp: distance between anterior side and posterior side of oropharynx; 15: HPha-HPhp: distance between anterior side and posterior side of hypopharynx; 16: PMi-NL: nasopharyngeal height; 17: PNS-AD1: distance between PNS to the nearest adenoid tissue along PNS-BA; 18: PNS-AD2: distance between PNS and the nearest adenoid tissue along the line perpendicular to S-BA
Oral appliance (passive MFT)
The appliance is a one-piece, custom-made adjustable oral device for advancing the mandible. A bead is mounted on the lower end of the frame for the tip of the tongue to roll, which in turn places the tongue in a forward position so as to open the airway [17, 18]. The amount of mandibular advancement from wearing the device is 50% of the maximum mandibular advancement (European Patent No. 288,82,384, October 12, 2016; US Patent No. 10,105,2056, October 23, 2018). When construction bites were taken, the participants were asked to advance their mandibles to 50% of the maximum mandibular advancement (to provide space for the device and tongue’s forward movement) with a vertical opening of 2 mm (which is the thickness of the device and the minimum requirement for the device to work properly). Study participants were instructed to wear their appliances and use their tongue to roll the bead (i.e., passive MFT) during sleep every night. Parents recorded in sleep logs the nightly wear by children in the treatment group for 1 year. Recall appointments were arranged for each participant every 3 months to check the condition and fitting of the oral device as well as any side effect or discomfort from wearing the device. The oral device would be fixed or adjusted, if needed. Study participants did not receive traditional MFT.
Statistical analysis
Data were analyzed using a statistical software package (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0, Chicago: SPSS Inc.). Descriptive statistics were presented as means and standard deviations. Chi-square test was used to examine any gender difference between the treatment group and the control group. Mann-Whitney U test was used to examine any significant differences in basic clinical data at the baseline and any differences in the AHI and cephalometric data (T1y − T0) between the two groups. Wilcoxon signed-rank test was used to examine any significant differences in the PSG and cephalometric data before and after the study within each group. The level of significance was set at p < 0.05.
Results
As shown in Table 1, 57 children (44 boys and 13 girls) with OSA (mean age 7.86 ± 3.09 years old; BMI 18.09 ± 3.84; mean AHI 3.56 ± 2.50; mean RDI 6.61 ± 4.49) participated in the study. There were 40 children in the treatment group and 17 children in the control group. There was no significant difference between the two groups in the baseline data. The rate of compliance among participants was high at 80%.
After wearing the oral appliance for 1 year (T1y), the percentages of stage 1 sleep and lowest SaO2 in the treatment group increased significantly. The following parameters also decreased significantly in the treatment group: AHI during sleep, respiratory disturbance index (RDI), AHI in REM sleep, hypopnea index (HI), desaturation index (DI), time in bed, total sleep period (TSP), total sleep time, obstructive sleep apnea count, hypopnea count, respiratory effort-related arousal (RERA) count, and desaturation count. In contrast, only the mean heart rate decreased significantly in the control group after the study period (Table 2).
According to the intergroup comparison, the differences in the change in AHI during sleep, AHI in REM sleep, hypopnea count, and desaturation count were statistically significant between the oral device group and the control group. However, the changes in the following were not statistically significant between the two groups: RDI during sleep, HI, DI, total time in bed, TSP, total sleep time, obstructive apnea count, RERA count, and lowest SaO2 (%) (Table 2).
The cephalometric analysis (Table 3) found no statistically significant difference in the craniofacial angular measurement between the two groups over the study period. However, for the linear measurement, participants’ overall skeletal growth in the treatment group was significant after they wore the oral appliance for 1 year, for example, the length of cranial base (NBa), S-N, nasal line, Co-Gn, length of the palate (ANS-PNS), S-Go, N-Me, Go-Gn, Ar-A, and Ar-Gn. In the control group, Go-Gn increased significantly at T1y. As demonstrated by the intergroup comparison, the length of mandible (Co-Gn) and anterior facial height (N-Me) became significantly greater in the treatment group.
Meanwhile, the airway analysis (Table 3) revealed that the size of the upper airway (PNS-AD2, minRGA, OPha-Ophp) became significantly larger in the treatment group after participants wore the oral appliance for 1 year. In the control group, the size of the upper airway (PNS-AD1) became significantly larger after 1 year. The intergroup comparison showed that the difference in the change in OPha-Ophp (distance between anterior and posterior sides of oropharynx) between the treatment and control groups was statistically significant. In terms of the change in tonsil and adenoid, 12.5% adenoid and tonsil hypertrophy improved in the treatment group, which contrasted with improvement in 5.8% tonsil hypertrophy in the control group. The improvement in hypertrophic tonsil in the treatment group was significant compared to the control group (Table 4).
For quality of life, significant improvement was seen in the following in the treatment group, based on the OSA-18 survey: loud snoring, choking or gasping, fragmented sleep, frequent colds, rhinorrhea, dysphagia, mood swings, aggression/hyperactivity, difficulty awakening, caregiver worrying about child’s health, caregiver being concerned that the child does not have enough air, caregiver frustration, quality of life, total score for the sleep disturbance portion, total score for the physical symptom portion, total score for the emotional distress portion, total score for the daytime problems portion, total score for the caregiver concerns portion, and total survey score (Table 5). In the control group, no statistically significant improvement was found in symptoms. Between the 2 groups, statistically significant improvements were seen in the treatment group in loud snoring, dysphagia, mood swings, discipline problems, difficulty awakening, total score for the emotional distress portion, and total survey score.
Discussions
A previous randomized study compared the results of MFT performed by parents at home with supervision of trained therapists (active MFT) with those using the same oral device as in the current study (passive MFT). The results from that study echoed the findings in the current study where children in the treatment group (wearing the dental device) saw their breathing during sleep improve significantly (AHI dropping from 3.75 to 2.16/h) [17]. The results of the present study also mirrored the findings by Villa et al. [20] which reported a reduction in the AHI from 7.1 to 2.6 as well as improvement in clinical respiratory symptoms in children with OSA after they were treated with a classic mandibular advancement device (MAD) for 6 months.
During the study period, the craniofacial skeletal linear measurements increased significantly in both the treatment and control groups due to spontaneous growth. However, the intergroup comparison found that the changes in Co-Gn and N-Me in the treatment group were statistically significant, which indicates that the mandible has grown more in the treatment group. For intragroup comparisons, maxillary growth (Ar-A) and mandibular growth (Ar-Gn and Co-Gn) were seen in both groups, and yet, only the growth in the treatment group was significant, especially in mandibular arch (Ar-A/Ar-Gn). Similarly, both groups showed vertical facial height growth (S-Go and N-Me) during the study period, and yet, only the growth in the treatment group was significant, especially in anterior facial height (S-Go/N-Me).
For the angular measurement, neither the intragroup nor the intergroup comparison has yielded any significant differences. The larger standard deviations, the wide range of participants’ ages, or the different initial facial patterns may be the reason for the absence of statistical differences. Meanwhile, the mandibular plane angle (SN-MP) appeared to have decreased in the control group after the study period, which was indicative of a trend of counterclockwise rotation. In the treatment group, however, more vertical growth in the anterior facial height was seen, indicating a trend of clockwise rotation. According to the study by Fan [21], the mandibular plane angle does decrease slowly over time (at an average rate of − 0.5°/year) among Taiwanese children between the ages of 8 and 14 and with a normal facial profile. As there were more hyperdivergent facial growth patterns observed in the treatment group (50.0% vs 41.2%) at the start of this study, it is unclear whether little increase in children’s mandibular plane angle in this group is attributable to children’s initial facial pattern or to the oral appliance used. A study with a larger sample and a longer follow-up period will be needed to delineate respective contributions of these factors. Furthermore, studies on dental and skeletal changes after wearing oral appliances by adults with OSA have reported an increase in the incidence of posterior open bite and facial height [22]. However, the effect to the facial profile and occlusion could be the function of patients’ initial skeletal and dental morphology. For example, individuals with a high angle vertical growth pattern appear to be more at risk, whereas individuals with large overjet and deep bite may actually display more favorable changes [23,24,25].
In a study conducted by Arens et al. [26, 27], midsagittal magnetic resonance images (MRI) were taken of a child’s and an adult’s heads (both with OSAS). The image showed a narrowing airway in nasopharyngeal and high oropharyngeal regions in the child where the adenoid and tonsils overlap with the airway. In the adult, the airway narrowing was most pronounced in low retropalatal and retroglossal regions. Based on the cephalometric X-rays taken in the present study, the 1-year treatment using the oral device significantly improved the size of airway in nasopharynx (PNS-AD2) and oropharynx (low oropharyngeal region: OPha-Ophp). The cephalometric results in this study thus echoed the MRI findings by Arens et al. After 1 year, the increase in the size of airway in nasopharynx (PNS-AD1) was more significant in the control group than in the treatment group. It seems that the oral device with a tongue bead did not improve the nasopharyngeal diameter beyond the spontaneous growth. In addition, OPha-Ophp became larger in the treatment group and smaller in the control group. This indicates stronger tongue muscles which pushed the tongue base forward after 1 year of passive MFT using the oral device. Thus, it stands to reason that the repositioning of the jaw and improved tongue posture might have increased the retropharyngeal space [16]. The LSP did decrease in the treatment group and increase in the control group, although the changes were not statistically significant. This result again mirrored findings by Arens et al. [28] where the volume of the soft palate of children with mild to moderate OSAS increased by 30%, compared to the control group. They surmised that the larger palatal volume might have been due to the edema and inflammatory changes secondary to chronic snoring. More improvements in hypertrophic adenoid and tonsil tissues were also found in the treatment group after participants wore the oral device for 1 year.
Hypotonic muscles are often seen in pediatric OSAS, including the tongue muscle. The hypotonic tongue would drop back and obstruct the oropharyngeal airway when children lie down or sleep. The tongue bead in the oral device used in this study thus functions like a foreign object which targets the swallowing reflex and increases the front-to-back movement of the tongue, mimicking the myofunctional therapy. As the tongue moves forward during sleep, the oropharyngeal airway could then reopen (Fig. 4), hence the “passive” myofunctional therapy. Nevertheless, it remains unclear whether the aforementioned treatment effects stemmed solely from the tongue bead in the device, the 50% maximum mandibular advancement, the vertical opening, or any combination of these factors. Future studies to isolate the effect of 50% maximum mandibular advancement will be needed to shed light on this causal link.
To address the current gap in the literature, the present study also examined changes in quality of life using the OSA-18 questionnaire and found improvement among treated participants. In the survey, significant improvements were associated with many indicators in the oral device group, especially for the emotional distress portion.
Meanwhile, there are a number of limitations in this study. First, as sleep MRI [29] was not utilized during the study, the study results would not apply to OSA cases with dynamic upper airway collapse. Second, participants in the control group suffered milder forms of OSA than those in the treatment group, and the sample size of the control group was much smaller than that of the treatment group. Third, the initial facial patterns were different in the two groups. There were more children with hyperdivergent facial growth patterns in the treatment group and more children with normal mandibular plane angles in the control group. Forth, the adenoid and tonsil tissue patterns were different in the two groups at the start of the study, with more such tissues in the control group. Fifth, the wide range of participants’ ages made it difficult to explain the facial growth patterns.
Conclusions
Passive MFT for 1 year can improve the mandibular growth and upper airway morphology in the oropharyngeal region as well as nasal breathing during sleep. Treated patients’ quality of life (as measured by the OSA-18 survey) also improves significantly, especially as related to emotional distress.
References
Capua M, Ahmadi N, Shapiro C (2009) Overview of obstructive sleep apnea in children: exploring the role of dentists in diagnosis and treatment. J Can Dent Assoc 75(4):285–289
Bahammam A (2011) Obstructive sleep apnea: from simple upper airway obstruction to systemic inflammation. Ann Saudi Med 31(1):1–2
Lal C, Strange C, Bachman D (2012) Neurocognitive impairment in obstructive sleep apnea. Chest. 141(6):1601–1610
Katz ES, D’Ambrosio CM (2008) Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 5(2):253–262
Huang YS, Guilleminault C (2013) Pediatric obstructive sleep apnea and the critical role of oral-facial growth: evidences. Front Neurol 3(184):1–7
Harvold EP, Tomer BS, Vargervik K, Chierici G (1981) Primate experiments on oral respiration. Am J Orthod 79(4):359–372
Miller AJ, Vargervik K, Chierici G (1984) Experimentally induced neuromuscular changes during and after nasal airway obstruction. Am J Orthod 85(5):385–392
Vargervik K, Miller AJ, Chierici G, Harvold E, Tomer BS (1984) Morphologic response to changes in neuromuscular patterns experimentally induced by altered modes of respiration. Am J Orthod 85(2):115–124
Rubin RM (1987) Effects of nasal airway obstruction on facial growth. Ear Nose Throat J 66(5):212–219
Vargervik K, Harvold EP (1987) Experiments on the interaction between orofacial function and morphology. Ear Nose Throat J. 66(5):201–208
Katyal V, Pamula Y, Martin AJ, Daynes CN, Kennedy JD, Sampson WJ (2013) Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: systematic review and meta-analysis. Am J Orthod Dentofac Orthop 143(1):20–30
Flores-Mir C, Korayem M, Heo G, Witmans M, Major MP, Major PW (2013) Craniofacial morphological characteristics in children with obstructive sleep apnea syndrome: a systematic review and meta-analysis. J Am Dent Assoc 144(3):269–277
Huang YS, Guilleminault C, Lee LA, Lin CH, Hwang FM (2014) Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep. 37(1):71–76
Pirelli P, Saponara M, Guilleminault C (2004) Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep. 27(4):761–766
Camacho M, Certal V, Abdullatif J, Zaghi S, Ruoff CM, Capasso R, Kushida CA (2015) Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 38(5):669–675
Villa MP, Brasili L, Ferretti A, Vitelli O, Rabasco J, Mazzotta AR, Pietropaoli N, Martella S (2015) Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep Breath 19(1):281–289
Huang YS, Chuang LC, Hervy-Auboiron M, Paiva P, Lin CH, Guilleminault C (2019) Neutral supporting mandibular advancement device with tongue bead for passive myofunctional therapy: a long term follow-up study. Sleep Med 60:69–74. https://doi.org/10.1016/j.sleep.2018.09.013
Chuang LC, Lian YC, Hervy-Auboiron M, Guilleminault C, Huang YS (2017) Passive myofunctional therapy applied on children with obstructive sleep apnea: a 6-month follow-up. J Formos Med Assoc 116(7):536–541
Lian YC, Huang YS, Guilleminault C, Chen KT, Hervy-Auboiron M, Chuang LC, Tsai AI (2017) The preliminary results of the differences in craniofacial and airway morphology between preterm and full-term children with obstructive sleep apnea. J Dent Sci 12(3):253–260
Villa MP, Bernkopf E, Pagani J et al (2002) Randomized controlled study of an oral jaw- positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. Am J Respir Crit Care Med 165(1):123–127
Fan Y C (1995) A study of radiographic cephalometric analysis and Taiwanese standards [thesis]. Taipei: National Taiwan University
Perez CV, de Leeuw R, Okeson JP, Carlson CR, Li HF, Bush HM, Falace DA (2013) The incidence and prevalence of temporomandibular disorders and posterior open bite in patients receiving mandibular advancement device therapy for obstructive sleep apnea. Sleep Breath 17(1):323–332
De Almeida FR, Lowe AA, Tsuiki S et al (2005) Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med 1(2):143–152
Chen H, Lowe AA, de Almeida FR, Fleetham JA, Wang JA (2008) Three-dimensional computer assisted study model analysis of long-term oral-appliance wear. Part 2. Side effects of oral appliances in obstructive sleep apnea patients. Am J Orthod Dentofac Orthop 134(3):408–417
Ngiam J, Balasubramaniam R, Darendeliler MA (2013) Clinical guidelines for oral appliance therapy in the treatment of snoring and obstructive sleep apnoea. Aust Dent J 58(4):408–419
Arens R, Marcus CL (2004) Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 27(5):997–1019
Arens R, McDonough JM, Corbin AM et al (2003) Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 167(1):65–70
Arens R, McDonough JM, Costarino AT et al (2001) Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 164(4):698–703
Huon LK, Liu SY, Shih TT et al (2016) Dynamic upper airway collapse observed from sleep MRI: BMI-matched severe and mild OSA patients. Eur Arch Otorhinolaryngol 273(11):4021–4026
Funding
This study was supported by the Chang Gung Memorial Hospital (CMRPG3G1951 and CMRPG3H1591; Grant Recipient: Li-Chuan Chuang). The data of the study were first presented at the 5th International Pediatric Sleep Association Congress, Paris, April, 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of the Human Investigation Committee of Chang Gung Memorial Hospital and Chang Gung University (IRB 104-9308A3), as well as the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent from the legal guardian of each participant was obtained prior to the investigation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chuang, LC., Hwang, YJ., Lian, YC. et al. Changes in craniofacial and airway morphology as well as quality of life after passive myofunctional therapy in children with obstructive sleep apnea: a comparative cohort study. Sleep Breath 23, 1359–1369 (2019). https://doi.org/10.1007/s11325-019-01929-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-019-01929-w