Abstract
Purpose
To compare the diagnostic performance of the synthetic amino acid analogue PET radiotracer anti-3-[18F]FACBC (fluciclovine) with that of CT in the detection of recurrent prostate carcinoma.
Methods
This was a retrospective analysis of 53 bone scan-negative patients with suspected recurrent prostate carcinoma who underwent fluciclovine PET/CT and routine clinical CT within 90 days of each other. The correlation between imaging findings and histology and clinical follow-up was evaluated. Positivity rates and diagnostic performance were calculated for fluciclovine PET/CT and CT.
Results
Of 53 fluciclovine PET/CT and 53 CT examinations, 41 (77.4 %) and 10 (18.9 %), respectively, had positive findings for recurrent disease. Positivity rates were higher with fluciclovine PET/CT than with CT at all prostate-specific antigen (PSA) levels, PSA doubling times and original Gleason scores. In the prostate/bed, fluciclovine PET/CT was true-positive in 31 and CT was true-positive in 4 of 51 patients who met the reference standard. In extraprostatic regions, fluciclovine PET/CT was true-positive in 12 and CT was true-positive in 3 of 41 patients who met the reference standard. Of the 43 index lesions used to prove positivity, 42 (97.7 %) had histological proof. In 51 patients with sufficient follow-up to calculate diagnostic performance in the prostate/bed, fluciclovine PET/CT demonstrated a sensitivity of 88.6 %, a specificity of 56.3 %, an accuracy of 78.4 %, a positive predictive value (PPV) of 81.6 %, and a negative predictive value (NPV) of 69.2 %; the respective values for CT were 11.4 %, 87.5 %, 35.3 %, 66.7 % and 31.1 %. In 41 patients with sufficient follow-up to calculate diagnostic performance in extraprostatic regions, fluciclovine PET/CT demonstrated a sensitivity of 46.2 %, a specificity of 100 %, an accuracy of 65.9 %, a PPV of 100 %, and an NPV of 51.7 %; the respective values for CT were 11.5 %, 100 %, 43.9 %, 100 % and 39.5 %.
Conclusion
The diagnostic performance of fluciclovine PET/CT in recurrent prostate cancer is superior to that of CT and fluciclovine PET/CT provides better delineation of prostatic from extraprostatic recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When prostate cancer recurs, differentiating prostatic from extraprostatic disease, typically metastasis to lymph nodes or bone, can be challenging. Yet determining whether the recurrence is local, locoregional, or systemic influences the type of therapy offered [1–3]. While conventional imaging such as CT, bone scan and MRI can be useful in delineating local and distant prostate cancer recurrence, these modalities are also limited by suboptimal diagnostic performance [1–8]. As a result, the performance of molecular imaging techniques in restaging of recurrent prostate cancer is currently being explored [2, 8, 9]. With the use of different radiotracers such as choline and now PSMA ligands, PET holds promise for identifying recurrence earlier and more accurately than conventional imaging techniques [10–12].
Anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (FACBC, fluciclovine) is an investigational amino acid-based PET radiotracer that has been studied in the staging and restaging of prostate cancer [13–17]. The mechanism of uptake of fluciclovine is via transmembrane amino acid transporters, primarily ASCT2 and LAT1, which have been reported to be upregulated in prostate cancer [18]. We have previously reported a completed clinical trial which demonstrated that the diagnostic performance of fluciclovine PET/CT is superior to that of 111In-capromab pendetide (ProstaScint) [14]. Yet conventional CT is still more commonly used in patients with biochemical (prostate-specific antigen, PSA) failure than 111In-capromab pendetide. In this analysis, we compared the performance of fluciclovine PET/CT with that of CT in the detection and restaging of recurrent prostate carcinoma in a subset of patients who had been part of the previous clinical trial and who also had undergone routine CT scanning as standard of care for the detection of prostate cancer recurrence.
Materials and methods
Patient selection and informed consent
After Emory University Institutional Review Board approval and informed consent had been obtained, patients with suspected prostate cancer recurrence were recruited to receive fluciclovine PET/CT scans as part of a prospective parent clinical trial (NCT00562315) of fluciclovine PET/CT for the detection of recurrent prostate cancer compared to conventional imaging, with emphasis on 111In-capromab pendetide [14]. Inclusion criteria were: (1) an original diagnosis of localized prostate carcinoma with subsequent definitive treatment, (2) a suspicion of prostate cancer recurrence, based on the previous American Society for Radiation Oncology (ASTRO) criteria of three consecutive PSA increases and/or the more recent ASTRO/Phoenix criteria of PSA of at least nadir plus 2.0 ng/ml after radiotherapy or cryotherapy and/or >0.2 ng/ml after prostatectomy, and (3) a negative bone scan. The results of this clinical trial comparing the diagnostic performance of fluciclovine PET/CT with that of 111In-capromab pendetide in recurrent prostate cancer have been previously published [14].
For the purpose of this retrospective analysis, out of the original cohort of patients in the parent trial, we analysed patients who had also undergone standard of care routine CT scans within 90 days of the fluciclovine PET/CT scan.
Fluciclovine PET/CT and CT imaging protocols
Fluciclovine preparation and PET/CT imaging protocols have been previously reported [14, 15, 19, 20]. The radiotracer was produced under US Food and Drug Administration investigational new drug application 72,437. No adverse events were reported among the study participants. Scanning was performed on a Discovery DLS or a 690 PET/CT scanner (GE Healthcare, Milwaukee, WI). All patients fasted for 4 to 6 h before the fluciclovine PET/CT scan. A CT scan without administration of intravenous or oral contrast agent was obtained for anatomical localization and attenuation correction at approximately 100 mAs and 120 kVp. Fluciclovine (mean ± SD dose 358 ± 52.9 MBq, range 163.9 – 469.9 MBq) was then injected intravenously over 2 min. After a 3-min delay for blood pool clearance, abdominopelvic PET/CT imaging was performed with an early acquisition from 5 to 16 min, and delayed acquisitions from 17 to 28 min (delayed 1) and 29 to 40 min (delayed 2).
Routine clinical CT at our facility was performed on a 16-slice GE-BS or a 64-slice GE-VCT CT scanner with multiple contiguous 5-mm axial images from the diaphragm to the upper thigh for abdominopelvic CT and from the lower abdomen to the upper thigh for pelvic CT with 120 kVp and autoadjusted tube current (mA) with administration of intravenous and oral contrast agent unless contraindicated. Contraindications for the use of intravenous contrast agent at our facility include allergic history, elevated creatinine, and patient refusal. The choice of abdominopelvic or pelvic CT was deferred to the ordering clinician. Scan results from outside facilities were included in the analysis if diagnostic protocols were similar. The interval between the fluciclovine PET/CT and CT scans was (mean ± SD) 45.5 ± 23.9 days (range 0 – 88 days).
Image interpretation
As part of the parent cohort, fluciclovine PET/CT images were prospectively interpreted individually on a MIM-Vista workstation (MIM Software, Cleveland, OH) by two board-certified nuclear medicine physicians blinded to other imaging and reference validation. Disagreement was resolved by consensus. Abnormal moderate (greater than marrow at L3) focal uptake deviating from the expected biodistribution and persisting from early to delayed images was interpreted as positive, as previously reported [14, 15]. Anatomical findings on the CT portion of the PET/CT scans (e.g. lymph node size and shape) did not influence individual lesion interpretation. The fluciclovine PET/CT scan interpretations obtained at the time of the parent trial were utilized in this subanalysis.
CT imaging had been interpreted as part of standard clinical workflow by a board-certified radiologist without knowledge of the fluciclovine PET/CT study results, but with ability to access all other patient imaging and clinical data. For the purpose of this retrospective analysis, data from clinical reports were utilized and it was assumed that outside facility reports were based on similar standard interpretative guidelines. CT images were not reinterpreted for the purpose of this study. All lesions reported as equivocal on CT were considered positive.
Reference standard
As part of the completed parent prospective cohort, patients were followed up for up to 5 years, and final consensus was achieved on the presence or absence of prostatic and extraprostatic disease by a multidisciplinary board comprising one nuclear medicine physician, two urologists and two radiation oncologists, who utilized the histological, imaging and clinical follow-up results to achieve consensus for the presence or absence of recurrent disease [14]. For this subset analysis, this consensus was used as the reference standard to calculate the diagnostic performance of both CT and fluciclovine PET/CT. Briefly, and as previously reported in detail, the consensus criteria were as follows [14]. In the prostate/bed, the standard of truth was histological sampling with transrectal ultrasonography/biopsy. Absence of tissue to biopsy was deemed negative. A patient in whom, despite a negative biopsy, PSA control was achieved (PSA <0.2 ng/ml after prostatectomy or less than PSA nadir plus 2 ng/ml without prostatectomy) after salvage therapy to the prostate/bed alone, was deemed positive for the presence of prostate/bed disease.
For extraprostatic involvement, histological sampling via image-guided needle biopsy, or laparoscopic or open dissection was the primary verification standard for the presence of disease with deliberate tracking of the index lesion to ensure concordance between positivity on the scan and the site of biopsy. For bone only, concordant findings on two or more correlative imaging studies (MR, CT and/or subsequent bone scan) were accepted in lieu of histology. Similarly, as with the prostate/bed, achieving durable PSA control after directed therapy only to the scan-positive site was accepted as proof of disease in lieu of biopsy. Absence of extraprostatic disease was biochemically confirmed by achievement of durable PSA control after salvage therapy to the prostate/bed only.
Spontaneous decline in PSA without therapy was interpreted as an overall absence of disease in both prostate and extraprostatic locations. Patients with inadequate follow-up data to establish the presence or absence of disease in the prostate and extraprostatic regions were excluded from the respective analysis of diagnostic performance [14, 15].
Statistical analyses
On whole-body analysis, positivity rates were calculated for the fluciclovine PET/CT and CT scans across PSA levels, PSA doubling time (DT) and original Gleason scores (GS) at diagnosis. According to the contemporary prostate cancer grading system, GS 3 + 4 = 7 was classified as grade group 2, while GS 4 + 3 = 7 was classified as grade group 3 [21]. The McNemar test was used to determine the statistical significance of differences in positivity rates between fluciclovine PET/CT and CT for these measures.
The diagnostic performance of fluciclovine PET/CT and CT and, in a subset analysis, of CT with contrast agent for recurrent prostatic/bed and extraprostatic disease detection were determined on a per-patient basis. The exact binomial proportions were used to compute the 95 % confidence intervals (CI) of each performance measure. The McNemar test was used to determine the statistical significance of the differences in sensitivity and specificity between fluciclovine PET/CT and CT. In addition, the chi-squared test was used to evaluate the statistical significance of differences in the accuracy of the two tests, while the generalized score statistic method was used to determine the statistical significance of differences in positive predictive value (PPV) and negative predictive value (NPV). A type I error rate of α = 0.05 was used. Analysis was done using Statistical Analysis Software (SAS version 9.3; SAS Institute Inc. Cary, NC) and Microsoft Excel 2010.
Results
Demographics
Table 1 shows selected demographic characteristics of the 53 patients who met the inclusion criteria for this retrospective subanalysis. Their median (mean ± SD) PSA was 4.0 ng/ml (7.2 ± 8.3 ng/ml). Of the 53 patients, 7 (13.2 %) had undergone prostatectomy, 5 (9.4 %) external beam radiation therapy, 6 (11.3 %) brachytherapy, 4 (7.5 %) cryotherapy and 1 (1.9 %) hormone therapy, while 30 (56.6 %) had received a combination of two or more treatment modalities.
All CT scans were performed within 45.53 ± 23.94 days (range 0 – 88 days) prior to the fluciclovine PET/CT scan. Of the 53 CT scans, 39 were performed at our facility and 14 were performed at outside facilities; 36 were pelvic scans and 17 were abdominopelvic scans. There were no patients in whom disease was found on the fluciclovine PET/CT study outside the clinical CT field of view. Intravenous contrast agent was used in 30 of the 53 CT scans, and no contrast agent was administered in 23 scans due to contraindications.
Reference standard
Of 115 patients in the parent study, 53 had a routine CT scan according to the standard of care within 90 days of the fluciclovine PET/CT scan. There was sufficient follow-up data and histological proof to determine the presence or absence of local prostatic disease recurrence in 51 patients and for extraprostatic disease in 41 patients (Fig. 1). Of the 51 patients, 35 were determined to have disease in the prostate bed. In 33 of these 35 patients the disease was biopsy-proven while in 2 patients PSA control was achieved after local salvage therapy. The remaining 16 of the 51 patients were determined to have no disease in the prostate bed. In 15 of these 16 patients, the absence of disease was biopsy-proven while 1 patient showed spontaneous PSA decline over time without therapy.
Of the 41 patients with sufficient follow-up data, 26 were determined to have extraprostatic disease: 11 with histological proof, 4 with a bone lesion confirmed on correlative imaging, and 11 with biochemical failure and no evidence of active local disease who were categorized conservatively as having occult extraprostatic disease. The remaining 15 patients were determined to have no extraprostatic disease, 14 with PSA control after local salvage therapy to the prostate/bed only, and 1 patient (same patient as above) had spontaneous PSA decline with no therapy.
Positivity rates
On a whole-body basis, 41 of 53 fluciclovine PET/CT scans (77.4 %) were positive, of which 26 (63.4 %) were positive in the prostate/bed only, 3 (7.3 %) in extraprostatic regions only and 12 (29.3 %) in both the prostate/bed and extraprostatic regions. On CT, 10 of 53 scans (18.9 %) were reported as positive: 5 (50.0 %) in the prostate/bed only, 4 (40.0 %) in extraprostatic regions only and 1 (10.0 %) in both the prostate/bed and extraprostatic regions.
The positivity rates of both fluciclovine PET/CT and CT increased as the absolute PSA value increased (Fig. 2a). In addition, the positivity rate of fluciclovine PET/CT increased with shorter DT, but the positivity rate of CT decreased with shorter DT (Fig. 2b). The positivity rate of fluciclovine PET/CT was also significantly higher with higher GS, but that of CT was not significantly higher with higher GS (Fig. 2c). The positivity rates of fluciclovine PET/CT in relation to absolute PSAs, DT and GS were significantly different from those of CT, except in relation to PSA <1 ng/ml.
Test outcome and diagnostic performance
Of the 43 index lesions used for determining true positivity on fluciclovine PET/CT, 42 (97.7 %) had histological proof. All lesions that were true-positive on CT were also true-positive on fluciclovine PET/CT.
Prostate/bed disease
In the 51 patients with an adequate reference standard, fluciclovine PET/CT was true-positive in 31, true-negative in 9, false-positive in 7, and false-negative in 4. CT was true-positive in 4,true-negative in 14, false-positive in 2 and false-negative in 31 (Fig. 1). Of 33 patients with histological proof of disease, fluciclovine PET/CT detected disease in 31 (93.9 %) but CT detected disease in 4 (12.1 %).
In the detection of prostate/bed disease, fluciclovine PET/CT had a sensitivity of 88.6 % (95 % CI 72.3 – 96.3), a specificity of 56.3 % (95 % CI 30.6 – 79.2), an accuracy of 78.4 % (95 % CI 64.7 – 88.7), a PPV of 81.6 % (95 % CI 65.1 – 91.7), and a NPV of 69.2 % (95 % CI 38.9 – 89.6). CT had a sensitivity of 11.4 % (95 % CI 3.7 – 27.7), a specificity of 87.5 % (95 % CI 60.4 – 97.8), an accuracy of 35.3 % (95 % CI 22.4 – 49.9), a PPV of 66.7 % (95 % CI 24.1 – 94.0), and an NPV of 31.1 % (95 % CI 18.6 – 46.8). The sensitivity, specificity, accuracy, PPV and NPV differed significantly between fluciclovine PET/CT and CT (Table 2). Figure 3 shows a biopsy-proven prostate lesion.
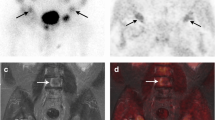
True-positive fluciclovine PET/CT and false-negative CT in a 66-year-old patient who had had external beam radiation therapy and brachytherapy for prostate cancer with a subsequent rise in PSA to 4.85 ng/ml. The prostate was positive on biopsy (not shown). The fluciclovine PET/CT image (a) shows abnormal uptake in the prostate (yellow arrow) meeting criteria for positivity. The CT image (b) is negative in the prostate (yellow arrow)
Extraprostatic disease
Of the 41 patients with an adequate reference standard, on fluciclovine PET/CT 12 were true-positive, 15 were true-negative, none was false-positive, and 14 were false-negative. On CT 3 were true positive, 15 were true-negative, none was false-positive and 23 were false-negative (Fig. 1). Of the 12 patients true-positive on fluciclovine PET/CT, 10 had positive lymph nodes on biopsy, one had a positive bone biopsy, and 1 had skeletal disease confirmed on correlative imaging. In the three patients who were true-positive on CT, disease was confirmed histologically via index nodes. Of the 11 patients with histological proof of extraprostatic disease, fluciclovine PET/CT detected disease in 11 (100 %) while CT detected disease in 3 (27.3 %). The short-axis diameters of the smallest nodes considered positive on fluciclovine PET/CT and CT were 0.4 cm and 0.9 cm, respectively. Figure 4 shows a subcentimetre lymph node with biopsy-proven disease interpreted as positive on fluciclovine PET/CT but not on CT.
Subcentimetre lymph node true-positive on the fluciclovine PET/CT and false-negative on CT in a 72-year-old patient who had had prostatectomy, external beam radiation therapy and hormone therapy for prostate cancer with a subsequent rise in PSA to 1.78 ng/ml. The 0.4 × 0.6 cm left obturator lymph node (yellow arrow) is positive on the fluciclovine PET/CT image (a) but negative on the CT image (b). c The cytology specimen (×400) of the lymph node biopsy shows metastatic prostatic adenocarcinoma
For detecting extraprostatic disease, fluciclovine PET/CT had a sensitivity of 46.2 % (95 % CI 37.7 – 68.8), a specificity of 100 % (95 % CI 74.7 – 100), an accuracy of 65.9 % (95 % CI 49.4 – 79.9), a PPV of 100 % (95 % CI 69.8 – 100) and an NPV of 51.7 % (95 % CI 32.9 – 70.1). CT had a sensitivity of 11.5 % (95 % CI 3.0 – 31.3), a specificity of 100.0 % (95 % CI 74.7 – 100.0), an accuracy of 43.9 % (95 % CI 28.5 – 60.3), a PPV of 100.0 % (95 % CI 31.0 – 100.0) and an NPV of 39.5 % (95 % CI 24.5 – 56.5). Sensitivity and NPV differed significantly between fluciclovine PET/CT and CT (Table 2). Figure 5 shows fluciclovine PET/CT and CT images of biopsy-proven nodal disease.
True-positive lymph node on both fluciclovine PET/CT and CT in a 76-year-old patient who had had external beam radiation therapy for prostate cancer with a subsequent rise in PSA to 5.3 ng/ml. Both the fluciclovine PET/CT image (a) and the CT image (b) are positive in a 1.0-cm round right obturator lymph node (yellow arrows). c The H&E stained section (×100) of the lymph node shows metastatic prostatic adenocarcinoma with extranodal extension
Subanalysis of fluciclovine PET/CT versus CT with intravenous contrast agent
Since 30 of the 53 clinical CT scans were performed with intravenous contrast agent, these patients were analysed separately. The diagnostic performance of CT with contrast and that of fluciclovine PET/CT are compared in Table 3. Compared with CT, fluciclovine PET/CT had significantly higher sensitivity, accuracy, PPV and NPV in the detection of prostate/bed disease as well as significantly higher sensitivity in the detection of extraprostatic disease. Fluciclovine PET/CT detected 11 more true-positive prostate lesions and 6 more extraprostatic lesions than CT with contrast, further confirming the overall observation of better disease detection by fluciclovine PET/CT than by CT.
Discussion
We set out to compare the differences in whole-body positivity rates between fluciclovine PET/CT and CT at varying absolute PSA levels, DT, and GS, as well as diagnostic performance in the detection of recurrent prostate carcinoma in prostatic and extraprostatic locations. The overall positivity rate of CT was 18.9 % (10 of 53 patients), but that of fluciclovine PET/CT was 77.4 % (41 of 53 patients). This difference in positivity was sustained across absolute PSA levels, DT and GS. Fluciclovine PET/CT also had a better overall performance than CT in the detection of both prostatic and extraprostatic disease. Fluciclovine PET/CT detected local prostatic disease in 31 patients compared with 4 patients detected by CT, and also detected 12 patients with extraprostatic disease compared with 3 detected by CT. Although both imaging modalities had similar PPV in the extraprostatic region, fluciclovine PET/CT detected more patients with disease than CT. Thus, fluciclovine PET/CT differentiated prostatic from extraprostatic recurrence better than CT. One of the strengths of this study was that of the 43 index lesions (31 prostatic, 12 extraprostatic) used to establish the high PPV of fluciclovine PET/CT, 97.7 % had histological proof.
Our findings are important since therapy for prostate cancer recurrence depends on whether the recurrence is located in the prostate/bed, pelvis, and/or extrapelvic regions [2, 22]. Conventional imaging plays an important role in this process [23, 24]. Yet studies have shown low positivity rates of CT in the evaluation of patients with recurrent prostate cancer [5, 6]. While 18F-fluorodeoxyglucose (FDG) is currently the most commonly used PET radiotracer in cancer imaging, it is of limited value in the detection of prostate cancer [12, 25–27]. Because of these limitations, molecular techniques using fluciclovine, choline, acetate, PSMA and other radiotracers are being investigated for prostate cancer imaging [1, 12, 28–30].
Although PSA level is a critical factor in prostate cancer detection, PSA DT and GS also affect the detection rate, as reported for conventional and molecular imaging, for example choline PET/CT [12, 25, 31, 32]. In our study, positivity rates with fluciclovine PET/CT also correlated with increasing absolute PSA, shorter DT, and higher GS. In a systemic review of 1,000 patients, choline PET demonstrated positivity rates of 31 %, 43 % and 81 % % at PSA levels <1, 1 – 2, and >2 ng/ml, respectively, comparing favourably with those of fluciclovine PET/CT (37.5 %, 77.8 % and 83.3 – 91.7 %, respectively) [33]. Despite reports of an increase in disease detection on CT with shorter DT, a similar trend was not observed in this study possibly due to the overall low disease detection rate on CT and small sample size [34].
In a recent meta-analysis, choline PET/CT was found to have a pooled sensitivity of 75.4 % and a specificity of 82 % for disease in the prostate bed and a sensitivity of 100 % and specificity of 81.8 % for extraprostatic disease [11]. Although this reported diagnostic performance of choline PET/CT seems to be superior to the diagnostic performance found in this study for fluciclovine PET/CT, a comparison between radiotracers using the literature alone should be viewed with caution. Due to differences in study design, PSA kinetics and reference standards, the most reliable comparison is best done by a trial in which radiotracers are utilized within the same patient. Such a study was recently reported by Nanni et al. who noted significantly better performance of fluciclovine PET/CT in comparison with 11C-choline PET/CT in patients with recurrence after prostatectomy [35].
For detection of disease after therapy in the prostate/bed and in extraprostatic locations, it is not surprising that a molecular technique such as fluciclovine PET/CT will have greater sensitivity than CT. In this study, the 11.4 % and 11.5 % sensitivity of CT in the detection of recurrent tumour in the prostatic/bed and extraprostatic regions, respectively, is similar to that reported elsewhere in the literature [36]. Even though the sensitivity of CT was higher with intravenous contrast agent, the sensitivity was still significantly lower than with fluciclovine PET/CT. In the prostate/bed, specificity was higher with CT than with fluciclovine PET/CT probably because CT images must show significant abnormality in the prostate/bed to reach the threshold of suspicion. Yet only four true-positive prostate/beds were detected on CT compared with 31 true-positive detected on fluciclovine PET/CT. As previously reported, fluciclovine PET/CT does have lower specificity in the prostate, probably because of the confounding effect of prostatic hypertrophy and inflammation [13–15, 37, 38].
For detection of extraprostatic recurrence, CT had equally high specificity and PPV compared to fluciclovine PET/CT, probably because a larger nodal size threshold was used to designate positivity on CT. Yet CT detected only three patients with extraprostatic disease compared to 12 patients with fluciclovine PET/CT, which has the added advantage of being able to detect metabolic activity. Hovels et al. found that the specificity of CT for the diagnosis of nodal metastasis decreases as the threshold size of lymph node positivity decreases, but specificity remains high for lymph node threshold sizes ≥1.5 cm [26]. In our cohort, all nodes positive on CT were also positive on fluciclovine PET/CT and small nodes considered benign on CT were detected as positive on fluciclovine PET/CT.
The limitations of this study include the fact that the CT scans were performed an average of 45.53 days before the fluciclovine PET/CT scans, but although lesions may have grown during the period before fluciclovine PET/CT imaging, prostate cancer is typically indolent. While all 53 patients had fluciclovine PET/CT of the abdomen and pelvis, 36 of the 53 CT scans were pelvis only. However, no disease detected on fluciclovine PET/CT was outside the CT field of view in any patient. Intravenous contrast agent could not be used in CT imaging in 23 patients because of contraindications. Although this mirrors the limitations in routine clinical practice, it reduced the overall detection rate of CT. However, separate analysis of the CT with contrast data did not change the overall conclusion of this study of greater sensitivity of fluciclovine PET/CT compared with CT. Clinical interpretation of the CT scans may have also benefited from access to other clinical imaging and data which were unavailable during interpretation of the fluciclovine PET/CT scans. Despite detection of bone lesions with fluciclovine PET/CT not seen on conventional imaging, only limited conclusions can be drawn regarding the diagnostic performance of fluciclovine PET/CT for bone metastasis, as a negative bone scan was an eligibility criterion for this study.
Furthermore, although the positivity rate of 37.5 % for molecular imaging with fluciclovine in detecting prostatic disease recurrence in patients with PSA <1 ng/ml is better than that with CT, continued improvement is required due to the trend of the increasing use of salvage radiotherapy in patients with PSA <1 ng/ml. Early reports have indicated that 68Ga-PSMA PET imaging is promising in this regard and may also improve specificity in the prostate/bed over current radiotracers [39].
In conclusion, having previously reported that the diagnostic performance of fluciclovine PET is superior to that of 111In-capromab pendetide in the restaging of prostate cancer, this subanalysis of patients who had also undergone routine clinical CT demonstrates that the performance of fluciclovine PET/CT is also superior to that of CT [14, 15].
References
Schiavina R, Brunocilla E, Borghesi M, Vagnoni V, Castellucci P, Nanni C, et al. Diagnostic imaging work-up for disease relapse after radical treatment for prostate cancer: how to differentiate local from systemic disease? The urologist point of view. Rev Esp Med Nucl Imagen Mol. 2013;32(5):310–3. doi:10.1016/j.remn.2013.06.003.
Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol). 2010;22(1):46–55. doi:10.1016/j.clon.2009.10.015.
Boukaram C, Hannoun-Levi JM. Management of prostate cancer recurrence after definitive radiation therapy. Cancer Treat Rev. 2010;36(2):91–100. doi:10.1016/j.ctrv.2009.06.006.
Jager GJ, Ruijter E, Van de Kaa C, de la Rosette J, Oosterhof G, Thornbury JR, et al. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. AJR Am J Roentgenol. 1996;166(4):845–52.
Kramer S, Gorich J, Gottfried HW, Riska P, Aschoff AJ, Rilinger N, et al. Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. Br J Radiol. 1997;70(838):995–9. doi:10.1259/bjr.70.838.9404201.
Kane CJ, Amling CL, Johnstone PA, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61(3):607–11.
Oyen RH, Van Poppel HP, Ameye FE, Van de Voorde WA, Baert AL, Baert LV. Lymph node staging of localized prostatic carcinoma with CT and CT-guided fine-needle aspiration biopsy: prospective study of 285 patients. Radiology. 1994;190(2):315–22. doi:10.1148/radiology.190.2.8284375.
Zukotynski K, Haider MA. Imaging in prostate carcinoma. Hematol Oncol Clin North Am. 2013;27(6):1163–87. doi:10.1016/j.hoc.2013.08.003.
Beer AJ, Eiber M, Souvatzoglou M, Schwaiger M, Krause BJ. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 2011;12(2):181–91. doi:10.1016/s1470-2045(10)70103-0.
Schiavina R, Ceci F, Borghesi M, Brunocilla E, Vagnoni V, Gacci M, et al. The dilemma of localizing disease relapse after radical treatment for prostate cancer: which is the value of the actual imaging techniques? Curr Radiopharm. 2013;6(2):92–5.
Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38(5):305–14. doi:10.1097/RLU.0b013e3182867f3c.
Ceci F, Castellucci P, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, et al. 11C-choline PET/CT detects the site of relapse in the majority of prostate cancer patients showing biochemical recurrence after EBRT. Eur J Nucl Med Mol Imaging. 2014;41(5):878–86. doi:10.1007/s00259-013-2655-9.
Odewole OA, Oyenuga OA, Tade F, Savir-Baruch B, Nieh PT, Master V, et al. Reproducibility and reliability of anti-3-[18F]FACBC uptake measurements in background structures and malignant lesions on follow-up PET-CT in prostate carcinoma: an exploratory analysis. Mol Imaging Biol. 2015;17(2):277–83. doi:10.1007/s11307-014-0797-1.
Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191(5):1446–53. doi:10.1016/j.juro.2013.10.065.
Schuster DM, Savir-Baruch B, Nieh PT, Master VA, Halkar RK, Rossi PJ, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259(3):852–61. doi:10.1148/radiol.11102023.
Turkbey B, Mena E, Aras O, Garvey B, Grant K, Choyke PL. Functional and molecular imaging: applications for diagnosis and staging of localised prostate cancer. Clin Oncol (R Coll Radiol). 2013;25(8):451–60. doi:10.1016/j.clon.2013.05.001.
Kairemo K, Rasulova N, Partanen K, Joensuu T. Preliminary clinical experience of trans-1-amino-3-(18)F-fluorocyclobutanecarboxylic acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. Biomed Res Int. 2014;2014:305182. doi:10.1155/2014/305182.
Okudaira H, Oka S, Ono M, Nakanishi T, Schuster DM, Kobayashi M, et al. Accumulation of trans-1-amino-3-[(18)F]fluorocyclobutanecarboxylic acid in prostate cancer due to androgen-induced expression of amino acid transporters. Mol Imaging Biol. 2014;16(6):756–64. doi:10.1007/s11307-014-0756-x.
McConathy J, Yu W, Jarkas N, Seo W, Schuster DM, Goodman MM. Radiohalogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Med Res Rev. 2012;32(4):868–905. doi:10.1002/med.20250.
McConathy J, Voll RJ, Yu W, Crowe RJ, Goodman MM. Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Appl Radiat Isot. 2003;58(6):657–66.
Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69(3):428–35. doi:10.1016/j.eururo.2015.06.046.
Talab SS, Preston MA, Elmi A, Tabatabaei S. Prostate cancer imaging: what the urologist wants to know. Radiol Clin N Am. 2012;50(6):1015–41. doi:10.1016/j.rcl.2012.08.004.
Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179(3):906–10. doi:10.1016/j.juro.2007.10.059.
Ferguson JK, Oesterling JE. Patient evaluation if prostate-specific antigen becomes elevated following radical prostatectomy or radiation therapy. Urol Clin North Am. 1994;21(4):677–85.
Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, et al. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer patients with very low PSA level <0.5 ng/mL. Clin Nucl Med. 2013;38(9):342–5. doi:10.1097/RLU.0b013e31829af913.
Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–95. doi:10.1016/j.crad.2007.05.022.
Zaorsky NG, Raj GV, Trabulsi EJ, Lin J, Den RB. The dilemma of a rising prostate-specific antigen level after local therapy: what are our options? Semin Oncol. 2013;40(3):322–36. doi:10.1053/j.seminoncol.2013.04.011.
Bouchelouche K, Turkbey B, Choyke P, Capala J. Imaging prostate cancer: an update on positron emission tomography and magnetic resonance imaging. Curr Urol Rep. 2010;11(3):180–90. doi:10.1007/s11934-010-0105-9.
Oyama N, Miller TR, Dehdashti F, Siegel BA, Fischer KC, Michalski JM, et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med. 2003;44(4):549–55.
Avanesov M, Karul M, Derlin T. (68)Ga-PSMA as a new tracer for evaluation of prostate cancer: comparison between PET-CT and PET-MRI in biochemical recurrence. Radiologe. 2015;55(2):89–91. doi:10.1007/s00117-014-2792-6.
Treglia G, Ceriani L, Sadeghi R, Giovacchini G, Giovanella L. Relationship between prostate-specific antigen kinetics and detection rate of radiolabelled choline PET/CT in restaging prostate cancer patients: a meta-analysis. Clin Chem Lab Med. 2014;52(5):725–33. doi:10.1515/cclm-2013-0675.
Chen J, Zhao Y, Li X, Sun P, Wang M, Wang R, et al. Imaging primary prostate cancer with 11C-choline PET/CT: relation to tumour stage, Gleason score and biomarkers of biologic aggressiveness. Radiol Oncol. 2012;46(3):179–88. doi:10.2478/v10019-012-0034-y.
Cimitan M, Evangelista L, Hodolic M, Mariani G, Baseric T, Bodanza V, et al. Gleason score at diagnosis predicts the rate of detection of 18F-choline PET/CT performed when biochemical evidence indicates recurrence of prostate cancer: experience with 1,000 patients. J Nucl Med. 2015;56(2):209–15. doi:10.2967/jnumed.114.141887.
Okotie OT, Aronson WJ, Wieder JA, Liao Y, Dorey F, De KJ, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol. 2004;171(6 Pt 1):2260–4.
Nanni C, Schiavina R, Brunocilla E, Boschi S, Borghesi M, Zanoni L, et al. 18F-Fluciclovine PET/CT for the detection of prostate cancer relapse: a comparison to 11C-choline PET/CT. Clin Nucl Med. 2015;40(8):e386–91. doi:10.1097/rlu.0000000000000849.
Johnstone PA, Tarman GJ, Riffenburgh R, Rohde DC, Puckett ML, Kane CJ. Yield of imaging and scintigraphy assessing biochemical failure in prostate cancer patients. Urol Oncol. 1997;3(4):108–12.
Schuster DM, Taleghani PA, Nieh PT, Master VA, Amzat R, Savir-Baruch B, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F] -fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am J Nucl Med Mol Imaging. 2013;3(1):85–96.
Turkbey B, Mena E, Shih J, Pinto PA, Merino MJ, Lindenberg ML, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology. 2014;270(3):849–56. doi:10.1148/radiol.13130240.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668–74. doi:10.2967/jnumed.115.154153.
Acknowledgments
We acknowledge the significant contributions of Rianot Amzat, MD, Pooneh Taleghani, MD, Delicia Votaw, CNMT, Fenton G. Ingram, RT(R), CNMT, PET, Seraphinah Lawal, RT(R), CNMT, PET, Adam Brown, RT(N), CNMT, Ronald J. Crowe, RPh, BCNP, and the entire cyclotron and synthesis team from the Emory Center for Systems Imaging, Beverly Hunter, RN, Michelle Faurot, BS, James R. Galt, PhD, John R. Votaw, PhD, our research nurse Leah-Madge Bellamy, RN, MSN, and Pardeep Mittal, MD, who provided professional and technical information on conventional imaging protocols.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by a grant from the National Institutes of Health (R01CA129356) and by the Georgia Cancer Coalition.
Additional information
Oluwaseun A. Odewole and Funmilayo I. Tade are co-first authors.
Rights and permissions
About this article
Cite this article
Odewole, O.A., Tade, F.I., Nieh, P.T. et al. Recurrent prostate cancer detection with anti-3-[18F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 43, 1773–1783 (2016). https://doi.org/10.1007/s00259-016-3383-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3383-8