Abstract
Purpose
To evaluate the diagnostic performance and clinical utility of 18F-fluciclovine PET/CT in patients with biochemical recurrence (BCR) of prostate cancer (PC).
Methods
18F-Fluciclovine scans of 165 consecutive men with BCR after primary definitive treatment with prostatectomy (n = 102) or radiotherapy (n = 63) were retrospectively evaluated. Seventy patients had concurrent imaging with at least one other conventional modality (CT (n = 31), MRI (n = 31), or bone scan (n = 26)). Findings from 18F-fluciclovine PET were compared with those from conventional imaging modalities. The positivity rate and impact of 18F-fluciclovine PET on patient management were recorded. In 33 patients who underwent at least one other PET imaging (18F-NaF PET/CT (n = 12), 68Ga-PSMA11 PET/CT (n = 5), 18F-DCFPyL PET/CT (n = 20), and 68Ga-RM2 PET/MRI (n = 5)), additional findings were evaluated.
Results
The overall positivity rate of 18F-fluciclovine PET was 67 %, which, as expected, increased with higher prostate-specific antigen (PSA) levels (ng/ml): 15 % (PSA < 0.5), 50 % (0.5 ≤ PSA < 1), 56 % (1 ≤ PSA < 2), 68 % (2 ≤ PSA < 5), and 94 % (PSA ≥ 5), respectively. One hundred and two patients (62 %) had changes in clinical management based on 18F-fluciclovine PET findings. Twelve of these patients (12 %) had lesion localization on 18F-fluciclovine PET, despite negative conventional imaging. Treatment plans of 14 patients with negative 18F-fluciclovine PET were changed based on additional PET imaging with a different radiopharmaceutical.
Conclusion
18F-Fluciclovine PET/CT remains a useful diagnostic tool in the workup of patients with BCR PC, changing clinical management in 62 % of participants in our cohort.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although significant advances in primary definitive treatment of prostate cancer (PC) have improved the prognosis [1], recurrence of PC is still common, occurring in 20 to 40 % of treated patients [2, 3]. Monitoring serum PSA after treatment helps identify recurrences in otherwise asymptomatic patients. Rising PSA after initial curative intent therapy (termed biochemical recurrence, BCR) is a risk factor for development of distant metastases and prostate cancer–specific mortality [4]. Over the past decade, PET imaging of patients with BCR PC has evolved with the development of various promising radiotracers [5]. 18F-Fluciclovine (18F-FACBC, anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, Axumin®) was approved by the US Food and Drug Administration (FDA) in 2016 and by the European Medicines Agency (EMA) in 2017 for use in BCR PC. 18F-Fluciclovine is a synthetic amino acid analog that is actively transported into cells, specifically by LAT-1 (large amino acid transporter) and ASCT2 (alanine-serine-cysteine transporter), which are both upregulated in prostate cancer [6,7,8]. The FDA approval was based on high diagnostic performance and histologically confirmed data in patients with BCR showing 68 % detection rate on patient basis, 62 % positive predictive value (greater than 90 % for extraprostatic disease), and 70 % specificity [9]. Prospective trials reported that 59 % of patients with BCR PC had a change in management after the 18F-fluciclovine PET scan [10]. A recent update to the NCCN Guidelines® recommends that 18F-fluciclovine PET be considered in the workup of patients with BCR PC [11].
Although studies prior to FDA approval have shown the usefulness and limitations of 18F-fluciclovine PET/CT, it is important to evaluate its performance in real-world clinical scenarios. Here, we report our experience in an academic center of 18F-fluciclovine PET/CT in patients with BCR PC, with a focus on the rate of disease localization and changes in clinical management.
Materials and Methods
Patients
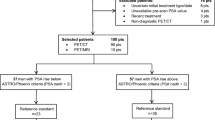
We reviewed retrospective data from 297 men with histologically confirmed PC who underwent 18F-fluciclovine PET/CT between September 2017 and December 2019. Inclusion criteria were patients with PC suspected of BCR after primary definitive treatment (radical prostatectomy or radiation therapy). BCR was diagnosed after prostatectomy with or without adjuvant radiotherapy at a PSA level of 0.2 ng/ml or greater, with a second confirmatory PSA level of at least 0.2 ng/ml [12]. For post radiation therapy patients, BCR was diagnosed as rise of PSA measurement of 2 or more ng/ml over the nadir [13]. The following patients were excluded: (1) received non-primary treatment such as androgen deprivation therapy (ADT) or chemotherapy (n = 61), (2) underwent 18F-fluciclovine PET for initial staging (n = 42), (3) did not experience the first BCR based on the abovementioned criteria yet (n = 23), and (4) had no documented follow-up management plan (n = 6). Thus, a total of 165 patients were included in the final analysis. Figure 1 shows a flow chart of the study population. The Institutional Review Board approved this retrospective study and waived the requirement for obtaining written informed consent.
18F-Fluciclovine PET/CT Protocol
We followed published ACR-ACNM practice parameters [14]. In brief, patients were advised to avoid any significant exercise 1 day prior and to not eat or drink for a minimum of 4 h prior to imaging. Approximately 3–5 min following IV administration of the radiopharmaceutical, images were acquired using the Discovery 600, 690, or MI models (GE Healthcare, Waukesha, WI, USA) from the mid-thigh to the base of the skull. A low-dose CT scan was performed for attenuation correction and anatomic correlation. The mean dosage of 18F-fluciclovine administered was 389 ± 59 MBq.
Image Analysis
18F-Fluciclovine PET images were visually evaluated by two board-certified nuclear medicine physicians (RN, with 11 years of experience; AI, with 15 years of experience) by consensus and according to guidelines [15], blinded to the clinical information, including other imaging. The positivity rate of 18F-fluciclovine PET was correlated with PSA values, PSA doubling time, and anatomic region (prostate bed, lymph node, bone, lung, or other) on a per-patient basis. Locations of positive lesions were also divided into 2 categories: (1) prostate and (2) extraprostatic regions. In addition, positive lesions were classified into 3 categories based on their location: (1) prostate bed only, (2) pelvic-confined (pelvic-confined lymph nodes with or without prostate lesion), and (3) extra-pelvic (abdominal and retroperitoneal lymph nodes, osseous lesions, pulmonary lesions, and other visceral/soft tissue lesions with or without pelvic-confined PC). Imaging findings from abdomen and pelvis CT, pelvic MRI, and bone scan were compared to those from 18F-fluciclovine PET where the field of view overlapped. Findings were considered congruent if both scans compared were negative or if the same lesions were identified on both scans.
Impact on Patient Management
The impact of 18F-fluciclovine PET on patient management was evaluated by clinical chart review.
Additional PET Imaging with Other Radiopharmaceuticals
In patients who underwent other PET scans (18F-NaF PET/CT, 68Ga-PSMA11 PET/CT, 18F-DCFPyL PET/CT, and 68Ga-RM2 PET/MRI), additional findings were evaluated.
Standard of Reference
Pathological confirmation of 18F-fluciclovine PET findings was only available in a small number of cases (n = 7). Therefore, the diagnostic performance of clinical 18F-fluciclovine PET/CT could not be evaluated.
Statistical Analysis
Continuous variables are summarized as medians and interquartile ranges (IQR) or means ± standard deviations. Values between groups were compared using the Mann-Whitney U test, Fisher’s exact test, chi-squared test, or McNemar’s test. Receiver operating characteristic (ROC) curves were used to calculate the area under the curves (AUCs) and to ascertain the optimal cutoff value of PSA doubling time for predicting positive 18F-fluciclovine PET results, defined as the value providing the largest sum of sensitivity and specificity (Youden’s index). Multivariate logistic regression analysis was also performed to identify independent predictors of positive 18F-fluciclovine PET results. All statistical analyses were performed using statistical software, MedCalc version 19.2.1 (MedCalc Software, Ostend, Belgium), in which P values < 0.05 were considered statistically significant.
Results
Patients
Table 1 shows the patients’ characteristics of the 165 men (50–91 years old, 71.1 ± 8.8) analyzed in this study. Primary definitive treatment included radical prostatectomy with or without adjuvant pelvic radiation in 102 patients (62 %) and radiation therapy in 63 patients (38 %). Of the 63 patients treated with radiotherapy, only five patients received external beam radiation therapy (EBRT) within 1 year or brachytherapy within 2 years. PSA levels at the time of 18F-fluciclovine PET ranged from 0.02 to 2975 ng/ml (median 3.1 ng/ml, IQR 1.0–9.6). PSA levels of 5 patients who had a previously confirmed diagnosis of BCR were below the BCR criteria at the time of the scan.
Lesions Detection
A total of 110 patients had positive 18F-fluciclovine PET findings in putative sites of disease, and the remaining 55 patients had no 18F-fluciclovine avid lesions. PSA in 18F-fluciclovine positive cases (median 5.4 ng/ml, IQR 2.3–14.3) was significantly higher than that of negative cases (median 0.69 ng/ml, IQR 0.32–2.46) (P < 0.05). The overall positivity rate was 66.7 % (110/165) (95 % CI 54.8–80.4), increasing with higher PSA levels (ng/ml): 15.4 % (PSA < 0.5), 50 % (0.5 ≤ PSA < 1), 55.6 % (1 ≤ PSA < 2), 68.2 % (2 ≤ PSA < 5), and 93.7 % (PSA ≥ 5), respectively (P < 0.0001). The positivity rate of 18F-fluciclovine PET for different PSA doubling time was 70.8 %, 69.7 %, 65.7 %, and 65.4 % for doubling time of 0–3 month, 3–6 months, 6–12 months, and greater than 12 months, respectively (P = 0.94). Table 2 shows the positivity rate by PSA level and doubling time. The positivity rate of patients who underwent SiPM-based PET/CT (GE Discovery MI, n = 93) was significantly higher than that of patients who underwent standard PET/CT (GE Discovery 690 or 600, n = 72) (73.1 % vs. 58.3 %, P = 0.046) (Table 3), although their PSA levels were not significantly different (median 2.9 vs. 3.1 ng/ml, P = 0.22). Supplemental Table 1 shows the 18F-fluciclovine PET results in 40 patients with low PSA levels (< 1 ng/ml). In these 40 patients, patients who underwent SiPM-based PET had a significantly higher positivity rate of 18F-fluciclovine PET (8/19, 42.1 %) than those who underwent standard PET (3/21, 14.3 %) (P = 0.029). In addition, the performance of 18F-fluciclovine PET was higher (sensitivity 80 %, specificity 66.7 %, AUC = 0.705) when a PSA doubling time threshold of 7 months or less was used. However, multivariate analysis revealed that the combination of a PSA doubling time threshold and SiPM-based PET did not improve lesion detection on 18F-fluciclovine PET in this cohort. Forty-six (27.9 %) patients had lesions in the prostate/prostate bed. Eighty-six (52.1 %) patients had lesions in the extraprostatic region. Of these, lesions with 18F-fluciclovine uptake were detected in lymph nodes in 69 (41.8 %) patients, in bone in 41 (24.8 %), and in the lung in 9 (5.5 %) patients. When 110 patients with positive 18F-fluciclovine PET scan were divided into three groups according to the sites of lesions (prostate bed only, pelvic-confined, and extra-pelvic), the number of patients in each site was 28 (14.5 %), 28 (14.5 %), and 68 (37.6 %), respectively. These findings are summarized in Table 4.
Comparison with Conventional Imaging
Seventy (42.4 %) patients had at least one other concurrent conventional imaging study within 3 months before or after the 18F-fluciclovine PET scan: abdomen-pelvis CT (n = 31), pelvic MRI (n = 31), and bone scan (n = 26). Of the three conventional scans, 55 patients had one, 12 had two, and 3 had all three scans. The lesion detection rates of CT, MR, and bone scan were 25.8 % (8/31), 64.5 % (20/31), and 26.9 % (7/26), respectively. The detection rate of 18F-fluciclovine PET (54.8 %, 17/31) was significantly higher than that of CT (P = 0.0039); 9/31 (29 %) patients with negative CT had positive 18F-fluciclovine PET finding(s). The detection rate of 18F-fluciclovine PET (83.9 %, 26/31) was significantly higher than that of MR (P = 0.031); 6/31 (19 %) patients with negative MRI had positive 18F-fluciclovine PET finding(s). There were no patients with negative 18F-fluciclovine PET who had lesions identified on CT or MRI in our cohort. Figure 2 shows an example patient who had subcentimeter local recurrence in a 18F-fluciclovine avid lesion that was not detected on MRI. For dedicated bone imaging, 25/26 (96.2 %) bone scans were congruent with 18F-fluciclovine PET, while 1/26 (3.8 %) patient with negative bone scan had an osseous lesion identified on 18F-fluciclovine PET, as shown in Fig. 3. Table 5 summarizes these findings.
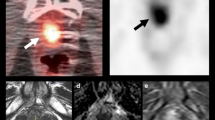
A 72-year-old man with suspected BCR 3 years after radical prostatectomy underwent 18F-fluciclovine PET/CT (a–c). The two most recent PSA values before the scan were 0.75 ng/ml and 1.3 ng/ml, respectively. Focal 18F-fluciclovine uptake in the prostate bed was seen on axial PET and fused PET/CT (a, c, arrows). No definite lesion was found on T2-weighted MRI (d). The patients underwent salvage radiation therapy and PSA became undetectable.
A 69-year-old man with suspected BCR 10 years after radical prostatectomy underwent 18F-fluciclovine PET/CT (a–d). The two most recent PSA values before the scan were 7.82 ng/ml and 11.12 ng/ml, respectively. 18F-Fluciclovine PET/CT revealed not only right pelvic nodal metastases (a, arrowhead) but also small bone metastasis in T6 (a, b, d, arrows). Bone scan (posterior view) was negative (e). The patient started androgen deprivation therapy, and PSA decreased to less than 0.01 ng/ml.
Impact on Patient Management
One hundred and two patients (61.8 %, 95 % CI 53.9–69.3) had a resulting change in clinical management due to 18F-fluciclovine PET findings. These included 41 of the 102 patients (40.2 %) referred for radiation therapy with or without concurrent ADT and 51 patients (50 %) who started ADT only. Figure 4 shows the flow chart of changes in management based on 18F-fluciclovine PET findings. Twelve of the 102 patients (12 %) who had a change in management had lesion localization on 18F-fluciclovine PET, despite negative conventional imaging (CT, MRI, and/or bone scan).
Additional PET Imaging with Other Radiopharmaceuticals
Additional PET imaging was performed at a median of 36 days (IQR 19–69) after 18F-fluciclovine PET scan. Thirty-three (20 %) patients underwent at least one other PET imaging (18F-NaF PET/CT (n = 12), 68Ga-PSMA11 PET/CT (n = 5), 18F-DCFPyL PET/CT (n = 20), and 68Ga-RM2 PET/MRI (n = 5)). In 14 of these patients with negative 18F-fluciclovine PET, additional PET imaging revealed new lesion(s). Table 6 summarizes these findings. Figure 5 shows a representative case that was negative on 18F-fluciclovine PET but positive on 18F-DCFPyL PET. The PSA level of the 14 patients (median 2.3 ng/ml, IQR 1.3–3.1) was significantly lower than that of patients with positive 18F-fluciclovine PET scan (median 5.4 ng/ml, IQR 2.3–14.3) (P < 0.0028). Treatment plans of all these 14 patients were changed, and subsequent treatment resulted in decreased PSA levels.
A 69-year-old man with suspected BCR 6 months after radiation therapy underwent 18F-fluciclovine PET/CT (a–c) that was negative for suspicious lesions. The PSA nadir was 1.0 ng/ml and it had risen to 8.6 ng/ml prior to the scan. 18F-DCFPyL PET/CT done 1 month later showed focal 18F-DCFPyL uptake in multiple small (subcentimeter) paraaortic lymph nodes, as well as along the right iliac chain (d–f, arrows). The patient started androgen deprivation therapy, and PSA decreased to 0.7 ng/ml.
Discussion
In our cohort, 18F-fluciclovine had an overall positivity rate of 66.7 % on a per-patient basis, which was higher than that of 57 % reported in a recent prospective study of 213 patients with BCR PC [10]. One possible reason for this is that our population had a higher PSA level than the population in the recent study (median 3.1 vs. 1.0 ng/ml). Another possible reason is that SiPM-based PET/CT, which has higher spatial resolution compared with standard PET/CT [16], was used for more than half (55 %) of our patients. The SiPM-based PET/CT had a higher positivity rate than standard PET/CT in our population (73.1 % vs. 58.3 %, P = 0.046). Furthermore, the subanalysis highlighted the advantage of SiPM-based PET/CT over standard PET in patients with low PSA levels (< 1 ng/ml) (42.1 % vs. 14.3 %, P = 0.029). The scanner used for 18F-fluciclovine PET/CT was randomly assigned based on clinical workflow.
It is known that the lesion detection rate of 18F-fluciclovine PET depends on PSA levels; this was confirmed by our results. According to published data, in patients with PSA values < 1 ng/ml, 18F-fluciclovine PET has relatively low sensitivity for extraprostatic disease, ranging from 21 to 39 % [9, 17]. In patients with PSA values higher than 5, sensitivity is overall approximately 85 % (approximately 60 % for extraprostatic disease) [9]. Similarly to other reports [18, 19], no statistically significant higher positivity rate of 18F-fluciclovine was found in patients with shorter PSA doubling time, although it is known as a prognostic factor for patients with BCR PC [20]. This may be attributed to the relatively small cohort size. The positivity rate for the prostate region was 27.9 %, which is lower than the 60.5 % (92/152) reported in the recent retrospective study by Savir-Baruch et al. [18] but comparable to 30 % (64/213) reported in the recent prospective trial by Andriole et al. [10]. In the study by Andriole et al., to minimize false-positive tracer activity due to inflammatory uptake after radiation, patients who underwent EBRT within 1 year or brachytherapy within 2 years were excluded, while patients who have recently undergone radiation therapy were included in the study by Savir-Baruch et al. The coincidence of only 5 patients in our cohort who had recently undergone radiotherapy may have been responsible for the low positivity rate in the prostate region, as well as in Andriole et al.
Our results confirm the superiority of 18F-fluciclovine PET in lesion detection over CT and MR. A previous study reported that, in 53 patients with BCR PC, recurrent lesions were detected in 41 patients (77.4 %) by 18F-fluciclovine PET and in 10 patients (18.9 %) by CT alone [21]. Another study reported that, in a cohort of 24 men with BCR PC, the disease detection rate was 94.7 % using 18F-fluciclovine PET compared to 31.6–36.8 % for multiparametric MR [22]. The main advantage of 18F-fluciclovine PET over conventional CT or MR is that 18F-fluciclovine PET often detects subcentimeter lesions with increased uptake as shown in Fig. 2. For bone lesions, 18F-fluciclovine PET findings were congruent with bone scan in all but one case. Although 18F-fluciclovine PET typically demonstrates intense focal uptake in lytic osseous lesions, it shows variable activity in sclerotic lesions [15]. In addition, compared with 18F-FDG PET, 18F-fluciclovine PET has greater heterogeneity in physiologic bone marrow uptake [15]. Accordingly, mild 18F-fluciclovine uptake in dense sclerotic lesions may be masked by physiologic distribution in normal bone marrow.
18F-Fluciclovine PET altered clinical management in 102 patients (62 %) including 92 patients treated with targeted radiotherapy and/or ADT (Fig. 4). Similar clinical impact of 18F-fluciclovine PET was shown previously in a study where overall 126 of the 213 patients (59 %) had a change in management after 18F-fluciclovine PET scan [10]. A total of 12 patients who had lesion localization only on 18F-fluciclovine PET with no findings on conventional imaging had changes in clinical management. However, the extent to which patient-specific treatment decisions based on 18F-fluciclovine PET have a more favorable impact on progression-free survival or overall survival than conventional imaging has not yet been reported. Therefore, additional studies are necessary to assess the impact of 18F-fluciclovine PET on patient outcomes as a result of changes in patient management.
Additional PET imaging led to changes in the treatment strategy for 14 patients with negative 18F-fluciclovine uptake. As previously reported, our results demonstrate that the relatively lower detection rate of 18F-fluciclovine PET in patients with low PSA levels is one of its drawbacks [23]. The fact that additional PET imaging was obtained after 18F-fluciclovine PET (after a median of 36 days) may have favored the detection of lesions by additional PET imaging, but our results reiterated the usefulness of PSMA-based PET, which contributed to a change in treatment strategy in 11 of 14 patients. In light of the above, if 18F-fluciclovine PET is negative despite clinical suspicion of BCR, further evaluation with other imaging modalities, such as PSMA-based PET/CT in research protocols until FDA approval, should be considered [15].
A growing number of studies in recent years have reported the superiority of PSMA-based PET to 18F-fluciclovine PET [23,24,25]. The advantage of PSMA-based PET stands out in the detection of lymph node metastases in patients with low PSA concentrations (< 2.0 ng/ml) [23]. Overexpression of PSMA that is seen in not only advanced castrate–resistant PC but also early castrate–sensitive PC results in higher SUVmax and lesion-to-background ratios for concordantly PET positive lesions with PSMA than 18F-fluciclovine [23]. On the other hand, 18F-fluciclovine PET is non-inferior to PSMA PET in detecting distant metastases of patients with high PSA levels [24]. Furthermore, previous data have shown that 18F-fluciclovine PET and PSMA-based PET are complementary to each other with strengths in different areas [9, 24], although, in our cohort, the small number of PSMA-based PET scans (n = 25) did not allow for a sufficient comparison. The advantage of 18F-fluciclovine is reported to be its ability to detect curable localized disease in close anatomical relation to the urinary bladder due to relatively slow physiological urinary excretion of 18F-fluciclovine in comparison to PSMA-based PET [26], which was also shown in Fig. 5.
Our study has several limitations. Firstly, it is a retrospective single-center study. However, to reduce selection bias, all patients during the period between the initiation of the 18F-fluciclovine PET at our facility and the start of the analysis were selected for inclusion in the study. Secondly, pathological confirmation of 18F-fluciclovine PET findings was only available in a small number of cases (n = 7), because biopsy was often difficult since lesions detected on 18F-fluciclovine PET were frequently subcentimeter in size. In addition, when multiple lesions are detected on 18F-fluciclovine PET in putative sites of disease for PC recurrence or metastases, treating physicians often rely on post-treatment PSA changes for confirmation of positive 18F-fluciclovine lesions rather than on biopsy. Therefore, we only indicated the positivity rate of 18F-fluciclovine PET. Thirdly, we conducted a review of clinical charts to determine the impact of 18F-fuciclovine PET on clinical management, while that several prior studies used a survey of oncologists to determine change of intended clinical management [10]. Our approach, although we assessed the impact of 18F-fluciclovine PET on the clinical management based on actual changes after we confirmed in the medical records that physicians were referring to the findings of 18F-fluciclovine PET, has the limitation that it is difficult to determine whether the decisions made by the treating physicians were based on imaging alone or other contributing factors such as PSA, risk and benefit of treatments, and patient preference. Further prospective studies using a questionnaire to physicians are needed to accurately assess the impact of 18F-fluciclovine PET/CT.
Conclusions
18F-Fluciclovine PET/CT is a useful diagnostic tool in the workup of patients with BCR PC as it changed clinical management in 64 % of participants in our cohort. In the setting of a negative 18F-fluciclovine PET despite clinical suspicion of BCR, further evaluation with alternative imaging modalities including currently investigational PSMA-based PET should be considered for a more complete evaluation.
References
Kim MM, Hoffman KE, Levy LB, Frank SJ, Pugh TJ, Choi S, Nguyen QN, McGuire SE, Lee AK, Kuban DA (2012) Improvement in prostate cancer survival over time: a 20-year analysis. The Cancer Journal 18:1–8
Bruce JY, Lang JM, McNeel DG, Liu G (2012) Current controversies in the management of biochemical failure in prostate cancer. Clin Adv Hematol Oncol 10:716–722
Simmons MN, Stephenson AJ, Klein EA (2007) Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 51:1175–1184
Van den Broeck T, Mottet N, Lam T (2019) Reply to Xueliang Zhou and Xinwei Hans letter to the editor re: Thomas Van den Broeck, Roderick C.N. van den Bergh, Nicolas Arfi, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur Urol 2019;75:967-87. Eur Urol 76:e16
Evans JD, Jethwa KR, Ost P, Williams S, Kwon ED, Lowe VJ, Davis BJ (2018) Prostate cancer–specific PET radiotracers: a review on the clinical utility in recurrent disease. Practical radiation oncology 8:28–39
Ono M, Oka S, Okudaira H, Nakanishi T, Mizokami A, Kobayashi M, Schuster DM, Goodman MM, Shirakami Y, Kawai K (2015) [(14)C] Fluciclovine (alias anti-[(14)C]FACBC) uptake and ASCT2 expression in castration-resistant prostate cancer cells. Nucl Med Biol 42:887–892
Oka S, Hattori R, Kurosaki F, Toyama M, Williams LA, Yu W, Votaw JR, Yoshida Y, Goodman MM, Ito O (2007) A preliminary study of anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med 48:46–55
Fuchs B, Bode B (2005) Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 15:254–266
Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H, Korsan KA, Kieboom J, Tade FI, Odewole O, Chau A, Ward P, Goodman MM, Fanti S, Schuster DM, Willoch F (2017) Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol 197:676–683
Andriole GL, Kostakoglu L, Chau A, Duan F, Mahmood U, Mankoff DA, Schuster DM, Siegel BA, Adler LP, Belkoff LH, Burzon D, Dato P, Farwell M, Fogelson S, Gardiner P, Hanna L, Hoffman JM, Intenzo C, Josephson D, Kaminetsky J, Kipper M, Krynyckyi B, Linder KE, Marques H, Melnick J, Miller MP, Oh W, Philips S, Rose J, Savir-Baruch B, Stevens DJ, Tewari A, Twardowski P, Ward P, Wasserman M, Weick S, (Michael) Yu JQ (2019) The impact of positron emission tomography with 18F-fluciclovine on the treatment of biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol 201:322–331
Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel MR, Lang JM, McKenney J, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J, Shead DA, Freedman-Cass DA (2019) Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 17:479–505
Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus SR, Moul JW, Tangen C, Thrasher JB, Thompson I (2007) Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 177:540–545
Roach III M, Hanks G, Thames Jr H, et al. (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. International Journal of Radiation Oncology* Biology* Physics 65:965-974
Savir-Baruch B, Banks KP, McConathy JE et al (2018) ACR-ACNM practice parameter for the performance of fluorine-18 fluciclovine-PET/CT for recurrent prostate cancer. Clin Nucl Med 43:909–917
Nanni C, Zanoni L, Bach-Gansmo T, Minn H, Willoch F, Bogsrud TV, Edward EP, Savir-Baruch B, Teoh E, Ingram F, Fanti S, Schuster DM (2020) [18 F] Fluciclovine PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging—version 1.0. Eur J Nucl Med Mol Imaging 47:579–591
Baratto L, Park SY, Hatami N, Davidzon G, Srinivas S, Gambhir SS, Iagaru A (2017) 18F-FDG silicon photomultiplier PET/CT: a pilot study comparing semi-quantitative measurements with standard PET/CT. PLoS One 12:e0178936
Nanni C, Zanoni L, Pultrone C, Schiavina R, Brunocilla E, Lodi F, Malizia C, Ferrari M, Rigatti P, Fonti C, Martorana G, Fanti S (2016) 18 F-FACBC (anti1-amino-3-18 F-fluorocyclobutane-1-carboxylic acid) versus 11 C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging 43:1601–1610
Savir-Baruch B, Lovrec P, Solanki AA, Adams WH, Yonover PM, Gupta G, Schuster DM (2019) Fluorine-18-labeled fluciclovine PET/CT in clinical practice: factors affecting the rate of detection of recurrent prostate cancer. Am J Roentgenol 213:851–858
Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FDB, Halkar RK, Master VA, Nye JA, Odewole OA, Osunkoya AO, Savir-Baruch B, Alaei-Taleghani P, Goodman MM (2014) Anti-3-[(18)F] FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 191:1446–1453
Okotie OT, Aronson WJ, Wieder JA et al (2004) Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol 171:2260–2264
Odewole OA, Tade FI, Nieh PT, Savir-Baruch B, Jani AB, Master VA, Rossi PJ, Halkar RK, Osunkoya AO, Akin-Akintayo O, Zhang C, Chen Z, Goodman MM, Schuster DM (2016) Recurrent prostate cancer detection with anti-3-[18 F] FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 43:1773–1783
Akin-Akintayo O, Tade F, Mittal P, Moreno C, Nieh PT, Rossi P, Patil D, Halkar R, Fei B, Master V, Jani AB, Kitajima H, Osunkoya AO, Ormenisan-Gherasim C, Goodman MM, Schuster DM (2018) Prospective evaluation of fluciclovine ((18)F) PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur J Radiol 102:1–8
Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, Bach-Gansmo T, Nanni C, Savir-Baruch B, Elashoff D, Grogan T, Dahlbom M, Slavik R, Gartmann J, Nguyen K, Lok V, Jadvar H, Kishan AU, Rettig MB, Reiter RE, Fendler WP, Czernin J (2019) (18)F-fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-Centre, single-arm, comparative imaging trial. Lancet Oncol 20:1286–1294
Pernthaler B, Kulnik R, Gstettner C, Salamon S, Aigner RM, Kvaternik H (2019) A prospective head-to-head comparison of 18F-fluciclovine with 68Ga-PSMA-11 in biochemical recurrence of prostate cancer in PET/CT. Clin Nucl Med 44:e566–e573
Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F (2018) Comparison of 68Ga-PSMA-11 and 18F-fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med 59:789–794
Freitag MT, Radtke JP, Afshar-Oromieh A, Roethke MC, Hadaschik BA, Gleave M, Bonekamp D, Kopka K, Eder M, Heusser T, Kachelriess M, Wieczorek K, Sachpekidis C, Flechsig P, Giesel F, Hohenfellner M, Haberkorn U, Schlemmer HP, Dimitrakopoulou-Strauss A (2017) Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68 Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging 44:776–787
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Nakamoto, R., Harrison, C., Song, H. et al. The Clinical Utility of 18F-Fluciclovine PET/CT in Biochemically Recurrent Prostate Cancer: an Academic Center Experience Post FDA Approval. Mol Imaging Biol 23, 614–623 (2021). https://doi.org/10.1007/s11307-021-01583-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01583-3