Abstract
Purpose
This study investigated the properties of blood pressure (BP) fluctuation and sympathovagal imbalance with the severity of OSAS.
Methods
Nocturnal BP was continuously monitored by polysomnography for mild (n = 33), moderate (n = 34), and severe (n = 37) OSAS patients. Apnea-related systolic BP elevation (△SBP) indicated the amplitude of BP fluctuation. The SBP index, number of △SBP > 10 mmHg/h of sleep, indicated the frequency of significant BP fluctuations. The low frequency/high frequency (LF/HF) ratios indicated heart rate variability and sympathovagal imbalance.
Results
△SBP and the SBP index were the highest in severe OSAS (12.9 ± 2.3 mmHg and 33.7 ± 14.7/h), followed by moderate OSAS (9.5 ± 2.6 mmHg and 7.1 ± 4.4/h), and mild OSAS (8.3 ± 1.6 mmHg and 3.4 ± 2.1/h). The LF/HF ratios in severe OSAS were significantly higher than that in moderate and mild OSAS. In mild OSAS, arousal played a more important role in BP fluctuation. In moderate OSAS, the oxygen desaturation index (ODI) and the SBP index were correlated. The difference in △SBP induced by hypoxia or by arousal was not significant. In severe OSAS, the apnea-hypopnea index (AHI) and LF/HF ratio were correlated with the SBP index, and △SBP was larger with hypoxia than arousal.
Conclusions
BP fluctuation and sympathovagal imbalance were both related to obstructive sleep apnea severity. The influence of arousal and hypoxia on BP fluctuation varied with OSAS severity.
Trial registration
NCT02876471
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is characterized by recurrent episodes of upper-airway obstruction with large fluctuations in arterial blood pressure (BP) during sleep [1]. Increased fluctuation of nocturnal BP may increase the risk of organ damage independent of the mean BP [2, 3]. Assuming that repetitive hemodynamic oscillations during apnea adversely affect cardiovascular structure and function, it becomes important to clarify the causes of nocturnal BP fluctuation. Abnormal sympathovagal balance has been noted in OSAS patients, and it may directly elevate BP [4, 5]. However, the ways in which BP fluctuation and sympathovagal balance vary with OSAS severity are not clear.
Some evidences indicate that arousal and not hypoxia and mechanical factors that are the primary influence of acute increases in BP associated with obstructive apneic events [6, 7]. However, the amplitude of post-apneic BP elevations is closely related to the degree of hypoxia [8], with the maximal BP elevation occurring at the time of minimal pulse oxyhemoglobin saturation (SpO2) during sleep [9]. Different severity of OSAS was characterized with different degrees of hypoxia. Thus, we hypothesized that the effects of hypoxia and arousal on BP fluctuation may vary with OSAS severity. Few of the previous studies of the amplitude of post-apneic BP have reported the frequency of overnight BP fluctuations. Consequently, this study continuously monitored nocturnal dynamic BP fluctuations during sleep by polysomnography (PSG) and pulse transfer time (PTT). Overnight apnea and hypopnea events were analyzed to assess the effects of oxygen desaturation and arousal on the amplitude of nocturnal BP fluctuation in patients with OSAS of differing severity. The association of the frequency of BP fluctuation and sympathovagal imbalance with OSAS severity was also evaluated.
Methods
Subjects
A total of 217 subjects with suspected sleep-disordered breathing were recruited were recruited at our sleep center between March 2016 and April 2017 following an overnight PSG examination. Four patients were taking antihypertensive drugs. To avoid a drug effect on the results, they were instructed to stop treatment 3 days prior to enrollment, and their BP was closely monitored (three cuff measurements on two separate occasions) after resting seated for ≥ 5 min. Individuals whose BP was consistently ≥ 180/110 mmHg or who complained of dizziness, headache, palpitations, and other clinical syndromes were excluded from the protocol, placed back on their BP medications, or given clinical intervention immediately. Patients between 18 and 75 years of age with BP ≤ 180/110 mmHg and with newly diagnosed OSAS or with OSAS without treatment were eligible. Patients who had been hospitalized for cardiac or respiratory exacerbations less than 6 weeks previously; those with autonomic nervous system diseases or with endocrine disorders (that might influence BP variability); or who were unwilling to participate in the study were excluded. In total, 104 patients were enrolled and diagnosed with mild (AHI 5–14, n = 33); moderate (AHI 15–29, n = 34), or severe (AHI ≥ 30, n = 37) OSAS. Figure 1 displayed a flowchart of study design and process. The study was approved by the Scientific Research and Technology Ethics Committee of Huai’an First People’s Hospital (IRB-KPJ 2016–005-01) and registered in ClinicalTrials.gov. (ID: NCT02876471); all subjects gave their informed consent.
PSG data
Patients underwent the PSG (SOMNOmedics GmbH, Randersacker, Germany) study. Sleep recordings included electroencephalograms (EEGs), left and right electrooculogram, and bilateral chin electromyography. Respiratory variables were recorded with nasal cannulas, thoracic and abdominal strain gauges, and SpO2.
Cardiovascular testing parameters
Apart from the continuous ECG monitoring during PSG, noninvasive frequency-domain analysis was used for assessing the autonomic nervous system (ANS) regulation of the heart. The spectral analysis of HR variability we used allows some degree of separation of the sympathetic and vagal contributions: low-frequency (0.04–0.15 Hz) fluctuations are mediated by an unknown mixture of sympathetic and vagal influences, whereas high-frequency (0.15–0.40 Hz) fluctuations are believed to be solely vagally mediated. Commonly, a balance of sympathetic and vagal contributions to HR variability is computed from these low- and high-frequency fluctuations. The LF/HF ratio was used as an index of sympathovagal balance [10,11,12,13,14]. Office BP was measured with an automatic sphygmomanometer on three separate occasions with a 1-min interval. Hypertension was defined as systolic (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg following American Society of Hypertension guidelines [15]. Beat-to-beat BP was monitored during PSG by PTT. The novel cuffless continuous BP monitor derived BP from the PTT measurement using a previously described stretch-strain model in which the PTT reflects short-term changes of BP and hemodynamic parameters [16,17,18]. BP was measured once with a BP cuff and that value was used to calibrate the simultaneously measured PTT value. Using this one-point calibration, accurate systolic and DBP values were calculated for each PTT value, were synchronized with PSG, and were analyzed by “DOMINO” software (version2.7.0, GmbH, Randersacker, Germany).
Data analysis
Tracings were scored manually following the American Academy of Sleep Medicine guidelines [19]. Arousal was defined as an abrupt shift in EEG lasting > 3 s. RERAs was the respiratory event-related arousals. Obstructive apnea involved absence of airflow for >10 s. Hypopnea involved a reduction of airflow of > 30% for 10 s that was associated with ≥ 3% desaturation or with arousal. The oxygen desaturation index (ODI) was the average number of oxygen desaturation events of ≥ 3%/h during sleep. TST90 was the percentage of sleep time with oxygen saturation < 90%. Total AHI was the number of apnea and hypopnea events/hour of sleep. OSA was defined as an AHI of ≥ 5, and OSA severity was determined by the AHI score. Mild OSA was indicated by an AHI of 5–14; moderate OSA by an AHI of 15–29; and severe OSA by an AHI of ≥ 30. Event-related systolic BP elevation (△SBP) was calculated as the gap between the peak post-apneic SBP and the lowest SBP recorded during an obstructive respiratory event. The SBP index was the number of △SBPs of > 10 mmHg that was recorded per hour of sleep time. △SBP was used to represent the amplitude of BP fluctuation, and the SBP index was the frequency of BP fluctuations. Overnight apnea and hypopnea events were classified as type 1, with only oxygen desaturation; type 2, with arousal and without oxygen desaturation; and type 3, with both oxygen desaturation and arousal. To investigate the influence of oxygen desaturation and arousal on BP fluctuations, differences in the △SBP observed in mild, moderate, and severe OSAS were compared. For each patient, the 7-h PSG recording was divided into multiple 5-min intervals, 84 points were sampled. The resulting data was to calculate a mean value for each interval of LF/HF ratio.
Statistical analysis
Continuous data were presented as means ± SD. Categorical data were presented as numbers and percentages. The Kolmogorov–Smirnov and Levine tests were used to assess the normality and homogeneity of distributions. Nonnormal distributions were transformed prior to regression analysis and comparison. Chi-square (χ2) tests were used to assess differences in categorical data values. Differences of continuous variables observed in mild, moderate, and severe OSAS were tested by one-way ANOVA. The Student–Newman–Keuls method was used for post hoc correction of multiple comparisons. The interrelation of variables was evaluated by Pearson’s correlation coefficient and multiple linear regression. A p value of < 0.05 was considered statistically significant. The statistical analysis was performed with the SPSS 16.0 (SSPS Inc., Chicago, IL, USA).
Results
Patient characteristics
The patient characteristics and BP data are shown in Table 1. The mean age was 48.9 ± 11.3 (range 26–74) years, mean body mass index (BMI) was 27.0 ± 3.8 (range 19.7–35.9) kg/m2. Seventeen patients (16.3%) were hypertensive; 87 (83.7%) were normotensive. The mean age and sex distribution in each group were not significantly different, but the mean BMI, Epworth Sleepiness Scale (ESS) score, and BP were significantly higher in patients with severe OSAS than in patients with moderate or mild OSAS. Compared with mild OSAS, the prevalence of hypertension was two times higher in patients with moderate OSAS and five times higher in those with severe OSAS.
PSG and BP variability
As shown in Table 2, patients with a high AHI tended to have significantly increased RERAs occurrence, ODI, and TST90 and decreased SpO2 and minimum SpO2 (all p < 0.01). Apnea, hypopnea, and hypoxia durations were longer in severe OSAS than in mild and moderate OSAS (all p < 0.05). Both △SBP (12.9 ± 2.3 mmHg) and the SBP index (33.7 ± 14.7/h) were the highest in severe OSAS followed by moderate OSAS (9.5 ± 2.6 mmHg and 7.1 ± 4.4/h) and mild OSAS (8.3 ± 1.6 mmHg and 3.4 ± 2.1/h). The differences were all significant (all p < 0.05). In severe OSAS, awake (2.4 ± 0.8%) and asleep (2.6 ± 1.0%) LF/HF ratios were significantly higher than in moderate (2.2 ± 1.1% and 1.7 ± 0.7%) and mild (1.9 ± 0.6% and 1.6 ± 0.6%) (all p < 0.05) OSAS. The differences in mild and moderate OSAS were not significant.
Event-related SBP elevations in patients vary with OSAS severity
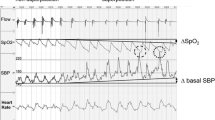
The beat-to-beat BP changes in response to arousal and hypoxia in patients with different OSAS severity are shown in Fig. 2. In mild OSAS, the amplitude of SBP change, i.e., △SBP induced by arousal (type 2, 8.5 ± 1.6 mmHg) was larger than that induced by oxygen desaturation (type 1, 6.5 ± 1.9 mmHg, p < 0.01), but △SBP in response to arousal (8.7 ± 2.2 mmHg) and oxygen desaturation (9.3 ± 2.6 mmHg) were not significantly different in moderate OSAS (p = 0.54). In severe OSAS, △SBP in type 1 (12.7 ± 2.6 mmHg) was significantly larger than that in type 2 (10.6 ± 2.4 mmHg, p < 0.01). In all three groups, △SBP was significantly larger following both oxygen desaturation and arousal (type 3) events than following either type 1 or type 2 events alone.
Mean values of overnight apnea-related SBP elevation caused by hypoxia, arousal, and both factors in mild, moderate, and severe OSA. Type 1, apnea or hypopnea events ended with oxygen desaturation only; type 2, arousal followed apnea or hypopnea; and type3, apnea or hypopnea events ended with hypoxia and arousal; *p < 0.05
Correlation and multiple linear regression analysis of variables associated with SBP fluctuation
Correlation (r) and multiple linear regression (r2) results are shown in Table 3. The SBP index was the dependent variable. In mild OSAS, RERAs, oxygen desaturation duration, and minimum SpO2 were all correlated with the SBP index. Following multiple linear regression, only RERAs remained significantly correlated with the SBP index (r2 = 0.614, p < 0.001). In moderate OSAS, the SBP index was correlated with the ODI, AHI, sleeping LF/HF, and desaturation duration. Regression analysis found that only ODI was correlated with the SBP index (r2 = 0.480, p < 0.001). In severe OSAS, AHI, RERAs, ODI, LF/HF, meanSpO2, TST90, event length, and desaturation duration were all correlated with BP fluctuation. After multiple regression analysis, only AHI (r2 = 0.593, p < 0.001) and LF/HF (r2 = 0.034, p < 0.001) were independently associated with the SBP index.
Discussion
We believe this to be the first study to evaluate the effects of hypoxia and arousal on the amplitude and frequency of beat-to-beat BP fluctuation in patients with OSAS of differing severity. The study population included patients with mild-to-severe OSAS, who were free of antihypertensive medications; this avoided the influence of medications of BP variability. Ambulatory BP monitor (ABPM), which is used to monitor nocturnal BP during sleep in previous studies, measures BP at fixed intervals without synchronization with sleep apnea episodes [20, 21]. Consequently, it often fails to detect apnea-induced BP surges. In addition, cuff inflation and noise caused by cuff inflation may induce an arousal; however, arousal itself could induce a BP elevation. In present study, beat-to-beat recording of BP synchronized with polysomnography using the PTT method, which is a noninvasive technique that has been shown accurately monitoring acute BP changes with only minimal sleep disturbance. Therefore, PTT may better reflect dynamic BP changes and the study design may allow more objective and accurate evaluation of the effects of OSAS on BP fluctuation than previous reports.
We found that patients with a high AHI had significantly increased mean BPs and LF/HF ratios, which confirmed and extended the results of previous findings that OSAS increased the risk of hypertension [22, 23].
Both SBP and DBP were higher in severe OSAS than in mild and moderate OSAS. Enhanced sympathetic nerve activity can induce hypertension in OSAS patients not only during sleep but also during periods of wakefulness [24, 25]. Sympathovagal balance was assessed by the LF/HF ratio. Compared with mild and moderate OSAS, patients with severe OSAS had higher LF/HF ratios both while sleeping and while awake, suggesting increased sympathetic activity. The LF/HF ratio was higher during sleep than when awake, but that was not observed in either mild or moderate group. This suggests that in these OSAS patients, sympathetic activity during sleep depended more on the severity of apnea rather than the stage of sleep. Frequent respiratory events while sleep could result in increased chemoreceptor reflex and sympathetic nerve activity compared with that while awake. This partly accounts for the “nondipping” of BP at night in many OSAS patients. Moreover, when breathing resumes, venous return, and cardiac output increase. The increased cardiac output passes through a highly constricted peripheral vasculature, exacerbating the acute BP fluctuation that manifested as the increased SBP index and larger △SBP seen in this study.
△SBP and the SBP index tended to be higher in severe than that in mild and moderate OSAS. The mean post-apneic BP elevation was 10.2 mmHg; however, Plane’s et al. have monitored beat-by-beat photoplethysmographic BP continuously with Finapres method, with the exception of 10-min periods every 2 h where the system was turned off for patient comfort. They have reported the average value of apnea-related SBP elevations as large as 30.7 ± 2.1 mmHg in normotensive patients and 44.7 ± 4.1 mmHg in hypertensive patients [9]. In another study, arterial BP was measured continuously by using a same BP monitor (Finapres) and mean BP following an obstructive apneic event can increase by as much as 32 mmHg during NREM and 42 mmHg during REM sleep [26]. Increases of that size were not observed in our patients, even in those with severe OSAS. The inconsistency could be associated with ethnic differences. In addition, previous studies selected only 20–30 events during sleep or in the first period of stage 2 NREM sleep for data analysis. A small sample of apnea events might not fully represent the extent of all the post-apneic BP elevations that occurred. Overnight apnea events were included in this study, but excluded evaluation of the effects of changes of body position and leg movements. In this study, large desaturations (SpO2 < 75%) had the strongest influence on apnea-related BP elevation (△SBP > 70 mmHg), which suggests that significant BP fluctuation could be induced by severe hypoxia. However, since not all apnea events included severe desaturation, short apnea or hypopnea events with mild desaturation might have had less effect on BP fluctuation, or may not have influenced post-apneic BP elevation; there are thus a number of reasons why the △SBP reported in our study was smaller than previously reported.
Oxygen desaturation, change in intrathoracic pressure, hypercapnia, and arousal have all been reported to cause post-apneic BP elevation [27]. Of these, hypoxia has been considered as the major influence [28, 29]. However, Ringler et al. failed to demonstrate an attenuated pressor response to apnea with administration of supplemental oxygen. The pressor responses to auditory-induced arousals and arousals terminating obstructive apneas were of similar magnitude [6]. Davies et al. reported that auditory stimulation to induce arousal in normal subjects evoked a rise in BP sufficiently large to explain most of the post-apneic BP increase seen in OSA [30], and Yoon et al. reported arousal had the strongest correlation with BP reactivity during sleep in OSAS patients [31]. Those studies demonstrate that arousal plays a major role in the amplitude of BP fluctuation induced by respiratory events. The reasons for these contradictory results are not clear. Okabe et al. reported that BP during apneic episodes was correlated with △SpO2 [26], and Planès et al. found that patients with the most severe desaturation (minimum SpO2 < 75%) also had the greatest apnea-related BP increases [9]. Both results indicate that the extent of oxygen desaturation was associated with post-apneic BP elevation and that significant BP fluctuation was induced by severe hypoxia. In patients who experience severe, intermittent nocturnal oxygen desaturation, hypoxia may be a stronger influence on BP fluctuation than arousal. If respiratory events ending with mild desaturation have a smaller effect on BP fluctuation, then arousal would play a more important role. The study results to support the viewpoint that in mild OSAS, arousal was the most relevant variable to △SBP and SBP index. In moderate OSAS, ODI completely displaced arousal as the chief determinant of the SBP index. In severe OSAS, AHI was the variable most closely correlated with the SBP index, and the effect of hypoxia on BP fluctuation was greater than that of arousal. The results of this study might explain the inconstancies of previous reports concerning the influence of hypoxia or arousal on post-apneic BP fluctuation in patients with OSAS of differing severity. It also demonstrated that the amplitude of BP fluctuation was increased when accompanied by both oxygen desaturation and arousal than by arousal or hypoxia alone. This suggested superimposed effects of both oxygen desaturation and arousal on BP fluctuation.
Limitation
Respiratory pattern also can significantly influence HF power. OSAS was characterized by dramatic respiratory fluctuation, which could affect HF power; thus, HRV assessed by spectral analysis in OSAS patient could not avoid the effect of obvious respiratory pattern fluctuation [32]. However, a number of studies employing spectral analysis of HRV in OSAS patients have generally reported some valuable results [33,34,35,36]. In addition, spectral analysis measurement is severely limited by advanced heart failure or complicated myocardial infarction, arrhythmia, diabetes, and autonomic nervous system disease, although the diseases mentioned above were excluded from our study.
Conclusions
In OSAS patients, sympathovagal balance and BP fluctuations were associated with OSAS severity. The variables that induced BP fluctuation differed with OSAS severity. In mild OSAS, arousal played a major role. In moderate OSAS, no difference was observed in post-apneic BP elevation evoked by arousal or hypoxia. ODI was the chief determinant of the frequency of BP fluctuation. In severe OSAS, hypoxia played a dominant role in post-apneic BP elevation. The variable most closely correlated with the frequency of BP fluctuation was the AHI. Hypoxia and arousal following apnea events had additive effects on the amplitude of BP fluctuation.
References
Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC (1976) Hemodynamic in sleep-induced apnea: studies during wakefulness and sleep. Ann Intern Med 85:714–719
Richter Y, Edelman ER (2006) Cardiology is flow. Circulation 113:2679–2682
Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA, International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators (2010) International database on ambulatory blood pressure in relation to cardiovascular outcomes investigators. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension 55:1049–1057
Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE (2001) Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest 120:887–893
Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA (1997) Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest 99:106–109
Ringer J, Basner RC, Shannon R et al (1990) Hypoxemia alone does not explain blood pressure elevations after obstructive apneas. J Appl Physiol 69:2143–2148
O’Donnell CP, Ayuse T, King ED, Schwartz AR, Smith PL, Robotham JL (1996) Airway obstruction during sleep increases blood pressure without arousal. J Appl Physiol 80:773–777
O’Donnell CP, King ED, Schwartz AR, Robotham JL, Smith PL (1994) Relationship between blood pressure and airway obstruction during sleep in the dog. J Appl Physiol 77:1819–1828
Planès C, Leroy M, Fayet G et al (2002) Exacerbation of sleep-apnoea related nocturnal blood-pressure fluctuations in hypertensive subjects. Eur Respir J 20:151–157
Lombardi F, Malliani A, Pagani M, Cerutti S (1996) Heart rate variability and its sympatho-vagal modulation. Cardiovasc Res 32:208–216
Hayano J, Sakakibara Y, Yamada A, Yamada M, Mukai S, Fujinami T, Yokoyama K, Watanabe Y, Takata K (1991) Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. Am J Cardiol 67:199–204
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17:354–381
Ori Z, Monir G, Weiss J, Sayhouni X, Singer DH (1992) Heart rate variability: frequency domain analysis. Cardiol Clin 10:496–499
Parati G, Saul JP, Rienzo MD, Mancia G (1995) Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation a critical appraisal. Hypertension 25:1276–1286
Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ (1993) Human blood pressure determination by sphygmomanometry. Circulation 88:2460–2470
Vlahandonis A, Biggs SN, Nixon GM, Davey MJ, Walter LM, Horne RSC (2014) Pulse transit time as a surrogate measure of changes in systolic arterial pressure in children during sleep. J Sleep Res 23:406–410
Wibmer T, Doering K, Kropfsanchen C et al (2014) Pulse transit time and blood pressure during cardiopulmonary exercise tests. Physiol Res 63:287–296
Wong MY, Poon CC, Zhang YT (2009) An evaluation of the cuffless blood pressure estimation based on pulse transit time technique: a half year study on normotensive subjects. Cardiovasc Eng Int J 9:32–38
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep Medicine. (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8:597–619
Steinhorst AP, Gonçalves SC, Oliveira AT, Massierer D, Gus M, Fuchs SC, Moreira LB, Martinez D, Fuchs FD (2014) Influence of sleep apnea severity on blood pressure variability of patients with hypertension. Sleep Breath 18:397–401
Martynowicz H, Porębska I, Poręba R et al (2016) Nocturnal blood pressure variability in patients with obstructive sleep apnea syndrome. Adv Exp Med Biol 952:9–15
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384
Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbé F, Vicente E, Wei Y, Nieto FJ, Jelic S (2012) Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 307:2169–2176
Somers VK, Dyken ME, Clary MP, Abboud FM (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96:1897–1904
Xie A, Skatrud JB, Puleo DS, Morgan BJ (2001) Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol 91:1555–1559
Okabe S, Hida W, Kikuchi Y, Taguchi O, Ogawa H, Mizusawa A, Miki H, Shirato K (1995) Role of hypoxia on increased blood pressure in patients with obstructive sleep apnoea. Thorax 50:28–34
Phillips CL, O’Driscoll DM (2003) Hypertension and obstructive sleep apnea. Curr Hypertens Rep 5:380–385
Narkiewicz K, Van PJ, Pesek CA et al (1999) Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99:1183–1189
Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L (1995) Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol 79:581–588
Davies RJ, Belt PJ, Roberts SJ, Ali NJ, Stradling JR (1993) Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol 74:1123–1113
Yoon IY, Jeong DU (2001) Degree of arousal is most correlated with blood pressure reactivity during sleep in obstructive sleep apnea. J Korean Med Sci 16:707–711
Rajendra AU, Paul JK, Kannathal N, Lim CM, Suri JS (2006) Heart rate variability: a review. Med Biol Eng Comput 44:1031–1051
Park DH, Shin CJ, Hong SC, Yu J, Ryu SH, Kim EJ, Shin HB, Shin BH (2008) Correlation between the severity of obstructive sleep apnea and heart rate variability indices. J Korean Med Sci 23:226–231
Aydin M, Altin R, Ozeren A, Kart L, Bilge M, Unalacak M (2004) Cardiac autonomic activity in obstructive sleep apnea: time-dependent and spectral analysis of heart rate variability using 24-hour Holter electrocardiograms. Tex Heart Inst J 31:132–136
Noda A, Yasuma F, Okada T, Yokota M (1998) Circadian rhythm of autonomic activity in patients with obstructive sleep apnea syndrome. Clin Cardiol 21:271–276
Gula LJ, Krahn AD, Skanes A et al (2015) Heart rate variability in obstructive sleep apnea: a prospective study and frequency domain analysis. Ann Noninvasive Electrocardiol 8:144–149
Acknowledgments
The authors would like to thank all patients included in this research.
Funding
The study was supported by National Natural Science Foundation of China (81500069).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Scientific Research and Technology Ethics Committee of Huai’an First People’s Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comment
In the present study, authors attempted to describe several factors (stimuli associated with repetitive obstructive apneas) which best determine cardiovascular response to apneas measured as HRV or BP-surrogate (pulse transit time) fluctuations. In this observational study, authors concluded that the severity of OSA denoted by standard AHI cutoff levels may have an impact on the findings which, as the authors state, may partially explain previous discrepancies reported in this regard. This is a novel finding which is worth further discussion.
Jacek Wolf
Gdansk, Poland
Rights and permissions
About this article
Cite this article
Xu, J., Ding, N., Chen, L. et al. Inducers of post-apneic blood pressure fluctuation monitored by pulse transfer time measurement in obstructive sleep apnea varied with syndrome severity. Sleep Breath 23, 769–776 (2019). https://doi.org/10.1007/s11325-018-1770-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1770-z