Abstract
Objective
To develop and compare pediatric upper airway three-dimensional normative values using the two most commonly used cone beam computed tomography (CBCT) software: Invivo5 (fixed threshold) and Dolphin 3D (interactive threshold).
Study design
Out of 3738 CBCT scans, scans of 81 pediatric patients were utilized after applying strict exclusion criteria. The sample was grouped into two age groups (7–11 and 12–17 years). Intra-class correlation coefficient was used to test intra-rater and inter-rater reliability and showed coefficients greater than 0.9 indicating good reliability of the methods used.
Results
Paired t tests showed that volumetric and area measurements obtained using Dolphin 3D were significantly larger than those obtained using Invivo5 (p < 0.05). The mean minimal cross-sectional areas (MCSA) for Dolphin 3D were 151 mm2 and 177 mm2 for age groups 1 and 2, respectively. The mean MCSA values for Invivo5 for age groups 1 and 2 were 120 mm2 and 145 mm2, respectively.
Conclusion
Pediatric upper airway volumetric, area, and linear measurements were reported after applying strict exclusion criteria including a validated sleep questionnaire. Our goal is that clinicians utilize the proposed-here normative values for screening and assist in the timely diagnosis and management of pediatric sleep apnea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Airway volume is increasingly of interest in the orthodontic field because it relates to craniofacial growth and development and responses to orthodontic treatment [1, 2]. The prevalence of pediatric obstructive sleep apnea (OSA) is on the rise especially with the increase in childhood obesity [3]. Orthodontists are in a unique position to assess the upper airway. Since orthodontists acquire radiographic records and screen children on a daily basis, they can recognize individuals who are at risk of OSA [2, 3]. Finding screening tools for timely diagnosis and management of pediatric OSA is important and may prevent associated comorbidities [4].
Different three-dimensional (3D) imaging modalities are available to investigate airway morphology and the surrounding soft tissues. This allows for the quantification of volumetric, area, and linear measurements [5]. Nowadays, the use of cone beam computed tomography (CBCT) is increasing in orthodontic practice due to its 3D diagnostic ability with the continued reduction in cost and radiation exposure [6].
Several factors influence upper airway analysis when using CBCT; airway segmentation technique is one of these factors [7, 8]. During airway segmentation, an appropriate threshold value is selected, which controls the degree of airway filling. The threshold value can either be fixed or interactive. A fixed threshold technique uses one threshold value for all patients. This single threshold value can be determined either manually by the operator [7, 9], or automatically by software algorithms [8]. On the other hand, an interactive threshold technique involves the operator choosing a threshold value for each patient. This is based on visual inspection of the airway in all 3D views for that specific patient [8]. Weissheimer et al. showed a statistically significant difference between measurements obtained using software programs that use different threshold techniques [8].
When analyzing anatomical structures, it is necessary to have normative values to define a deviation and to distinguish abnormalities. To develop normative values, a representative subgroup of the population should be selected [10]. Pediatric 3D upper airway normative values are lacking since most upper airway and sleep studies are conducted in adults [3]. The aims of this study were (1) to develop 3D upper airway normative values using two commonly used CBCT software in pediatric patients between the ages of 7 and 17, and (2) to compare pediatric 3D upper airway normative values measured using CBCT software that employs a fixed threshold technique or an interactive threshold technique.
Materials and methods
The sample utilized in this retrospective study consisted of CBCT scans obtained from two private orthodontic offices in Green Bay, Wisconsin. The two offices were operated by the same clinician and the CBCT scans were taken as part of routine initial diagnostic records. The inclusion criteria were being between 7 and 17 years of age and being Caucasian. Exclusion criteria were as follows: (1) craniofacial anomalies, (2) previous orthodontic treatment of any kind, (3) history of tonsillectomy or adenoidectomy, (4) posterior cross-bite, (5) CBCT scans not showing porion or the root of clivus superiorly, or the base of the epiglottis inferiorly, (6) ANB angle > 4.9 or < 0, (7) Frankfort mandibular plane angle (FMA) < 20 or > 29.9, (8) obesity based on body mass index (BMI) z score > 2 [11], and (9) pediatric sleep questionnaire (PSQ) score of 8 and above [12]. The University of Illinois at Chicago institutional review board reviewed and approved the study. Of a total of 3738 patient records that were reviewed, 81 met the eligibility criteria and were analyzed. The scans were divided into two groups based on age (7–11 and 12–17 years of age) to reflect different stages of pubertal growth.
All scans were taken with the same machine (iCAT FLX; Imaging Sciences International, Hatfield, Pa) following a standardized protocol (120 kV, 18.54 mAs, 0.3 mm voxel, 16 × 13 cm field of view, and 4.8–8.9 s scanning time). The machine has a chair and head support for scanning patients in an upright position. An incorporated mirror and a laser beam light are used to orient patients and ensure that the scan is obtained in natural head position. Also, patients were instructed to breathe lightly through the nose, rest the tongue in a relaxed position touching the upper front teeth, bite in maximum intercuspation which is an easily reproducible position for the mandible, avoid swallowing, and avoid any movement.
Two software programs were used to upload, re-orient, analyze, and obtain measurements. The first was Dolphin 3D (version 11.7, Dolphin Imaging, Chatsworth, CA), which employs an interactive threshold technique, and the second was Invivo5 (Version 5.4, Anatomage Inc, San Jose, CA), which employs a fixed threshold technique. Key differences between software programs are listed in Table 1. All measurements were performed by a single examiner.
Dolphin 3D
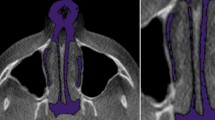
In Dolphin 3D, the scans were uploaded and re-oriented to a modified Frankfort horizontal (FH) axial plane determined by three landmarks: right porion, right orbitale, and left orbitale and checked in three views similar to previous reports [7]. On the right sagittal view, the horizontal reference line passed through porion and the right orbitale. On the frontal view, the horizontal reference line passed through the right and left orbitale, and the vertical reference line passed though Nasion (N) and the anterior nasal spine (ANS) perpendicular to the horizontal reference line. On the transverse view with the nose pointing downwards, the vertical reference line passed through crista galli and basion (BA) (Fig. 1).
Dolphin 3D skull orientation, right sagittal view (left), frontal view (middle), and transverse view (right). Images rendered using Dolphin (http://dolphinimaging.com)
Once the scans were re-oriented, the airway was defined. The airway was assessed from the root of the clivus superiorly to the base of the epiglottis inferiorly, and from PNS anteriorly to the pharyngeal wall posteriorly and laterally. The airway was divided into three subregions: nasopharynx (NP), oropharynx (OP), and hypopharynx (HP). Table 2 and Fig. 2 show the technical limits for each subregion adopted from the limits proposed by Guijarro-Martinez and Swennen [7]. The root of clivus was identified by the ventral end of the spheno-occipital synchondrosis. In younger subjects, when there was a gap at clivus, the lower margin of this gap was chosen.
Dolphin 3D technical limits of the nasopharyngeal (left), oropharyngeal (middle), and hypopharyngeal (right) airway subregions used during three-dimensional airway analysis. Images rendered using Dolphin (http://dolphinimaging.com)
Dolphin 3D uses semi-automatic segmentation, so after the technical limits were identified, a “seed” point was placed to identify the airway. Additional seed points were placed as needed based upon visual inspection in the sagittal, frontal, and transverse views. Regions that often needed additional seed points included the region of the nasal septum, the region anterior to the uvula, the epiglottis region, and the palatine tonsillar region if the tonsils were touching. An interactive thresholding technique was used with one threshold value assigned per subject. In an attempt to standardize airway sensitivity threshold values in 3D analysis, we decided to confine it to a range [7, 9]. The threshold value was kept between 40 and 80 selecting maximum airway volume yet limiting the noise (Fig. 3). To confirm that the appropriate sensitivity was selected, the primary investigator assessed airway slices in the sagittal, frontal, and transverse views. The threshold chosen in the nasopharynx was applied to the oropharynx and hypopharynx.
Dolphin 3D axial views showing underflow (left), overflow (middle) and adequate flow (right) during three-dimensional airway threshold selection. Images rendered using Dolphin (http://dolphinimaging.com)
In terms of volumetric and area measurements, Dolphin 3D calculated the nasopharyngeal volume (NPV), the oropharyngeal volume (OPV), the oropharyngeal minimum cross-sectional area (OCSA), the hypopharyngeal volume (HPV), and the hypopharyngeal minimum cross-sectional area (HCSA). The minimum cross-sectional area was not calculated in the nasopharynx due to extreme variability and the fact that the nasopharynx looks like a pyramid rather than a tube. The total airway volume was calculated by adding the NPV, OPV, and HPV. OCSA and HCSA were identified in the sagittal view. Superior and inferior limits were selected using two horizontal red lines, which were placed 0.5 mm away from the upper and lower limits of the subregion to avoid locating OCSA or HCSA at the limits which can cause errors (Fig. 4). Once the OCSA and HCSA were recorded, those 2 measurements were compared and the smaller of the two was deemed the minimum cross-sectional area (MCSA) and its location was recorded.
Dolphin 3D horizontal red lines placed 0.5 mm away from the superior and inferior limits of the oropharynx during oropharyngeal minimum cross-sectional area measurement. Images rendered using Dolphin (http://dolphinimaging.com)
The sagittal and transverse dimensions of the MCSA were measured using the 2D line measuring function in the axial view. The sagittal 2D measuring line was defined by two points centered in MCSA transversely, one point anteriorly and one posteriorly. Care was taken to make sure this line was perpendicular to the coronal plane. The transverse 2D measuring line was defined by 2 points centered in the MCSA sagitally, one point on the right border and another on the left. Care was taken to make sure this line was perpendicular to the sagittal 2D measuring line. Both dimensions were recorded in millimeters by the software (Fig. 5). If there were 2 separate areas for the MCSA, one in front of the other, the sagittal dimensions of both areas were added but only the transverse dimension of larger area was recorded (Fig. 6). If the 2 separate areas for the MCSA were next to each other, side by side, the transverse dimensions of both areas were added but only the sagittal dimension of the larger area was recorded.
Dolphin 3D axial view showing the sagittal and transverse dimensions of the minimum cross-sectional area. Image rendered using Dolphin (http://dolphinimaging.com)
Dolphin 3D axial view showing the sagittal and transverse dimensions of the minimum cross-sectional area (MCSA) when there are 2 separate areas, one in front of the other. Image rendered using Dolphin (http://dolphinimaging.com)
Finally, the total airway length was measured from the upper limit of the nasopharynx (the root of the clivus) to the base of the epiglottis in the sagittal view. Care was taken to make sure the 2D measuring line was made perpendicular to FH (Fig. 7). In some younger subjects, the lower border of the oropharynx (anterior inferior border of C3) was inferior to the base of the epiglottis. In those subjects, the lower border of the oropharynx was set at the base of the epiglottis, where our airway analysis ended, and no hypopharyngeal measurements were performed nor were values given to HPV or HCSA (Fig. 8).
Dolphin 3D sagittal view showing the total airway length measured from the root of the clivus to the base of the epiglottis. Images rendered using Dolphin (http://dolphinimaging.com)
A young subject where the lower border of the oropharynx was more inferior to the base of the epiglottis. In this subject, the lower border of the oropharynx was set at the base of the epiglottis and no values were given to HPV or HCSA. Images rendered using Dolphin (http://dolphinimaging.com)
Invivo5
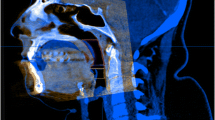
In Invivo5 the orientation technique was similar to that of Dolphin 3D (Fig. 9). For standardization, the default sagittal slice (50% clipping, Level/Brightness: 1650/0.00, Window/Contrast: 3300/0.00) was used to perform all the measurements in all scans. Unlike Dolphin 3D, no technical limits are needed to segment the airway, rather points are placed along the airway. These points form circular planes that act as limits to airway overflow superiorly and inferiorly. No threshold adjustment was used since Invivo5 employs a fixed threshold technique.
Similar to what was carried out in Dolphin 3D, the airway was divided into three subregions: NP, OP, and HP (Fig. 10). The NP limits were identified by placing points along the nasopharyngeal airway path. The first point formed a circular plane which acted as the anterior and superior limits of the nasopharynx. When placing this plane perpendicular to the modified FH axial plane, unwanted overflow occurred to the nasal cavity and surrounding areas which lead to an overestimation of the NPV (Fig. 11). To avoid such overestimation, this plane was rotated in a counterclockwise direction to extend from PNS to sella. This plane acted as the anterior superior limit of the NP. Other points were placed along the path of the nasopharynx with the last point forming a circular plane at the level of PNS parallel to the modified FH axial plane representing the inferior limit of the NP. The NPV was then automatically calculated.
The OP limits were identified superiorly with a circular transverse plane parallel to the modified FH axial plane passing through PNS, and inferiorly with a circular transverse plane parallel to the modified FH axial plane passing through the anterior inferior point of the body of C3. The HP limits were identified superiorly with a transverse circular plane parallel to the modified FH axial plane passing through the anterior inferior point of the body of C3, and inferiorly with a circular transverse plane parallel to the modified FH axial plane at the level of the base of the epiglottis. The software automatically calculated OPV, OCSA, HPV, and HCSA. Once the OCSA and HCSA were recorded, those 2 measurements were compared and the smaller of the two was deemed the MCSA and its location was recorded. The software also automatically calculated the sagittal and transverse dimension of each cross-sectional area by AP (anterior-posterior) and RL (right–left) lines which were perpendicular to each other (Fig. 12). Finally, the total airway length was measured on the default sagittal view, using a vertical line perpendicular to FH defined by two points extending from the root of clivus superiorly to the base of the epiglottis inferiorly (Fig. 13).
Statistical analysis
IBM SPSS Statistics for Windows, version 22.0 (Armonk, NY: IBM Corp.) was used for statistical analysis. To test reliability of the measurements used, 10 scans were analyzed using both software programs and all measurements were registered. 2 weeks later, measurements were repeated under the same conditions by the primary investigator, to establish intra-rater reliability, and by a co-investigator, to establish inter-rater reliability. Intra-class correlation coefficient (ICC) was used to test reliability. The distribution of the raw data was investigated using Shapiro–Wilk test of normality. Most data were normally distributed (p > 0.05) and parametric tests were used. Statistics were performed for the two age groups separately to show the corresponding normative values. Paired t test was performed to test the mean difference between variables measured using Dolphin 3D and Invivo5. Pearson correlation was used to test the association between age and different variables. When evaluating the strength of correlation, the following classification was used: strong if 0.7 < Ι r Ι ≤ 1.0, moderate if 0.4 ≤ Ι r Ι ≤ 0.7, and weak if 0.2 < Ι r Ι < 0.4. Statistical significance was set at α = 0.05.
Results
The total sample size was 81 subjects with a mean age of 12.1 (± 2.6) years. In age group 1 (7–11 years), there were 36 subjects with a mean age of 10.3 (± 1.3) years (12 males and 24 females), while in age group 2 (12–17 years) there were 45 subjects with a mean age of 14.1 (± 1.3) years (20 males and 25 females). The mean PSQ score was 2.0 (± 1.8) positive answers, and the mean BMI z score was 0.5 (± 0.9). ICC was higher than 0.9 for all the variables with 95% confidence intervals ranging from 0.80 to 0.99 for Dolphin 3D, and 0.632 to 0.99 for Invivo5 indicating good reliability of the methods used.
Dolphin 3D threshold values ranged from 40 to 60, with a mean of 47.00 (± 4.51). Tables 3 and 4 show normative upper airway values for both software programs for age groups 1 and 2, respectively. Results of paired t tests to compare between Dolphin 3D and Invivo5 mean values are shown in Table 5. MCSA locations were similar using both Dolphin 3D and Invivo5 software programs. MCSA was located in the oropharynx in 72 subjects (88.9%) and the hypopharynx in 9 subjects (11.1%). Correlation coefficients between age and different upper airway variables are displayed in Table 6.
Discussion
According to Merriam-Webster Collegiate Dictionary, normality is defined as “a state or form conforming to natural order or law and having a typical structure”. In this study, with a total sample size of 81, we attempted to develop pediatric 3D normative values for upper airway evaluation using two CBCT software programs. The sample size when developing orthodontic normative values varies in the literature. Some of the most prominent analyses in the field of orthodontics belong to Steiner, Downs, Tweed, and Sassouni who used 1, 20, 95, and 100 subjects, respectively [10]. Aboudara et al. [13] and Kim et al. [14] evaluated 3D airway measures in pediatric subjects with normal skeletal patterns using sample sizes of 11 and 14, respectively. Schendel et al. used a larger sample size with 474 subjects under the age of 20 years. However, they did not include the nasopharynx in their analysis [15]. The nasopharynx is very important to consider in children where adenotonsillectomy is often recommended as the first line of treatment [3, 16]. Additionally, none of above-mentioned pediatric 3D airway studies used a method to exclude sleep-disordered breathing such as a sleep study or a sleep questionnaire as was used in the present study. Certainly using a sleep study would have been the gold standard but it would be very costly.
Tables 3 and 4 show that values were larger in age group 2 compared to age group 1. Additionally, Table 6 shows statistically significant correlations between almost all measurements and age. The findings confirm what has been reported by Schendel et al. that airway size and length continue to increase until the age of 20 [15]. Authors have stated that during growth spurts, faces grow vertically, and the pharyngeal airway increases in the vertical dimension [17]. In Table 6, the total airway length had the highest correlation coefficient (Dolphin 3D, r = 0.703; Invivo5, r = 0.683) which is in line with this statement.
A previous study showed an underestimation of the airway volume when both fixed and interactive threshold techniques were used compared to the actual physical volume of the oropharynx acrylic phantom which was considered the gold standard. However, software programs with an interactive threshold, i.e., Dolphin 3D, had a smaller error difference and larger volumes compared with software programs that used fixed threshold, i.e., Invivo5 [8]. Similarly, in our study, Table 5 shows a statistically significant difference in all volumetric and area measurements between Dolphin 3D and Invivo5, with larger measurements produced by Dolphin 3D. Interactive threshold was used in Dolphin 3D and the operator manually increased the threshold to include all airway extensions yet limiting the noise. Accordingly, a linear measurement not dependent on threshold (i.e., total airway length) would not be expected to show significant difference between the two software programs which was the case as can be seen in Table 5 (p = 0.075).
Many researchers choose to exclude the NPV from their analysis due to its complexity [3, 15]. The NPV might not be critical in adults but, as previously mentioned, is very much so in children. Unlike Dolphin 3D, Invivo5 does not allow drawing borders. This led to overflow and overestimation of NPV when using Invivo5 with the same limits as Dolphin 3D (Fig. 11). To overcome this issue, a different anterior limit was used for Invivo5 (Table 1) which evidently produced a smaller NPV with Invivo5. Moreover, because of the difference in the anterior limit we could not properly compare our Invivo5 NPV values to other values in the literature. Finally, because of the pyramidal shape of the nasopharynx, the cross-sectional area was not recorded in this subregion.
Three-dimensional airway analysis using CBCT requires a large field of view. This exposes the patient to more radiation compared to the more conventional, yet limited, 2D airway analysis using cephalometric images. This might be the reason why the literature lacks 3D airway studies in children especially with regard to normative values [3]. Nonetheless, Kim et al. calculated the NPV in 14 skeletal class I patients with a mean age of 11 years and found an NPV of 2620.8 mm3 [14]. The mean NPV when using Dolphin 3D in the current study for age group 1 was larger at 3723.4 mm3. Aboudara et al. measured the NPV in 11 normal children with a mean age of 12.63 years. They reported a mean NPV of 3845.9 mm3 [13]. The mean NPV when using Dolphin 3D in the current study for age group 2 was also larger at 5061.6 mm3. It is important to note that no sleep questionnaire or sleep study was used in both studies and some of these patients may have had symptoms or even undiagnosed OSA.
Li et al. reported a statistically significant yet weak correlation between the MCSA in adults and the respiratory disturbance index [18]. Ogawa et al. and Avarhami and Englender measured the MCSA in non-OSA adults documented by a sleep study and reported averages of 147 mm2 and 174 mm2, respectively [19, 20]. Schendel et al. measured the MCSA in normal children, but as previously mentioned no sleep study or sleep questionnaire was used to rule out sleep-disordered breathing. They reported an MCSA of 83 mm2 for subjects aged 6–11 and an MCSA of 149 mm2 for subjects aged 12–17. Furthermore, they reported little to no change in the MCSA after the age of 14 until the age of 40 [15]. In the present study, a sleep questionnaire was used in an attempt to rule out sleep-disordered breathing. The mean MCSA for Dolphin 3D was 151 mm2 and 177 mm2 for age groups 1 and 2, respectively. The mean MCSA for Invivo5, respectively, for age groups 1 and 2 was 120 mm2 and 145 mm2. From the above, we can infer that the MCSA in pediatric subjects, especially in age group 2, is not very different from what has been reported in non-OSA adults.
In adults, measurements for MCSA in OSA patients have been published but not for NPV. To our knowledge, measurements for MCSA or NPV do not exist for pediatric patients with OSA. In the present study, we propose 3D normative values for pediatric patients and a future study should evaluate these 3D upper airway measures in pediatric patients with OSA. Such a study might help validate the use of CBCT for OSA screening in pediatric patients, especially those who already have CBCT scans taken for other purposes such as orthodontic treatment.
Conclusion
Pediatric upper airway volumetric, area, and linear measurements were reported after applying strict exclusion criteria including a validated sleep questionnaire. Comparing the two CBCT software programs, volumetric and area measurements were significantly larger when using an interactive threshold technique used in Dolphin 3D compared to a fixed threshold technique used in Inivo5. Our goal is that clinicians utilize the proposed-here upper airway normative values for screening and assist in the timely diagnosis and management of pediatric sleep apnea.
References
Aboudara C, Nielsen I, Huang JC, Maki K, Miller AJ, Hatcher D. Comparison of airway space with conventional lateral headfilms and 3-dimensional reconstruction from cone-beam computed tomography. Am J Orthod Dentofac Orthop. 2009;135(4):468–79.
Pliska B, Lowe AA, Almeida FR. The orthodontist and the obstructive sleep apnea patient. Int J Orthod. 2012;23(3):19–22.
Masoud AI, Jackson GW, Carley DW. Sleep and airway assessment: a review for dentists. Cranio. 2017;35(4):206–22.
Massicotte C, Al-Saleh S, Witmans M, Narang I. The utility of a portable sleep monitor to diagnose sleep-disordered breathing in a pediatric population. Can Respir J. 2014;21(1):31–5.
El H, Palomo JM. Measuring the airway in 3 dimensions: a reliability and accuracy study. Am J Orthod Dentofac Orthop. 2010;137(4 Suppl):S50 e1–9 (discussion S-2).
Garib DG, Calil LR, Leal CR, Janson G. Is there a consensus for CBCT use in Orthodontics? Dental Press J Orthod. 2014;19(5):136–49.
Guijarro-Martinez R, Swennen GR. Three-dimensional cone beam computed tomography definition of the anatomical subregions of the upper airway: a validation study. Int J Oral Maxillofac Surg. 2013;42(9):1140–9.
Weissheimer A, Menezes LM, Sameshima GT, Enciso R, Pham J, Grauer D. Imaging software accuracy for 3-dimensional analysis of the upper airway. Am J Orthod Dentofac Orthop. 2012;142(6):801–13.
Alves M Jr, Baratieri C, Mattos CT, Brunetto D, Fontes Rda C, Santos JR, et al. Is the airway volume being correctly analyzed? Am J Orthod Dentofac Orthop. 2012;141(5):657–61.
Athanasiou AE. Orthodontic cephalometry. London: Mosby-Wolfe; 1995.
Flegal KM, Ogden CL. Childhood obesity: are we all speaking the same language? Adv Nutr. 2011;2(2):159S–66S.
Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32.
Aboudara CA, Hatcher D, Nielsen IL, Miller A. A three-dimensional evaluation of the upper airway in adolescents. Orthod Craniofac Res. 2003;6(Suppl 1):173–5.
Kim YJ, Hong JS, Hwang YI, Park YH. Three-dimensional analysis of pharyngeal airway in preadolescent children with different anteroposterior skeletal patterns. Am J Orthod Dentofac Orthop. 2010;137(3):306 e1–11 (discussion 7).
Schendel SA, Jacobson R, Khalessi S. Airway growth and development: a computerized 3-dimensional analysis. J Oral Maxillofac Surg. 2012;70(9):2174–83.
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–55.
Shprintzen RJ. The origin of speech ease: evolution of the human upper airway and its functional implications for obstructive sleep apnea. Editorial commentary: the great leap forward: the anatomic basis for the acquisition of speech and obstructive sleep apnea by Terence M. Davidson. Sleep Med. 2003;4(3):171–3.
Li HY, Chen NH, Wang CR, Shu YH, Wang PC. Use of 3-dimensional computed tomography scan to evaluate upper airway patency for patients undergoing sleep-disordered breathing surgery. Otolaryngol Head Neck Surg. 2003;129(4):336–42.
Avrahami E, Englender M. Relation between CT axial cross-sectional area of the oropharynx and obstructive sleep apnea syndrome in adults. AJNR Am J Neuroradiol. 1995;16(1):135–40.
Ogawa T, Enciso R, Shintaku WH, Clark GT. Evaluation of cross-section airway configuration of obstructive sleep apnea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(1):102–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masoud, A.I., Alwadei, F.H., Alwadei, A.H. et al. Developing pediatric three-dimensional upper airway normative values using fixed and interactive thresholds. Oral Radiol 36, 89–99 (2020). https://doi.org/10.1007/s11282-019-00384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-019-00384-3