Abstract
Purpose of Review
The effect of continuous positive airway pressure (CPAP) on resistant hypertension in patients at high risk with obstructive sleep apnea (OSA) needs further investigation. We aimed to determine the effect of CPAP on blood pressure in patients with resistant hypertension and OSA. Databases including PubMed, EMBASE, MEDLINE, the Cochrane Library, and CMB were searched. Data were pooled using a random-effects or fixed-effects model to derive weighted mean differences (WMDs) and 95% confidence intervals (CIs).
Recent Findings
A total of 12 trials and 718 participants were included. Compared with control, CPAP significantly reduced 24-h systolic blood pressure (SBP) (WMD: − 5.92 mmHg [ − 8.72, − 3.11]; P<0.001), 24-h diastolic blood pressure (DBP) (WMD: − 4.44 mmHg [− 6.26 , − 2.62]; P <0.001), daytime SBP (WMD: − 5.76 mmHg [ − 9.16, − 2.36]; P <0.001), daytime DBP (WMD: − 3.92 mmHg [− 5.55, − 2.30]; nighttime SBP (WMD: − 4.87 mmHg [ − 7.96 , − 1.78]; P = 0.002), and nighttime DBP (WMD: − 2.05 mmHg [− 2.99, − 1.11]; P<0.001) in patients with resistant hypertension and OSA. CPAP improved the blood pressure both in the short (<3 months) and long term (≥ 3 months). No significant impact on mean heart rate was noted (WMD: -2.76 beats per min [− 7.50, 1.97]; P = 0.25).
Summary
CPAP treatment was associated with BP reduction in patients with resistant hypertension and OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resistant hypertension is defined as uncontrolled systolic blood pressure (SBP) despite therapy with more than three antihypertensive agents from at least three different classes including a diuretic [1,2,3]. In most studies, 10 to 15% of hypertensive subjects, and as high as 20% of the hypertensive population in some publications [4], are diagnosed with resistant hypertension, particularly those with advanced age, obesity, diabetes mellitus, sleep apnea, and chronic kidney disease [5,6,7].
Several epidemiological studies have demonstrated the association of obstructive sleep apnea (OSA) and hypertension [6, 7]. OSA is considered an etiologic factor in the development of hypertension and in the evolution of resistant hypertension [8, 9]. Additionally, OSA is independently associated with an increased risk of cardiovascular events, including atrial fibrillation, ischemic heart disease, heart failure, stroke, and sudden cardiac death [10]. Expert panels have recommended consideration of OSA and its treatment as part of the management of patients with resistant hypertension [11,12,13,14,15,16].
Continuous positive airway pressure (CPAP) is an effective way to treat OSA [17], and a recently published randomized controlled trial (RCT) [18] suggested a positive effect of CPAP treatment on 24-h BP in patients with resistant hypertension. Meta-analysis of RCTs [19,20,21, 22•] reported effects of CPAP on blood pressure in patients with resistant hypertension and OSA. However, the follow-uptime and observational studies were not analyzed. It was thought necessary to update the recently published papers. Therefore, the current study aimed to include newly published studies and to perform novel subgroup analyses.
Methods
Search Strategy
We performed a systematic review and meta-analysis in accordance with the standards set forth by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [23, 24]. The protocol was previously registered in November 2022 in the PROSPERO database (Review register: CRD42022379314). We searched PubMed, EMBASE, Web of Science, the Cochrane Library, and CMB using the keywords “continuous positive airway,” “CPAP,” “obstructive sleep apnea,” “blood pressure,” and “hypertension.” The search was restricted to articles published in the English or Chinese language. Titles and abstracts of retrieved citations were reviewed. Full texts of relevant citations were assessed for eligibility for inclusion in the review.
Inclusion Criteria
The inclusion criteria were as follows: (1) CPAP treatment can significantly reduce 24-h SBP and diastolic blood pressure (DBP) in patients with hypertension and OSA and diagnosis of hypertension and OSA using respiratory polygraph or polysomnography that shows apnea-hypopnea index (AHI) more than 5 times/hour (defined as SBP of at least 140 mmHg, DBP of at least 90 mmHg, or both according to the standard diagnosis of systemic hypertension), (2) RCT was used to compare the treatment of OSA with sham CPAP or without CPAP with that of CPAP in patients with hypertension, and (3) follow-up for 3 weeks or more to observe the changes of ambulatory blood pressure or heart rate.
Exclusion Criteria
The exclusion criteria were as follows: (1) serious concomitant diseases with poor functional status (e.g., advanced renal failure, severe liver failure, or New York Heart Association class III-IV heart failure), (2) sleep disorders other than OSA, (3) secondary systemic hypertension, (4) pregnancy or lactation, (5) occupation related to transportation, (6) current CPAP treatment, (7) contraindications to CPAP treatment (such as facial deformity or severe pulmonary disease), (8) severe somnolence requiring treatment (defined by the Epworth somnolence scale > 18), and (9) drug or alcohol abuse. The search strings used in the database are (“Obstructive Sleep Apnea” or “OSA” or “Sleep Apnea”), (“Hypertension” or “high blood pressure”), and (“Continuous Positive Airway Pressure” or “CPAP”).
Data Extraction and Quality Evaluation
Two independent reviewers (LS and YFC) extracted two pieces of data. Differences were resolved by consensus. The following information is extracted: the name of the first author, year of publication, number of participants, SBP and DBP measurements before and after intervention (standard deviation), country of origin, research design, and demographics including mean age, body mass index, gender distribution, presence of comorbidities and AHI, and blood pressure measurement methods or ambulatory blood pressure monitoring (ABPM).
Outcomes
The primary outcome measures were the SBP and DBP reductions following CPAP on 24-h ABPM recordings in patients with OSA and resistant hypertension compared to conventional treatment or pre-CPAP baseline values. The secondary outcome measures were the SBP and DBP reductions following CPAP on daytime and nocturnal recordings in OSA patients with resistant hypertension compared to conventional treatment or pre-CPAP baseline values.
Methodological Quality
To determine the quality of the included studies, we used the Cochrane Collaboration Risk of Bias Tool [25] for the 10 RCTs and the Newcastle-Ottawa Scale [26] for the 2 observational studys (OBs). The quality evaluation of the included studies is shown in Supplementary Tables S1 and S2.
Data Synthesis and Statistical Analysis
Extracted data from the included studies were entered into the Cochrane Collaboration Review Manager 5.3. Weighted mean differences (WMDs) in SBP and DBP following CPAP on 24 h, daytime, and nocturnal ABPM recordings in the CPAP group were compared to those in the non-CPAP group, pooling all included studies. Considering the clinical and statistical heterogeneity among studies, data were combined using a DerSimonian-Laird random-effects model with inverse variance weighting [27]. Estimates were reported as WMDs comparing the CPAP group with the non-CPAP group, with 95% confidence intervals (CIs). Differences were considered significant at a 2-sided P value < 0.05. Heterogeneity was investigated using I2 statistics. Then, we performed 2 sensitivity analyses, (1) assessing WMDs in SBP and DBP changes following CPAP on 24 h daytime and nocturnal ABPM recordings in the CPAP group compared to the non-CPAP group using data restricted to RCTs and (2) removing 1 study at a time and assessing the effect on the WMDs. Publication bias was assessed with the construction of a funnel plot and was further assessed with Egger’s test of the intercept and the Begg and Mazumdar rank correlation test [28]. P values less than 0.05 in these tests were considered statistically significant for the evidence of publication bias.
Results
Study Selection and Characteristics
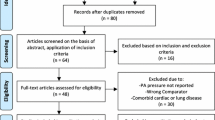
Twelve studies [18, 29,30,31,32,33,34, 35•, 36,37,38,39] with a total of 718 patients were included in the meta-analysis (Fig. 1). Table 1 summarizes the characteristics and the design of all included studies. Of the 12 analyzed studies, 10 were RCTs [18, 29,30,31,32,33,34, 35•, 36, 37] (n = 663) and 2 were OBs [38, 39] (n=55). Ambulatory BP monitors were employed to measure the primary outcome of BP response in 12 studies [18, 29, 32,33,34, 35•, 36,37,38,39], with these measurements being relatively better than office measurements, which were used as the primary outcome measure in the remaining study [31]. We used the ABPM data for 24-h SBP and DBP analyses from the 12 studies in which these data were available [18, 29,30,31,32,33,34, 35•, 36,37,38,39].
Mean Change in 24-h BP
Pooled analysis involving all 12 included studies showed that patients who underwent CPAP (n = 718) experienced reductions in 24-h SBP (WMD − 5.92 mmHg, 95% CI: − 8.72 ~ −3.11, P < 0.001, I2 = 79%) and DBP (WMD − 4.44 mmHg, 95% CI: − 6.26 ~ − 2.62, P < 0.001, I2 = 80%), compared with at the end of the follow-up period. When data were restricted to RCTs (n = 663), there was a significant reduction in 24-h SBP (WMD − 8.48 mmHg, 95% CI: − 13.24 ~ − 3.71, P = 0.0003, I2 = 82%) and DBP (WMD − 4.51 mmHg, 95% CI: − 6.83 ~ − 2.19, P < 0.001, I2 = 65%) at follow-up < 3 months and ≥ 3 months [24-h SBP (WMD − 5.01 mmHg, 95% CI: − 9.36 ~ − 0.67, P = 0.02, I2 = 76%) and DBP (WMD − 3.53 mmHg, 95% CI: − 6.73 ~ − 0.32, P = 0.03, I2 = 83%)]. When data were restricted to OBs (n = 55), included studies showed that patients who underwent CPAP experienced no statistical difference in 24-h SBP (WMD − 2.30 mmHg, 95% CI: − 12.50 ~ 7.89, P = 0.66, I2 = 67%) and 24-h DBP (WMD − 6.43 mmHg, 95% CI: − 17.60 ~ 4.74, P = 0.26, I2 = 93%) compared with the control group at the end of the follow-up period (Figs. 2 and 3).
Mean Change in Daytime BP
Pooled analysis involving all 9 included studies showed that patients who underwent CPAP (n = 621) experienced reductions in daytime SBP (WMD − 5.76 mmHg, 95% CI: − 9.16 ~ − 2.36, P < 0.001, I2 = 79%) and DBP (WMD − 3.92 mmHg, 95% CI: − 5.55 ~ − 2.30, P < 0.001, I2 = 79%) compared with control. When data were restricted to < 3 months (n = 346), there was a significant reduction in daytime SBP (WMD − 8.38 mmHg, 95% CI: − 14.91 ~ − 1.85, P = 0.01, I2 = 86%) and DBP (WMD − 4.00 mmHg, 95% CI: − 6.66 ~ − 1.34, P = 0.003, I2 = 51%). In the same way, when the data were limited to ≥ 3 months (n = 275), SBP (WMD − 4.11 mmHg, 95% CI: − 9.00 ~ 0.78, P = 0.10, I2 = 74%) and DBP (WMD − 3.73 mmHg, 95% CI: − 6.30 ~ − 1.17, P < 0.001, I2 = 64%) also decreased significantly (Figs. 4 and 5).
Mean Change in nighttime BP
Pooled analysis involving all 9 included studies showed that patients who underwent CPAP experienced reductions in nocturnal SBP (WMD − 4.87 mmHg, 95% CI: − 7.96 ~ − 1.78, P = 0.002, I2 = 67%) and DBP (WMD − 2.05 mmHg, 95% CI: − 2.99 ~ − 1.11, P < 0.001, I2 = 18%) compared with control. When data were restricted to < 3 months (n = 346), there was a significant reduction in nocturnal SBP (WMD − 9.38 mmHg, 95% CI: − 17.53 ~ − 1.24, P = 0.02, I2 = 85%) and DBP (WMD − 2.23 mmHg, 95% CI: − 3.38 ~ − 1.08, P < 0.001, I2 = 53%). In the same way, when the data were limited to ≥ 3 months (n = 275), SBP (WMD − 2.57 mmHg, 95% CI: − 4.64 ~ − 0.51, P = 0.01, I2 = 0%) and DBP (WMD − 1.70 mmHg, 95% CI: − 3.31 ~ − 0.09, P = 0.04, I2 = 0%) also decreased significantly (Figs. 6 and 7).
Mean Heart Rate
Pooled analysis involving 3 studies showed that patients who underwent CPAP (n = 244) experienced a statistical difference in mean heart rate (WMD − 2.76 beats per min, 95% CI: − 7.50 ~ 1.97, P = 0.25, I2 = 55%) compared with the control group at the end of the follow-up period (Fig. 8).
Publication Bias
The general characteristics and article quality evaluation of the included literature are shown in Table 1. The quality assessment of RCT studies is shown in Supplementary Table S1. The Newcastle-Ottawa Scale for OBs is shown in Supplementary Table S2. The funnel plot showed visual evidence of publication bias (Supplementary Fig. S1). Funnel plots and Begg’s test identified no strong evidence of publication bias.
Discussion
Hypertension is closely related to OSA, and these conditions share common risk factors such as age, alcohol consumption, obesity, and genetics. OSA is an independent risk factor for hypertension and has an effect on both high and low BP [40]. High BP is associated with changes in the circadian rhythm of blood pressure and heart function and the development of cardiac arrhythmias. In addition, the incidence of resistant hypertension, specifically at night, has been associated with OSA [41].
Haentjens et al.’s study showed that increased sympathetic nerve activity in patients with nocturnal apnea, hypoxemia, and hypercapnia leads to activation of the renin-angiotensin-aldosterone system and elevated levels of endothelin and catecholamines, which have been shown to lead to peripheral vasoconstriction [42]. This meta-analysis demonstrated that treatment using CPAP was associated with BP reduction. Therapeutic CPAP significantly lowered 24-h SBP and DBP, daytime SBP and DBP, and nighttime SBP and DBP.
Among our included studies, the study by Lozano et al. [32] compared 29 patients with resistant hypertension to 35 patients receiving 3 months of CPAP treatment; no significant drop in BP across study groups was observed. CPAP reduced the nocturnal DBP, which was close to the boundary of significance. However, in the subgroup with uncontrolled BP levels, there was a significant reduction in the 24-h DBP and borderline significant reductions in the 24-h SBP and daytime DBP. In the study by Pedrosa et al. [31], 19 resistant hypertensive patients receiving 6-month CPAP treatment were compared to 16 control patients, all with uncontrolled ambulatory BPs. Significant reductions were seen only for daytime BP levels (6.5 mmHg for SBP and 4.5 mmHg for DBP, respectively); the CPAP group did show a significant effect for 24 h or nocturnal BP measurements.
In this study, changes in BPs were not associated with CPAP adherence. In the largest and only multi-center study, Martínez-García et al. [29] randomized 98 resistant hypertensive patients to CPAP treatment for 3 months and 96 patients to the control arm; all patients had uncontrolled ambulatory BPs. Significant decreases were seen in 24-h average BP and 24-h DBP. However, only a critical reduction in 24-h SBP was observed in the intent-to-treat analysis. Our meta-analysis using data from these studies, reporting the difference in the BP-lowering effect of CPAP versus medical therapy, showed that patients treated with CPAP experienced significantly greater reductions in SBP and DBP at the end of the follow-up period. However, when the analysis was restricted to OBs, CPAP treatment had no significant effect on ambulatory BPs in patients with resistant hypertension and moderate/severe OSA.
In response to significant fluctuations in intrathoracic pressure apnea, the cardiovascular system adapts accordingly; however, if the sleep structure is destroyed, non-specific changes in nervous function may develop. Recurrent hypoxemia is also considered one of the important factors in the pathogenesis of resistant hypertension. CPAP represents an OSA-targeted therapy to potentially reverse the pathophysiologic mechanisms responsible for hypertension. CPAP treatment performed for OSA can effectively increase the patient’s functional residual capacity and reduce airway resistance to improve symptoms such as apnea and lack of oxygen, resulting in improved sympathetic responses to autonomic nervous system activity [43]. In addition, CPAP can improve sleep structure and reduce the patient’s nocturnal BP and the occurrence of arrhythmia [44].
Latest International Guidelines state that the BP decline can produce a clinically meaningful effect that significantly reduces the risk of incident cardiovascular and cerebrovascular diseases, such as stroke, coronary heart disease, and heart failure as well as heart failure hospitalization [45]. Our study shows that CPAP treatment can effectively reduce heart rate with hypertensive patients. Increased HR is associated with increased BP, increased risk for the development of hypertension, and cardiovascular and all-cause mortality in hypertensive patients [46]. As HR is a predictor of mortality, reducing HR is essential for prevention [47]. Multiple studies demonstrate a reduction of cardiovascular risk with CPAP use [15, 48].
Limitations
Twelve RCTs were included in this study, and although the results were measured with a blinded method, most of the experimental randomization methods and study blindness details were not clearly reported. In our studies, which included multi-joint antihypertensive drugs, obese patients, and those with unhealthy lifestyles such as drinking and smoking, it is possible that there were confounding factors regarding the effect of CPAP on BP. Additionally, the small sample sizes and short follow-up periods were important limitations in our meta-analysis. Further research and multi-center RCTs involving a larger number of study participants, followed over a longer period of time, are warranted to confirm our findings.
Conclusion
CPAP treatment significantly reduced BP in patients with OSA and resistant hypertension. Future research should increase the number of study participants and extend follow-up time to estimate the impact of CPAP on blood pressure, cardiovascular event incidence, mortality, or other adverse events in patients with OSA and resistant/refractory hypertension.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. American heart association professional education committee. resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the american heart association professional education committee of the council for high blood pressure research. Circulation. 2008;117(25):e510–e526.
Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57(6):1076–80.
Sierra A, Segura J, Banegas JR, Gorostidi M, Cruz JJ, Armario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902.
Roberie DR, Elliott WJ. What is the prevalence of resistant hypertension in the United States? Curr Opin Cardiol. 2012;27(4):386–91.
Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52(22):1749–57.
Sander GE, Giles TD. Resistant hypertension: concepts and approach to management. Curr Hypertens Rep. 2011;13(5):347–55.
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. a scientific statement from the american heart association professional education committee of the council for high blood pressure research. Hypertension. 2008;51(6):1403–1419.
Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84.
Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–7.
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation ScientificStatement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Circulation. 2008;118(10):1080–111.
Baguet JP, Barone-Rochette G, Pepin JL. Hypertension and obstructive sleep apnoea syndrome: current perspectives. J Hum Hypertens. 2009;23(7):431–43.
Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142(3):187–97.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–87.
Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93.
Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53.
Tsioufis C, Kasiakogias A, Thomopoulos C, Manolis A, Stefanadis C. Managing hypertension in obstructive sleep apnea: the interplay of continuous positive airway pressure, medication and chronotherapy. J Hypertens. 2010;28(5):875–82.
Butt M, Dwivedi G, Khair O, Lip GY. Obstructive sleep apnea and cardiovascular disease. Int J Cardiol. 2012;139(1):7–16.
Lloberes P, Sampol G, Espinel E, Segarra A, Ramon MA, Romero O, et al. A randomized controlled study of CPAP effect on plasma aldosterone concentration in patients with resistant hypertension and obstructive sleep apnea. J Hypertens. 2014;32(8):1650–7.
Iftikhar IH, Valentine CW, Bittencourt LRA, Cohen DL, Fedson AC, Gíslason T, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens. 2014;32(12):2341–50.
Liu L, Cao Q, Guo Z, Dai Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2016;18(2):153–8.
Lei Q, Lv Y, Li K, Ma L, Du G, Xiang Y, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. J Bras Pneumol. 2017;43(5):373–9.
• Labarca G, Schmidt A, Dreyse J, Jorquera J, Enos D, Torres G, et al. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): systematic review and meta-analysis. Sleep Med Rev. 2021;58:101446. This article presents that continuous positive airway pressure (CPAP) is no benefit for aortic stiffness, but it did lead to a mild reduction in aldosterone secretion. CPAP therapy improved BP, especially nighttime BP, in this population.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-34.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097.
Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine. 2009;34(18):1929–41.
Wells GA, Shea BJ, O'Connell D, Peterson J, Tugwell P. The Newcastle–Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. 2014.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Martínez-García MA, Capote F, Campos-Rodríguez F, Lloberes P, Díaz de Atauri MJ, Somoza M, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415.
Litvin AY, Sukmarova ZN, Elfimova EM, Aksenova AV, Galitsin PV, Rogoza AN, et al. Effects of CPAP on “vascular” risk factors in patients with obstructive sleep apnea and arterial hypertension. Vasc Health Risk Manag. 2013;9:229–35.
Pedrosa RP, Drager LF, de Paula LK, Amaro AC, Bortolotto LA, Lorenzi-Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144(5):1487–94.
Lozano L, Tovar JL, Sampol G, Romero O, Jurado MJ, Segarra A, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28(10):2161–8.
Muxfeldt ES, Margallo V, Costa LM, Guimarães G, Cavalcante AH, et al. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65(4):736–42.
Joyeux-Faure M, Baguet JP, Barone-Rochette G, Faure P, Sosner P, Mounier-Vehier C, et al. Continuous positive airway pressure reduces night-time blood pressure and heart rate in patients with obstructive sleep apnea and resistant hypertension: the RHOOSAS randomized controlled trial. Front Neurol. 2018;9:318.
• Lui MM, Tse HF, Lam DC, Lau KK, Chan CW, Ip MS. Continuous positive airway pressure improves blood pressure and serum cardiovascular biomarkers in obstructive sleep apnoea and hypertension. Eur Respir J. 2021;58(5):2003687. Findings from this study suggest that in a cohort with OSA and multiple cardiovascular risk factors including difficult-to-control hypertension, short-term CPAP treatment improved ambulatory BP and alleviated subclinical myocardial injury and strain.
Ruzicka M, Knoll G, Leenen FH, Leech J, Aaron SD, Hiremath S. Effects of CPAP on blood pressure and sympathetic activity in patients with diabetes mellitus, chronic kidney disease, and resistant hypertension. CJC Open. 2020;2(4):258–64.
Oliveira AC, Martinez D, Massierer D, Gus M, Gonçalves SC, Ghizzoni F, et al. The antihypertensive effect of positive airway pressure on resistant hypertension of patients with obstructive sleep apnea: a randomized, double-blind, clinical trial. 2014;190(3):345–7.
Logan AG, Tkacova R, Perlikowski SM, Leung RS, Tisler A, Floras JS, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21(2):241–7.
Martínez-García MA, Gómez-AldaravíR, Soler-Cataluña JJ, Martínez TG, Bernácer-Alpera B, Román-Sánchez P. Positive effect of CPAP treatment on the control of difficult-to-treat hypertension. Eur Respir J. 2007;29(5):951–957.
Redline S, Azarbarzin A, Peker Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat Rev Cardiol. 2023;20(8):560–73.
Dernaika TA, Kinasewitz GT, Tawk MM. Effects of nocturnal continuous positive airway pressure therapy in patients with resistant hypertension and obstructive sleep apnea. J Clin Sleep Med. 2009;5(2):103–7.
Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–64.
Koehler U, Reinke C, Sibai E, Hildebrandt O, Sohrabi K, Dette F, et al. Autonomic dysfunction and cardiac arrhythmia in patients with obstructive and central sleep apnea. Dtsch Med Wochenschr. 2011;136(50):2622–8.
Wang X, Qiu J, Wang Y, Cai Z, Lu X, Li T. Beneficial response of blood pressure to short-term continuous positive airway pressure in Chinese patients with obstructive sleep apnea-hypopnea syndrome. Blood Press Monit. 2018;23(4):175–84.
Alhabeeb W, Tash AA, Alshamiri M, Arafa M, Balghith MA, ALmasood A, Eltayeb A, Elghetany H, Hassan T, Alshemmari O. National Heart Center/Saudi Heart Association 2023 guidelines on the management of hypertension. J Saudi Heart Assoc. 2023;35(1):16–39.
Reule S, Drawz PE. Heart rate and blood pressure: any possible implications for management of hypertension? Curr Hypertens Rep. 2012;14:478–84.
Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Telera MP, Pede S, et al. Adverse prognostic value of a blunted circadian rhythm of heart rate in essential hypertension. J Hypertens. 1998;16:1335–43.
Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176:1274–80.
Author information
Authors and Affiliations
Contributions
LS, YFC, BL, and BN conceived the study. LS and YFC collected and analyzed the data. All authors edited and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not include any research conducted by the author on human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, L., Chang, YF., Wang, YF. et al. Effect of Continuous Positive Airway Pressure on Blood Pressure in Patients with Resistant Hypertension and Obstructive Sleep Apnea: An Updated Meta-analysis. Curr Hypertens Rep 26, 201–211 (2024). https://doi.org/10.1007/s11906-024-01294-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-024-01294-4