Abstract
Obstructive sleep apnea (OSA) has many serious consequences, and one of these may be the exacerbation of type 2 diabetes mellitus (T2DM). Reports on the effect of continuous positive airway pressure (CPAP) on glucose metabolism in people with T2DM and OSA are conflicting. Therefore, the purpose of this review was to examine the effect of CPAP treatment on glucose metabolism by synthesizing findings from randomized controlled trials. The PRISMA review protocol was developed and registered in PROSPERO. A systematic search of PubMed, CINAHL, Embase, Web of Science, PsycInfo, and Cochrane was conducted from inception to March 2017. The Cochrane risk of bias tool was used to assess the study quality. Review Manager (v5.2) was used for the meta-analyses, and the standardized mean difference was calculated. Six studies consisting of 496 participants were included in this review. The meta-analyses indicated that CPAP treatment did not have significant impact on glucose metabolism measured by A1C (mean difference = 0.05, 95% CI − 0.14 to 0.24, P = 0.61), fasting insulin level (mean difference = − 2.34, 95% CI − 8.19 to 3.51, P = 0.43), and fasting glucose (mean difference = − 0.05, 95% CI − 0.52 to 0.42, P = 0.84). As expected, CPAP treatment can improve daytime sleepiness (mean difference = − 2.68, 95% CI − 3.91 to − 1.54, P < 0.001). Findings of this meta-analysis do not substantiate a positive effect of CPAP on glucose metabolism in people with T2DM and coexisting OSA. Future large-scale clinical trials with a longer treatment duration and better CPAP compliance are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, 415 million people had diabetes in 2015, which is projected to be 642 million by 2040 [1]. Over 90% of all cases are type 2 diabetes mellitus (T2DM). Every 6 s, one person dies from diabetes, resulting in 5.0 million deaths in 2015 [1]. Around 12% of global health expenditure was spent on diabetes; the estimated direct costs for individuals with diabetes was estimated to be 2.3 times higher compared to their non-diabetic counterparts [2]. Multiple physiological and behavioral factors are considered responsible for the drastic increase in diabetes prevalence, among which obstructive sleep apnea (OSA) is shown to be an independent risk factor [3,4,5]. OSA is characterized by repetitive upper airway obstruction that results in a cessation or significant reduction in airflow during sleep, which causes intermittent hypoxia and sleep fragmentation [6]. With the rise of obesity and aging, the prevalence of OSA is expected to increase [7]. In the USA alone, 12% of the adult population is estimated to have OSA [8], with estimates rising to as high as 71% in people with T2DM [9]. OSA brings many serious health consequences, and one of these may be the exacerbation of T2DM.
OSA can be treated with various methods including oral appliances, surgery, and continuous positive airway pressure (CPAP). The most common treatment choice is CPAP, which helps to keep the airway open by pressurizing the air in the upper airway [10]. CPAP treatment is effective in reducing daytime sleepiness [11], but its effect on glucose metabolism (e.g., A1C, insulin sensitivity, and fasting glucose) is unknown. Theoretically, intermittent hypoxia and sleep fragmentation caused by OSA could influence the metabolism and contribute to the development of T2DM by activating the sympathetic nervous system, systemic inflammation, hypothalamic-pituitary-adrenal axis, and appetite-regulating hormone alterations [12, 13]. Empirical evidence on the effect of CPAP treatment on glucose metabolism is growing. Four reviews have included studies conducted in people with OSA [14,15,16,17], with inconsistent findings. One review concluded that CPAP could improve insulin sensitivity in non-diabetic and pre-diabetic patients [17]. In contrast, another report did not find a significant improvement in insulin sensitivity after CPAP treatment [16]. In people with T2DM and coexisting OSA, two previous meta-analyses found that CPAP treatment was effective in improving insulin sensitivity, with no effect on A1C [18, 19]. However, both reviews included the same studies, most of which were non-randomized.
Since the publication of previous reviews, more research has been published, particularly clinical trials. There is a need to review the emerging best evidence and provide an up-to-date examination of the effectiveness of CPAP treatment on glucose metabolism. Therefore, the objective of this systematic review and meta-analysis was to examine the effect of CPAP treatment on glucose metabolism including A1C, insulin sensitivity, fasting glucose, and mean glucose level. We synthesized findings from existing randomized controlled trials (RCTs) conducted in adults with T2DM and coexisting OSA.
Methods
We developed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol [20] and registered it in the international prospective register of systematic reviews (PROSPERO) (registration number: 42017059085). We followed the PRISMA guideline in developing this review [21].
Search strategy
A systematic search was conducted in PubMed, CINAHL, Embase, Web of Science, PsycInfo for dissertation/thesis, and Cochrane from inception to March 2017. ClinicalTrial.gov was searched for potential completed trials. A review of the reference lists from relevant studies was conducted to identify additional studies. There was no language restriction. Combinations of the following search terms were used: (1) sleep apnea, sleep apnoea, sleep disordered breathing, OSA, or SDB; (2) diabetes; and (3) CPAP or continuous positive airway pressure. The inclusion criteria were (1) RCT; (2) studies conducted in adults (aged 18 years or over) with T2DM and OSA; and (3) studies investigated CPAP treatment. The exclusion criteria were (1) glucose metabolism not measured; (2) review, abstract, editorial, and reply; or (3) secondary analysis.
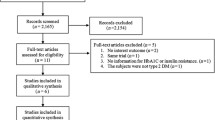
The PRISMA flow chart [21] was used to guide the selection of studies. Initial screening was conducted by one reviewer (BZ) based on the title/abstract. Full-text of potential studies was independently reviewed by two reviewers (BZ and CM) to determine the final inclusion based on the above inclusion and exclusion criteria. Disagreements were resolved by a third reviewer.
Data extraction
A standard matrix was developed by the team to extract information from each study. The extracted data included study characteristics (e.g., sample size, intervention, and outcomes) and participant characteristics (e.g., age, gender, and diabetes duration). Data were independently extracted by two reviewers (BZ and CM). Discrepancies were resolved by a third reviewer. If the data we needed could not be extracted directly from the text, we computed them using other available data. If the data were not reported in an original article, we attempted to contact the authors.
Outcome measures
The primary outcome was the change in the A1C level before and after treatment. A1C is an indicator of the overall glucose for the past 2 to 3 months and has been widely used as the “gold standard” for glycemic control [22]. Secondary outcomes included fasting glucose, mean glucose level, and insulin sensitivity measured by fasting insulin level or homeostasis model assessment (HOMA) index, such as HOMA-IR (insulin resistance). The HOMA index is a method assessing insulin sensitivity from fasting glucose and insulin [23]; it has been widely used as a robust, standard tool in diabetes research [24]. Body mass index (BMI) and daytime sleepiness measured by Epworth Sleepiness Scale (ESS) were also included as secondary outcomes. When the outcomes at different time points were reported, data at the later point were used.
Quality appraisal
The Cochrane risk of bias tool [25] was used to independently assess study quality by two reviewers (BZ and CS). Each study was evaluated from six aspects: random sequence generation, allocation concealment, blinding of the participant, blinding of outcome measures, incomplete data, and selective reporting. The discrepancy was resolved by a third reviewer.
Data analysis
Review Manager (v5.2 for Windows, Cochrane Collaboration, Oxford, U.K.) was used for statistical analyses. Statistical significance was set at P < 0.05. For outcomes reported in two or more studies, pooled mean differences with the 95% CI were calculated for each outcome, using the inverse variance method. Forest plots were used to present the results of individual studies and the pooled effect size. The funnel plot for the primary outcome (i.e., A1C) was used to examine publication bias, and asymmetry of the plot suggests publication bias. Heterogeneity among studies was examined by Cochrane Q test and I 2 value (I 2 > 50% considered significant) [26]. A fixed-effects model was used if no heterogeneity was detected, and a random-effects model was used otherwise [27]. In the case of heterogeneity, we performed sensitivity analyses to test the robustness of the pooled estimates, using leave-one-out approach.
Results
Searching results
The initial literature search yielded 2930 relevant records. A total of 23 underwent the full-text review, and 17 were excluded based on reasons listed in Fig. 1. Six RCTs [28,29,30,31,32,33] met the inclusion and exclusion criteria and thus were included in this review. No eligible studies were identified through other sources. The searching process is shown in Fig. 1.
Study characteristics
The six studies had a total of 496 participants, with the individual sample size between 19 and 298. Participants had a mean age between 55.0 and 62.4 years. The studies included a higher proportion of men, ranging from 53.8 to 100%. The intervention group received CPAP treatment, and the control group received usual care or sham-CPAP. The intervention duration ranged from 7 days to 6 months. Study characteristics are summarized in Table 1.
The risk of bias of each study is presented in Table 2. The studies typically had a low risk of selection bias and reporting bias. Three studies [30, 32, 33] used placebo or sham CPAP, and thus had a low risk of performance bias. The risk of detection bias was low in most of the studies, except in two [30, 32] where it was not clear. The risk of attrition bias was mostly low, with intention-to-treat analysis used.
Effect of CPAP treatment on glucose metabolism
Effect of CPAP treatment on A1C
Four studies [28, 29, 31, 32] were included in the meta-analysis of changes in A1C after CPAP treatment (Fig. 2a). The fixed-effects model was used, because no significant heterogeneity was detected (χ 2 = 1.33, P = 0.72). The pooled estimates of mean difference suggested no significant difference in A1C level between the CPAP and control group (mean difference = 0.05, 95% CI − 0.14 to 0.24, P = 0.61).
Effect of CPAP treatment on insulin sensitivity
Two studies [29, 32] were included in the meta-analysis of changes in fasting insulin level after CPAP treatment (Fig. 2b). Significant heterogeneity was detected (χ 2 = 4.39, P = 0.04). Therefore, the random-effects model was used. The pooled estimates of the mean difference suggested no significant difference in fasting insulin level between the CPAP and control group (mean difference = − 2.34, 95% CI − 8.19 to 3.51, P = 0.43). HOMA index was also used in those two studies. However, the use of different matrixes precluded us from getting a pooled result. HOMA-IR did not improve after 3-month CPAP treatment (P = 0.092) but had a significant improvement after 6-month intervention (intergroup adjusted difference = − 2.58, 95% CI − 4.75 to − 0.41, P = 0.023) [29]. In contrast, HOMA-%S did not change significantly after 3-month treatment (P = 0.2) [32].
Effect of CPAP treatment on fasting glucose
Four studies [28, 29, 31, 32] were included in the meta-analysis of changes in fasting glucose after CPAP treatment (Fig. 2c). The fixed-effects model was used, because no significant heterogeneity was detected (χ 2 = 0.84, P = 0.84). The pooled estimates of the mean difference suggested no significant difference in fasting glucose level between the CPAP and control group (mean difference = − 0.05, 95% CI − 0.52 to 0.42, P = 0.84).
Effect of CPAP treatment on mean glucose level
Two studies [30, 33] examined the effect of CPAP treatment on mean glucose level. Nevertheless, the use of mean glucose over different time spans (i.e., 24 h and 6 am–6 pm) precluded us from getting a pooled estimation. Mokhlesi et al. [30] found a positive effect of CPAP treatment on 24-h mean glucose. There was a 13.7 mg/dl decrease in the mean 24-h glucose in the CPAP treatment group as compared with the 2.9 mg/dl decrease in the control group (P = 0.01). In contrast, Morariu et al. [33] did not find a significant change in mean glucose from 6 am to 6 pm (P = 0.7).
Effect of CPAP treatment on other parameters
As predicted, there was a significant decrease in ESS score in the CPAP group compared to the control group (Fig. 3a): mean difference = − 2.68, 95% CI − 3.91 to − 1.45, P < 0.001. The effect of CPAP treatment on BMI was not significant: mean difference = 0.11, 95% CI − 0.49 to 0.71, P = 0.72 (Fig. 3b).
Sensitivity analysis and publication bias
Sensitivity analyses suggested the results were robust. A funnel plot was performed for the primary outcome (i.e., A1C). The plot indicated no publication bias (Fig. 4).
Discussion
The aim of this meta-analysis was to examine the effect of CPAP treatment on glucose metabolism in T2DM adults with OSA. We synthesized finding from six RCTs. Consistent with previous reviews, we found that CPAP treatment significantly reduced daytime sleepiness [11, 34]. Nevertheless, we did not find a significant effect of CPAP treatment on glucose metabolism.
In this review, we found that CPAP treatment did not affect A1C level. This finding is in line with previous systematic reviews [16, 18, 19]. Specifically, Feng et al. [18] and Chen et al. [19] found no significant difference in A1C before and after the CPAP treatment in people with T2DM and OSA. Likewise, in people with OSA only, Hecht et al. [16] found no improvement of A1C after CPAP treatment (P = 0.94). Taken together, we believe that current evidence does not support the beneficial effect of CPAP treatment on A1C. Nevertheless, the findings of this meta-analysis need to be interpreted carefully. Consensus has not been reached on which measures are most indicative of OSA-related changes in glucose metabolism. The use of a crude (i.e., A1C) measure of glucose metabolism does not fully capture the problems in glucose disposal [12]. Additionally, variability in the duration and adherence to CPAP treatment might also explain the inconsistent findings. A1C is a measure of overall glucose during the past 2–3 months, which takes time to change. In this review, the duration of CPAP treatment ranged from 1 week to 6 months. It has been suggested that a longer intervention is required for the structural changes caused by β-cell damage to be corrected [13]. The commonly used 3-month intervention might not be long enough to detect the changes in A1C [28]. More importantly, adherence to the CPAP treatment could largely influence the effect of CPAP treatment. In the three studies [28, 31, 32] that did not find a significant effect of CPAP on A1C, the usage of CPAP treatment per night was between 2.5 and 4.3 h, compared to 5.2 h in the study conducted by Martinez-Ceron et al. [29] where a significant decrease in A1C was observed. Additionally, the severity of OSA likely influences the effect of CPAP treatment on A1C. Tamura et al. [35] examined the relationship between the OSA severity and A1C. In that study, OSA-induced hypoxia was independently associated with A1C regardless of the glucose tolerance, and the association was stronger in those with diabetes. Similarly, in patients with T2DM, more severe OSA was associated with a higher A1C level, independent of confounders [36]. The adjusted A1C increased by 1.93% in those with moderate OSA and 3.60% in those with severe OSA. In this review, participants’ mean AHI ranged from 28.0 to 45.3 events/h. People with different OSA severity might show a different metabolic response to the CPAP, which could explain the negative finding.
We did not find an improvement in insulin sensitivity after CPAP treatment, which is inconsistent with two review findings. Feng et al. [18] synthesized findings from one RCT and two non-RCTs conducted in patients with T2DM and OSA. They found a significant improvement of insulin sensitivity, evaluated by euglycaemic hyperinsulinaemic clamp (combined difference = 0.330, 95% CI 0.001 to 0.658, P = 0.049). However, the wide CI indicates instability of the findings. Similar to the study of Feng et al., Chen et al. [19] included the same two non-RCTs and found that CPAP treatment significantly increased insulin sensitivity (combined difference = 0.522 μmmol/kg/min, SE = 0.196, P = 0.005). The inconsistency between our findings and those two reviews could be explained by study design and variances in the measurement of insulin sensitivity. In our analysis, we used fasting insulin level as opposed to the clamp. Although the use of fasting insulin level is the most practical method, it might result in a high proportion of false-positive results [37]. Participant characteristic, such as OSA severity, likely affect the effect of CPAP on insulin sensitivity. Patients with more severe OSA [38] might benefit more from the treatment. Participants in the two previous reviews [18, 19] all had moderated-to-severe OSA, while participants in the present review had newly diagnosed OSA with various disease severities.
Three previous reviews examined the effect of CPAP on insulin sensitivity in non-diabetic patients with OSA. Our finding is in line with the study of Hecht et al. [16] where no significant effect of CPAP on HOMA-IR was found. In contrast, the other two reviews suggested a favorable effect [14, 15]. Disease chronicity is a well-established key factor that can affect the response to treatment. Development of T2DM is a progressive process characterized by initial insulin resistance, compensatory hyperinsulinemia, and failure of pancreatic β-cells (i.e., impaired insulin secretion) [39]. Response to CPAP treatment on insulin sensitivity could differ for patients with various diabetes severity and duration. People with poorer glycemic control might benefit more from the treatment due to a larger potential for improvement [12]. In this review, participants’ mean baseline A1C ranged from 6.9 to 8.5%. Using a different A1C level as the inclusion criteria might have contributed to the inconsistency. Additionally, β-cell function progressively declines with diabetes duration [40], which likely requires various treatment regimens. In this review, diabetes duration ranged from 2.5 to 8.8 years. The inclusion of people under various treatment regimens (e.g., oral medication and insulin) could also account for the inconsistency. Moreover, even if CPAP treatment could improve insulin sensitivity in pre-diabetes or non-diabetes, it is also plausible that the same effect would not occur in established T2DM.
Consistent with previous findings, CPAP treatment did not have a significant effect on fasting glucose. Specifically, Yang et al. [14] found that 3 to 24 weeks of CPAP treatment did not improve the fasting glucose in non-diabetic patients compared to pre-CPAP. During CPAP treatment, an increase in growth hormone was observed [41, 42], and growth hormone has long been considered diabetogenic [43]. That might counter the effect of CPAP on the glucose, which helps to explain the negative findings of this review. Additionally, the sample size of included RCTs was typically determined by the primary outcome (i.e., A1C), which might not be large enough to capture the change in fasting glucose. Poor CPAP compliance could also account for the negative findings. That was further supported by the study of Mokhlesi et al. [30] where participants went through a 7-day in-lab CPAP treatment and had an average of 7.92 h CPAP usage. A significant decrease in mean 24-h glucose was observed in the CPAP group compared to the control (P = 0.01).
The role obesity plays in the relationship between OSA and glucose metabolism has been inconsistent. Some studies suggested the confounding role of obesity [44, 45], while others suggested that OSA was related to glucose metabolism independent of obesity [3, 46]. BMI has been, traditionally, considered an indicator of obesity. Nevertheless, controlling for BMI is not sufficient when evaluating the effect of CPAP treatment on glucose metabolism [12]. Consistent with previous findings, we did not find a significant effect of CPAP treatment on BMI in this review [14, 18].
To the best of our knowledge, this systematic review is the first that included only RCTs conducted in adults with T2DM and coexisting OSA. This meta-analysis clarifies our understanding about the causal relationship between T2DM and OSA. However, findings from this review need to be interpreted in light of the limitations. First, although we did an exhaustive literature search, the number of studies included in this review remains small, which suggests that more research in this area is needed. Second, insulin sensitivity was not measured using the hyperinsulinemic-euglycemic clamp. The clamp procedure has been considered the “gold standard” [23], but its use is limited in clinical trials due to high cost and complex operation. In this review, limiting the measure of insulin sensitivity to the clamp would result in no eligible RCTs that can be used for the meta-analysis. Third, confounding factors, including medication, eating, and physical activity, were not controlled in this review. CPAP treatment can alleviate OSA-related symptoms (e.g., daytime sleepiness and fatigue), which likely facilitate a healthier lifestyle that is beneficial for glycemic control [12]. Fourth, CPAP usage per night was not high in this review, which might have masked the beneficial effect of CPAP on glucose metabolism.
Findings from this review have important implications for both research and clinical practice. Given the small number of RCTs addressing this issue, more research is warranted. These studies ideally should be large-scale clinical trials with a longer treatment duration and better CPAP compliance. Target population could be those with more severe OSA and poorly controlled diabetes. Factors such as treatment regimen, physical activity, and eating behavior should be included as potential confounders. Although current evidence does not support the beneficial effect of CPAP treatment on glucose metabolism, CPAP is effective in reducing daytime sleepiness, which has a significant impact on daytime functioning. Therefore, timely sustained CPAP treatment of OSA should continue to be encouraged in clinical practice.
Conclusion
In view of the evidence from RCTs, CPAP is effective in alleviating daytime sleepiness. However, current findings do not substantiate a positive effect of CPAP treatment on glucose metabolism (e.g., A1C, insulin sensitivity, fasting glucose, and mean glucose level) in people with T2DM and coexisting OSA.
References
International Diabetes Federation (2015) Diabetes: Facts and figures http://www.idf.org/about-diabetes/facts-figures. Accessed 2 April 2017
Ozieh MN, Bishu KG, Dismuke CE, Egede LE (2015) Trends in health care expenditure in US adults with diabetes: 2002–2011. Diabetes Care 38:1844–1851
Nagayoshi M, Punjabi NM, Selvin E, Pankow JS, Shahar E, Iso H, Folsom AR, Lutsey PL (2016) Obstructive sleep apnea and incident type 2 diabetes. Sleep Med 25:156–161
Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A (2016) Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev 30:11–24
Rajan P, Greenberg H (2015) Obstructive sleep apnea as a risk factor for type 2 diabetes mellitus. Nat Sci Sleep 7:113–125
Flemons WW, Buysse D, Redline S, Oack A, Strohl K, Wheatley J, Young T, Douglas N, Levy P, McNicolas W (1999) Sleep-related breathing disorders in adults. Sleep 22:667–689
Franklin KA, Lindberg E (2015) Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J Thorac Dis 7:1311–1322
American Academy of Sleep Medicine (2016) Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system http://www.aasmnet.org/Resources/pdf/sleep-apnea-economic-crisis.pdf. Accessed 2 April 2017
Pamidi S, Tasali E (2012) Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol 3:126
National Institute of Health (2012) How is sleep apnea treated? https://www.nhlbi.nih.gov/health/health-topics/topics/sleepapnea/treatment. Accessed 2 April 2017
Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, Neill AM, Gander PH (2006) Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax 61:430–434
Aurora RN, Punjabi NM (2013) Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med 1:329–338
Martínez-Ceron E, Fernández-Navarro I, Garcia-Rio F (2016) Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med Rev 25:121–130
Yang D, Liu Z, Yang H, Luo Q (2013) Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath 17:33–38
Iftikhar IH, Khan MF, Das A, Magalang UJ (2013) Meta-analysis: continuous positive airway pressure improves insulin resistance in patients with sleep apnea without diabetes. Ann Am Thorac Soc 10:115–120
Hecht L, Möhler R, Meyer G (2011) Effects of CPAP-respiration on markers of glucose metabolism in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Ger Med Sci 9:1–13
Chen L, Kuang J, Pei J, Chen H, Chen Z, Li Z, Yang H, Fu X, Wang L, Chen Z (2017) Continuous positive airway pressure and diabetes risk in sleep apnea patients: a systemic review and meta-analysis. Eur J Intern Med 39:39–50
Feng Y, Zhang Z, Dong Z (2015) Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med 25:15005
Chen L, Pei J, Chen H (2014) Effects of continuous positive airway pressure treatment on glycaemic control and insulin sensitivity in patients with obstructive sleep apnoea and type 2 diabetes: a meta-analysis. Arch Med Sci 10:637–642
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Lenters-Westra E, Schindhelm RK, Bilo HJ, Slingerland RJ (2013) Haemoglobin A1C: historical overview and current concepts. Diabetes Res Clin Pract 99:75–84
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495
Higgins JPT, Altman DG, Sterne JAC (2011) Assessing risk of bias in included studies. In: Higgins JPT, Green S (eds) Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 edn
Higgins J, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Lam JCM, Lai AYK, Tam TCC, Yuen MMA, Lam KSL, Ip MSM (2016) CPAP therapy for patients with sleep apnea and type 2 diabetes mellitus improves control of blood pressure. Sleep Breath 21:377–386
Martinez-Ceron E, Barquiel B, Bezos AM, Casitas R, Galera R, Garcia-Benito C, Hernanz A, Alonso-Fernandez A, Garcia-Rio F (2016) Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes: a randomized clinical trial. Am J Respir Crit Care Med 194:476–485
Mokhlesi B, Grimaldi D, Beccuti G, Abraham V, Whitmore H, Delebecque F, Van Cauter E (2016) Effect of one week of 8-hour nightly continuous positive airway pressure treatment of obstructive sleep apnea on glycemic control in type 2 diabetes: a proof-of-concept study. Am J Respir Crit Care Med 194:516–519
Shaw JE, Punjabi NM, Naughton MT, Willes L, Bergenstal RM, Cistulli PA, Fulcher GR, Richards GN, Zimmet PZ (2016) The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med 194:486–492
West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR (2007) Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 62:969–974
Morariu EM, Chasens ER, Strollo PJ, Korytkowski M (2017) Effect of continuous positive airway pressure (CPAP) on glycemic control and variability in type 2 diabetes. Sleep Breath 21:145–147
Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, Williamson B, Windler S, McEvoy RD (2011) The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep 34:111–119
Tamura A, Kawano Y, Watanabe T, Kadota J (2012) Obstructive sleep apnea increases hemoglobin A1c levels regardless of glucose tolerance status. Sleep Med 13:1050–1055
Aronsohn RS, Whitmore H, Van Cauter E, Tasali E (2010) Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 181:507–513
Singh B, Saxena A (2010) Surrogate markers of insulin resistance: a review. World J Diabetes 1:36–47
Weinstock TG, Wang X, Rueschman M, Ismail-Beigi F, Aylor J, Babineau DC, Mehra R, Redline S (2012) A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep 35:617–625
Weir GC, Bonner-Weir S (2004) Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53:S16–S21
Saisho Y (2014) Importance of beta cell function for the treatment of type 2 diabetes. J Clin Med 3:923–943
Saini J, Krieger J, Brandenberger G, Wittersheim G, Simon C, Follenius M (1993) Continuous positive airway pressure treatment. Horm Metab Res 25:375–381
Hoyos CM, Killick R, Keenan DM, Baxter RC, Veldhuis JD, Liu PY (2014) Continuous positive airway pressure increases pulsatile growth hormone secretion and circulating insulin-like growth factor-1 in a time-dependent manner in men with obstructive sleep apnea: a randomized sham-controlled study. Sleep 37:733–741
Sperling MA (2016) Traditional and novel aspects of the metabolic actions of growth hormone. Growth Hormon IGF Res 28:69–75
Sánchez-de-la-Torre M, Mediano O, Barceló A, Piérola J, de la Peña M, Esquinas C, Miro A, Durán-Cantolla J, Agustí AG, Capote F (2012) The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath 16:649–656
Tasali E, Mokhlesi B, Van Cauter E (2008) Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133:496–506
Ip MSM, Lam B, Ng MMT, Lam WK, Tsang KWT, Lam KSL (2002) Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 165:670–676
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Zhu, B., Ma, C., Chaiard, J. et al. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 22, 287–295 (2018). https://doi.org/10.1007/s11325-017-1554-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1554-x