Abstract
Obstructive sleep apnea (OSA) is a common respiratory disorder associated with hypertension and cardiovascular complications. Blood pressure variability may be a sign of risk of cardiovascular events. The aim of this study was to investigate the hypothesis that severe OSA syndrome is associated with increased blood pressure variability. Based on respiratory polygraphy, 58 patients were categorized into two groups: severe OSA with apnea/hypopnea index (AHI) greater than 29 episodes per hour (mean 52.2 ± 19.0/h) and mild-to-moderate OSA with AHI between 5 and 30 episodes per hour (mean 20.2 ± 7.8/h). A 24-h noninvasive blood pressure monitoring was performed. The standard deviation of mean blood pressure was used as the indicator of blood pressure variability. In patients with severe, compared with mild-to-moderate OSA, a higher mean nocturnal systolic blood pressure (133.2 ± 17.4 mmHg vs. 117.7 ± 31.2 mmHg, p < 0.05) and diastolic blood pressure (80.9 ± 13.1 mmHg vs. 73.8 ± 9.2, p < 0.01), nocturnal systolic blood pressure variability (12.1 ± 6.0 vs. 7.6 ± 4.3, p < 0.01) and diastolic blood pressure variability (10.5 ± 6.1 vs. 7.3 ± 4.0 p < 0.05), nocturnal mean blood pressure variability (9.1 ± 4.9 mmHg vs. 6.8 ± 3.5 mmHg) were detected. The findings of the study point to increased nocturnal systolic and diastolic arterial blood pressure and blood pressure variability as risk factors of cardiovascular complications in patients with severe OSA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Apnea/hypopnea index
- Arterial blood pressure
- Arterial oxygen saturation
- Hypertension
- Obstructive sleep apnea
- Cardiovascular risk factor
- Sleep disordered breathing
1 Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by a collapse of upper airways in the setting of continued respiratory effort, leading to cessation of airflow, large swings in intrathoracic pressure, and arterial oxygen desaturation, often terminated by an arousal.
OSA has been independently associated with cardiovascular diseases, such as hypertension (Peppard et al. 2000), stroke (Munoz et al. 2006), myocardial ischemia (Peled et al. 1999), and arrhythmias (Mehra et al. 2006), which all increases risk for sudden cardiac death. OSA and hypertension, in turn, have common risk factors such as age, obesity, and sedentary lifestyle. OSA syndrome is found in about 50 % of patients with hypertension (Pedrosa et al. 2011) and in 70 % of patients with resistant hypertension (Florczak et al. 2013). There are some data indicating that OSA is associated with hypertension independent of age and obesity (Pankow et al. 1997). The pathogenesis of systemic arterial hypertension in the course of OSA is complex and not fully explained. The most important factor seems hypoxia observed during or immediately after apneic or hypopneic incidents underlying OSA. Sympathetic excitation, expressed by increased catecholamine level and chemoreceptor alterations, caused by intermittent hypoxia has been suggested as a hypertension promoting factor (Fung et al. 2014; Freet et al. 2013). Other pathogenic factors leading to hypertension in OSA patients include systemic inflammation and endothelial dysfunction (Chen et al. 2015).
Hypertension is the most common risk factor for cardiovascular disease and the single most important risk factor for stroke (Roger et al. 2012). Traditionally, cardiovascular risk in hypertension has been attributed to the mean blood pressure load (Taylor et al. 2015). However, inherent variability of an individual’s blood pressure may also be contributory (Rothwell 2010). Systemic arterial blood pressure undergoes marked variations during day and night (Mancia 2012). Blood pressure variability (BPV) is influenced by multiple factors, including neural dysregulation due to age, diabetes, or neuropathies, vascular, humoral, and central nervous system disorders, and mental and environmental stress (Kai et al. 2014). Association between BPV and organ damage (Matsui et al. 2011), cardiovascular events (Johansson et al. 2012), stroke (Shimbo et al. 2012), and mortality (Muntner et al. 2011) have been described. However, some other studies have failed to substantiate such associations or found the BPV of lesser importance than the actual level of blood pressure (Schutte et al. 2012).
Taking into account that OSA is accompanied by autonomic cardiovascular dysregulation which may be related to the frequency of obstructive apneic and hypopneic episodes and to increased sympathetic excitation, we hypothesized that BPV could be associated with the severity of OSA. In the present study we addresses this issue by examining BPV in the patients with mild-to-moderate and severe OSA during a 24-h period.
2 Methods
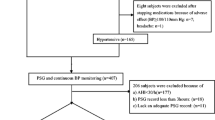
The study was approved by the Bioethics Committee of the Medical University in Wroclaw, Poland and was conducted according to the principles set in the Declaration of Helsinki for Human Research. Fifty eight patients, with newly diagnosed OSA syndrome, of the mean age of 54.3 ± 10.3 years and the mean body mass index (BMI) of 37.8 ± 6.7 kg/m2 were enrolled into the study. Thirty one (53 %) of the patients were on antihypertensive treatment. Based on the severity of OSA, patients were categorized into two groups: severe OSA with AHI ≥30/h (35 men, 5 women) and mild-to-moderate OSA with AHI between 5 and 30/h (4 men, 14 women). All patients underwent in-hospital, nocturnal polygraphic examination at the Department of Pulmonology and Lung Cancer of Medical University in Wroclaw. The following parameters were recorded during sleep: thoracic and abdominal respiratory movements, oro-nasal airflow, and arterial oxygen saturation (SaO2) with finger pulsoximetry. Abnormal respiratory events: apneas, hypopneas, and episodes of desaturations were evaluated according to the standard criteria of the American Academy of Sleep Medicine Task Force (Berry et al. 2012). The following parameters were calculated: apnea/hypopnea index (AHI) – mean number of apneic and hypopneic episodes per hour of sleep, oxygen desaturation index (ODI) – mean number of arterial oxygen desaturations per hour of sleep, and the mean of the minimal values of SaO2 at the end of apneic and hypopneic episodes.
During the day following the polygraphic examination, arterial blood (BP) pressure was monitored noninvasively, using the oscillometric method, along with pulse rate. Readings were obtained every 30 min during diurnal (6:00 a.m. to 10:00 p.m.) and every 60 min during nocturnal (10:00 p.m. to 6:00 a.m.). The BP data were calculated as means of total systolic (TSBP) and total diastolic blood pressure (TDBP) collected over the 24-h period, and separately as diurnal mean BP (DMBP), diurnal systolic (DSBP), diurnal diastolic BP (DDBP) and nocturnal mean BP (NMBP), nocturnal systolic (NSBP), nocturnal diastolic BP (NDBP). Based on the mean standard deviations (SD) of the data above listed, BP variability was calculated: total systolic BP variability (TSBPV), total diastolic BP variability (TDBPV), diurnal systolic BP variability (DSBPV), diurnal diastolic BP variability (DDBPV), nocturnal systolic BP variability (NSBPV), nocturnal diastolic BP variability (NDBPV), total mean BP variability (TMBPV), diurnal mean BP variability (DMBPV), and nocturnal mean BP variability (NMBPV). The thresholds for increases in blood pressure were taken as those set by the European Society of Hypertension/European Society of Cardiology 2013 criteria (Kjeldsen et al. 2013). Nocturnal BP changes were classified as follows: deep fall if a drop was greater than 10 % of the diurnal baseline, mild fall if a drop was between 0 and 10 % off the diurnal baseline level, and a rise if BP went over the diurnal baseline.
Data were presented as means ± SD. Distribution of variables was tested with the Shapiro-Wilk test. Inter-group data with normal distribution were statistically compared with a t-test, and those with skewed distribution were compared with the Mann-Whitney U test. Relationship between variables was estimated with the Spearman correlation coefficient. Significance of differences was considered at p < 0.05. Statistical analysis was performed using Statistica 6.0 software (StatSoft, Tulsa, OK).
3 Results
There were no significant differences in the anthropometric characteristics of patients with severe and milder OSA. The main OSA characteristics such as AHI, ODI, and SaO2 dips during breathless episodes were clearly intensified in the group of patients with severe OSA compared with those in milder OSA. However, due to a large scatter of individual data, the man values did not differ statistically between the two groups (Table 1).
The mean values of TSBP, TDBP, DSBP, and DDBP were similar in both groups of OSA patients. However, NSBP and NDBP were significantly higher in the severe OSA than those in the milder OSA patients (Table 2). The mean nocturnal falls in systolic and diastolic BP were between 0 and 10 % off the diurnal baseline levels in the severe OSA. These BP falls were greater than 10 % in the milder OSA.
Blood pressure variability, assessed from the magnitude of the standard deviation of the mean value, was significantly greater for the mean, systolic, and diastolic blood pressure during nighttime in the severe OSA than those in the milder OSA patients (Table 3). Positive linear correlations were found between AHI, on the one side, and NSBPV (r = 0.57, p < 0.05), TDBPV (r = 0.58, p < 0.05), and DDBPV (r = 0.70, p < 0.05), on the other side.
4 Discussion
The major finding of the present study is that nocturnal blood pressure variability was significantly greater in patients suffering from severe OSA, with the AHI over 29 episodes per hour of sleep, than that in milder forms of OSA. The potentially confounding factors such as age, weight, or BMI were similar in both groups of patients and thus may be excluded as the underlying reason of blood pressure variability, acting via increased sympathetic drive (Charkoudian and Rabbits 2009). There are a number of methods to assess blood pressure variability, such as based on the coefficient of variation, weighted standard deviation (mean of diurnal and nocturnal standard deviation values of blood pressure measurements weighted for the number of hours covered by these two periods during ambulatory monitoring), average real variability, or the difference between maximum and minimum blood pressure levels. In the present study we assessed blood pressure variability on the basis of the standard deviation of the mean values of the amplitude of blood pressure measured. This method is regarded as a good index of apnea-related blood pressure elevations (Planès et al. 2002). Our finding of increased nocturnal blood pressure variability in severe OSA is, generally, in line with that of Steinhorst et al. (2014), although those authors investigated clearly hypertensive OSA patients. Planès et al. (2002) have also shown that systemic hypertension is associated with increased short-term blood pressure variability during sleep in OSA patients.
The present study also unraveled some distortions in the day profile of arterial blood pressure in severe OSA consisting of the lack of a physiological decrease in blood pressure at night. Four categories of nocturnal blood pressure changes are considered: extreme dippers (a fall in blood pressure of more than 20 % compared with diurnal level), dippers (a fall greater than 10 % but less than 20 %), non-dippers (a fall less than 10 %), and reverse dippers, i.e., risers (blood pressure increases at night). Non-dipping pattern in the 24-h blood pressure monitoring has been largely described in patients with OSA syndrome (Loredo et al. 2001; Suzuki et al. 1996); the finding attributable to autonomic dysfunction. In addition, we also found that nocturnal systolic and diastolic blood pressure were higher in patients with severe OSA (AHI ≥30 episodes per hour) compared with those present in milder forms of OSA. Changes in the circadian rhythm of blood pressure have been described by Noda et al. (1993) who show that the severity of OSA has an impact on nocturnal blood pressure elevation. Lavie et al. (1993) have also shown that blood pressure during sleep significantly correlates with the apnea/hypopnea index.
The observed changes in blood pressure variability, impaired 24-h blood pressure profile and a greater nocturnal blood pressure, in patients with severe OSA give rise to cardiovascular complications. The lack of nocturnal dipping in blood pressure has been related to more pronounced target organ damage (Verdecchia et al. 1993) and increased risk of cardiovascular events (Parati and Valentini 2006). Moreover, findings of the International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO) have revealed that the night-to-day blood pressure ratio predicts both cardiovascular and non-cardiovascular mortality (Boggia et al. 2007). Sympathetic neural activity is increased in both OSA, even during the awake state, and hypertension. Sympathetic activation in patients with hypertension is associated with increases in cardiovascular risk and in end-organ damage (Mancia et al. 1999). The present study has some practical applications. The measurement of blood pressure performed once daily, or even several times a day, is clearly insufficient to determine the presence of nocturnal hypertension, the effectiveness of antihypertensive treatment, and the cardiovascular risk in OSA patients. In addition, assessment of blood pressure variability, based on the 24-h monitoring, enables to determine cardiovascular risk independently of the absolute values of blood pressure. The findings of the study indicate that in patients with severe OSA there are two important risk factors of cardiovascular complications occurring during sleep, i.e., increased nocturnal systolic and diastolic blood pressure as well as increased nocturnal blood pressure variability.
References
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep Medicine (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8:597–619
Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O’Brien E, Staessen JA, International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes (IDACO) investigators (2007) Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 370:1219–1229
Charkoudian N, Rabbits JA (2009) Sympathetic neural mechanism in human cardiovascular health and disease. Mayo Clin Proc 84:822–830
Chen HL, Lu CH, Lin HC, Chen PC, Chou KH, Lin WM, Tsai NW, Su YJ, Friedman M, Lin CP, Lin WC (2015) White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 38:361–370
Florczak E, Prejbisz A, Szwench-Pietrasz E, Sliwiński P, Bieleń P, Klisiewicz A, Michałowska I, Warchoł E, Januszewicz M, Kała M, Witkowski A, Więcek A, Narkiewicz K, Somers VK, Januszewicz A (2013) Clinical characteristics of patients with resistant hypertension: the RESIST-POL study. J Hum Hypertens 27:678–685
Freet CS, Stoner JF, Tang X (2013) Baroreflex and chemoreflex controls of sympathetic activity following intermittent hypoxia. Auton Neurosci 174:8–14
Fung ML, Tipoe GL, Leung PS (2014) Mechanisms of maladaptive responses of peripheral chemoreceptors to intermittent hypoxia in sleep-disordered breathing. Sheng Li Xue Bao 66:23–29
Johansson JK, Niiranen TJ, Puukka PJ, Jula AM (2012) Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home study. Hypertension 59:212–218
Kai H, Kudo H, Takayama N, Yasuoka S, Aoki Y, Imaizumi T (2014) Molecular mechanism of aggravation of hypertensive organ damages by short-term blood pressure variability. Curr Hyper Rev 10:125–133
Kjeldsen SE, Narkiewicz K, Oparil S, Hedner T (2013) European Society of Hypertension/European Society of Cardiology Hypertension guidelines. Blood Press 22:191–192
Lavie P, Yoffe N, Berger I, Peled R (1993) The relationship between the severity of sleep apnea syndrome and 24-h blood pressure values in patients with obstructive sleep apnea. Chest 103:1717–7721
Loredo JS, Ancoli-Israel S, Dimsdale JE (2001) Sleep quality and blood pressure dipping in obstructive sleep apnea. Am J Hypertens 14:887–892
Mancia G (2012) Short- and long-term blood pressure variability: present and future. Hypertension 60:512–517
Mancia G, Grassi G, Giannattasio C, Seravalle G (1999) Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 34:724–728
Matsui Y, Ishikawa J, Eguchi K, Shibasaki S, Shimada K, Kario K (2011) Maximum value of home blood pressure. A novel indicator of target organ damage in hypertension. Hypertension 57:1087–1093
Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S, Sleep Heart Health Study (2006) Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 173:910–916
Munoz R, Duran-Cantolla J, Martínez-Vila E, Gallego J, Rubio R, Aizpuru F, De La Torre G (2006) Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke 37:2317–2321
Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S (2011) The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: Findings from NHANES III, 1988 to 1994. Hypertension 57:160–166
Noda A, Okada T, Hayashi H, Yasuma F, Yokota M (1993) 24-hour ambulatory blood pressure variability in obstructive sleep apnea syndrome. Chest 103:1343–1347
Pankow W, Nabe B, Lies A, Becker H, Köhler U, Kohl FV, Lohmann FW (1997) Influence of sleep apnea on 24-hour blood pressure. Chest 112:1253–1258
Parati G, Valentini M (2006) Prognostic relevance of blood pressure variability. Hypertension 47:137–138
Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G (2011) Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 58:811–817
Peled N, Abinader EG, Pillar G, Sharif D, Lavie P (1999) Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol 34:1744–1749
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–8410
Planès C, Leroy M, Fayet G, Aegerter P, Foucher A, Raffestin B (2002) Exacerbation of sleep-apnoea related nocturnal blood-pressure fluctuations in hypertensive subjects. Eur Respir J 20:151–157
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, Committee AHAS, Stroke Statistics Subcommittee (2012) Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125:2–220
Rothwell PM (2010) Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 375:938–948
Schutte R, Thijs L, Liu YP, Asayama K, Jin Y, Odili A, Gu YM, Kuznetsova T, Jacobs L, Staessen JA (2012) Within-subject blood pressure level – not variability – predicts fatal and nonfatal outcomes in a general population. Hypertension 60:1138–1147
Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil-Smoller S (2012) Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the women’s health initiative. Hypertension 60:625–630
Steinhorst AP, Gonçalves SC, Oliveira AT, Massierer D, Gus M, Fuchs SC, Moreira LB, Martinez D, Fuchs FD (2014) Influence of sleep apnea severity on blood pressure variability of patients with hypertension. Sleep Breath 8:397–401
Suzuki M, Guilleminault C, Otsuka K, Shiomi T (1996) Blood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patients. Sleep 19:382–387
Taylor KS, Heneghan CJ, Stevens RJ, Adams EC, Nunan D, Ward A (2015) Heterogeneity of prognostic studies of 24-hour blood pressure variability: systematic review and meta-analysis. PLoS One 10(5):e0126375. doi:10.1371/journal.pone.0126375
Verdecchia P, Schillaci G, Gatteschi C, Zampi I, Battistelli M, Bartoccini C, Porcellati C (1993) Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation 88:986–992
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Martynowicz, H., Porębska, I., Poręba, R., Mazur, G., Brzecka, A. (2016). Nocturnal Blood Pressure Variability in Patients with Obstructive Sleep Apnea Syndrome. In: Pokorski, M. (eds) Advancements in Clinical Research. Advances in Experimental Medicine and Biology(), vol 952. Springer, Cham. https://doi.org/10.1007/5584_2016_64
Download citation
DOI: https://doi.org/10.1007/5584_2016_64
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48032-9
Online ISBN: 978-3-319-48033-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)