Abstract
The demand for environment-friendly cleanup techniques has arisen due to an increase in environmental pollutants. Fungi is the most prevalent and effective class of heavy metal-resistant microorganisms with the ability to leach metals. The objective of the present study was to isolate the fungi from the agricultural soil of Kashmir valley, investigate their multi-metal tolerance to heavy metals and evaluate the metal uptake capacities of the resistant fungi. The fungi were isolated and identified on the basis of morphological and molecular approach (ITS1 and ITS4). The tolerance limits of the isolated fungal strains to various doses of lead (Pb), cadmium (Cd), zinc (Zn), chromium (Cr), copper (Cu), nickel (Ni), and cobalt (Co) was evaluated. Five fungal strains, Aspergillus niger, Fusarium oxysporum, Fusarium verticillioides, Aspergillus fischeri, Epicoccum mackenziei were isolated from the soil samples. To the best of our knowledge, this is the first report on the study of metal resistance of Aspergillus fischeri and Epicoccum mackenziei. Among the identified fungal species, Aspergillus niger and Fusarium oxysporum were found to be most tolerant with a minimum inhibitory concentration (MIC) of 600 ppm against Cu and Cr respectively. Results indicated removal of considerable amount of heavy metals by some of the fungi. The highest metal uptake of 8.31 mg/g was found in Fusarium verticillioides for Zn. Surprisingly, these fungal strains demonstrated resistance to metal concentrations above the levels that are universally acceptable for polluted soils, and hence prove to be appealing contenders for use as bioremediation agents for cleaning up heavy metal-polluted environments.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental contamination has reached unprecedented levels in recent decades as a result of rapid industrialization, hazardous farming practices and increasing human activity. Metal poisoning of the soil is one of the most serious side effects of industrialisation (Amin et al. 2021). The existence of the harmful and non-biodegradable metals in our environment has always been a source of great concern (Uqab et al. 2022). Industrial wastes frequently contain heavy metals, such as Cd, Cu, Hg, Ni, Cr, Zn, Pb, and others, which leak into the environment and damage the ecology. In the past, a variety of methods, including ion exchange and precipitation, have been used to remove heavy metals from wastewater and soil (Kavita and Keharia 2012). There are several drawbacks to these methods, such as the ineffectiveness in removing heavy metals and possibility of chemical deterioration over time (Simonescu and Ferdes 2012). Moreover, it is much difficult to remove heavy metals from the soil and as such, biological approach is helpful in such circumstances. Thus, a procedure called as bioremediation uses microorganisms to detoxify specific metals and stop further contamination (Uqab et al. 2016). Microbes can endure metal stress due to their distinctive metal resistance capability (Birch and Bachofen 1990). Metal resistance is the capacity of an organism to survive metal toxicity by means of a mechanism that was developed in direct reaction to the metal species in question. Metal-tolerant microorganisms are typically found in abundance at locations contaminated by metals (Gadd and white 1993). Microorganisms have a significant role in metal reactivity. Microorganisms that can absorb contaminants include bacteria, fungi, yeast, algae, and many others. Among these microorganisms, fungi are recognised for their superior ability to synthesise a diverse array of extracellular proteins, enzymes, and organic acids that facilitate the sequestration of metals (Vaksmaa et al. 2023).
Fungi are common in subsurface and subaerial ecosystems, and they play a major role in habitats that are contaminated with metals, forming a key consortium. Fungi have the ecological and biochemical ability to break down environmental toxins and lower the dangers related to metals, metalloids, and radionuclides by modifying or regulating chemical bioavailability. Furthermore, fungi are perfect candidates for bio-remediation (also known as mycoremediation) because of their weak catabolic enzyme specificity, ability to form wide mycelial networks, and non-reliance on contaminants as a growth substrate (Akpasi et al. 2023). Numerous mechanisms, including active transport of metal ions outside the cell, chelation to mask metals, enzymatic transformation of metal ions, formation of vacuoles where metal ions are gathered and immobilized in the form of polyphosphates, increased production of melanin and other pigments, and production of specific metal binding compounds inside the cell, contribute to the development of fungal resistance to heavy metals (Balamurugan et al. 2006). Because of their easy synthesis process, low cost, and capacity to bioaccumulate metals, fungi are also becoming a focal point of study in the production of biological metal nanoparticles (Nazir et al. 2021). Numerous fungal species, including Penicillium and Aspergillus, have been documented to mycoremediate metals such as Cd, Cr, Cu, Ni, etc. (Congeevaram et al. 2007; Kumar et al. 2011). Similar bioremediation processes involving Aspergillus, Trichoderma, Rhizopus, and Fusarium have been reported elsewhere (Sim et al. 2016; Puglisi et al. 2012). Researchers are working to comprehend the remediation mechanism of different microorganisms. Numerous studies describe that microorganisms possess the capacity to either biosorb or bioaccumulate metals on their surfaces. Few research findings have also described the involvement of genes in imparting metal tolerance ability to different fungi (Gonen and Aksu, 2009). However, there are still many fungal strains which have not been explored for metal resistance capabilities.
Jammu & Kashmir is predominantly an agrarian economy with most of its inhabitants involved in agriculture and allied sectors. It is regarded as the largest industry of Kashmir region (India). The agricultural soil of Budgam district of Kashmir is surrounded by large number of brick kilns due to which the land is under tremendous pressure of heavy metals. Thus, the present study was designed to isolate fungi from the contaminated soil of the agricultural fields of Kashmir valley for the first time. This study further evaluated the tolerance levels of isolated fungi against toxic metals (Cd, Cu, Pb, Cr, Ni, Zn, Co) and determined their metal uptake capacities. It was assumed that screening for metal-tolerant fungi would result in new strains which have not been previously studied for metal resistance. Our hypothesis for choosing fungi from polluted areas is that the microorganisms in particular environments develop survival strategies to adapt to their surroundings, thus utilizing the capacity of these fungi for sequestration could be used as a bioremediation technique in future and expand our knowledge about mycoremediation science.
Materials and methods
Study site description
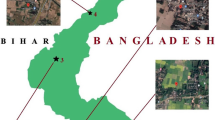
The agricultural region of Budgam district of Jammu and Kashmir was selected because of its intensive agricultural practices and proximity to a large number of brick kilns that discharge heavy metal containing effluents, which causes a notable build-up of metals in the surrounding soil. The soil has a loamy texture and is naturally rich in organic matter. Owing to the region’s climatic conditions, the soil is often acidic in nature, appropriate for a variety of crops including wheat, rice and fruits. Some areas grow vegetables to a large extent and few areas have also been exploited for saffron cultivation. Five different locations (Fig. 1) were selected according to the degree of influence for the collection of composite soil sample taken at a depth of 10–15 cm.
Soil sampling
Soil samples were collected from the designated places for a period of two years (2019–2020). Three soil samples were collected at each site in equal proportions (10 g each) using a sterile spoon, mixed systematically and pooled for the isolation of fungi by using the standard protocol (Uqab et al. 2022). For the following step of processing, the sample was taken to the Microbiology Research Laboratory, CORD, University of Kashmir. The samples were homogenised after being crushed in a mortar in sterile conditions (APHA 2017).
Culture and isolation
To make a soil suspension, 5 gram of soil sample was mixed with 10 ml of sterile normal saline and vortexed for 5 min. Each sample was serially diluted up to 104 times. Next, using the direct plating method, 0.1 ml of the inoculum from the serial dilution tubes was disseminated on petri plates with Potato Dextrose Agar (PDA) (APHA 2017). The Petri plates were incubated for 5–7 days at 25-30o C. In order to obtain pure cultures, the separated colonies were transferred to newly prepared PDA plates.
Morphological identification of fungal isolates
The pure fungal isolates were identified based on the macroscopic and microscopic features of preparations stained with Lacto phenol cotton blue (LCB) staining using direct microscopy (Olympus IX 71 Florescence microscope). Following that, the plates were examined for several cultural characteristics, including colour, topography, texture, and pattern on media plates. Macroscopic examination of the isolated fungal species was studied following the monographs for fungal identification (Domsch et al. 1980; Watanabe 2002).
Molecular identification and sequencing of fungal isolates
The fungal mycelia were used to extract genomic DNA directly using a HiMedia Genomic DNA Purification kit. To examine the separated DNA, an agarose gel was set up. In 1X TAE buffer, 1% agarose (w/v) was prepared. The agarose gel was stained with ethidium bromide and visualized under Gel Doc (Genosens 2100 Touch Clinx Inc.). The DNA content and ratio was recorded with the help of Nanodrop (Thermo Fisher Scientific). The primer pairs ITS1 and ITS4 were utilized to amplify the internal transcribed spacer (ITS) region (Nguyena et al. 2020). The primer pairs used in the PCR experiment had the following nucleotide sequences: ITS1 (Forward) 5’TCCGTAGGTGAACCTGCGG3’ ITS4 (Reverse) 5’TCCTCCGCTTATTGATATGC3’.
The reaction mixture of 50 µL was prepared which contained the reagent volume as follows: PCR Master mix (Thermo Fisher Scientific) 25 µL, Forward primer 1 µL, Reverse primer 1 µL, DNA template 5 µL, Milli Q water (nuclease free water) 9 µL. The polymerase chain reaction (PCR) conditions were as follows: 95° C for 5 min; 35 cycles: 95° C for 30 s, 58° C for 30 s and 72° C for 1 min; 72° C for 10 min and 4° C pause (White et al. 1990). The PCR used was Veriti 96 well Thermal cycler (Thermo Fisher Scientific). The resulting PCR products (500–700 bp) were examined using electrophoresis and sequenced by Sanger method from AgriGenome Labs Pvt. Ltd. India. The tools MEGA7.0 and BioEdit 7.2 were used to align the fungal sequences that were acquired. The ITS sequences of the fungal isolates were evaluated using the Basic Local Alignment Search Tool (BLAST) analysis to infer their taxonomic relatedness. The sequences were submitted to the GenBank National Centre for Biotechnology Information (NCBI) for the grant of accession numbers (Sheng-cui et al. 2015).
Phylogenetic analysis
The sequences obtained were aligned by ClustalW in MEGA7.0 and compared to other sequences of the same species or to the genus previously present in the NCBI GenBank database to generate phylogenetic trees. The phylogenetic relationships were inferred based on the Neighbor Joining method by using Tamura-Nei model in MEGA 7.0 (Kumar et al. 2016).
Determination of minimum inhibitory concentration (MIC) of fungal strains
The metal tolerance of the fungal isolates was evaluated against different concentrations of heavy metals following the standard protocol (Liaquat et al. 2020). Heavy metals were used in the form of salts: Cu as CuSO4, Zn as ZnSO4.7H2O, Co as CoCl2.6H2O, Pb as Pb (NO3)2, Ni as NiCl2.6H2O, Cr as CrCl3.6H2O, and Cd as CdCl2.H2O. PDA medium was used to assess the resistance to heavy metals. For the experiment, different concentrations of metal salts (50, 100, 200, 300, 400, 500, and 600 ppm) were used. The fungi were grown at each concentration per metal, and the lowest concentration of each metal that inhibited noticeable growth of the fungal species was reported as the minimum inhibitory concentration (MIC) (Agarwal et al. 2020).
Heavy metals tolerance index (TI) of fungi
TI measures an organism’s response to a metal dosage (Le et al. 2006). The higher the TI, the stronger the resistance (Fazli et al. 2015). The diameter of the fungal colony was measured from the point of inoculation to the expansion of the fungal colony. Following seven days of incubation at 25 °C, TI was ascertained for every plate on PDA for both colonies grown in heavy metal-containing media and colonies incubated in medium devoid of metals (control). The mean value was determined for each strain independently, and the TI was derived using the following formula:
TI = Dt/Du
Where, Dt = diameter of the radial extension (cm) of the treated fungal colony.
Du = radial extension (cm) of the untreated fungal colony (control).
Heavy metal uptake capacity of fungal species
The metal resistant fungi were taken for further research and validated for their metal uptake capacity. The fungal strains were inoculated in Potato Dextrose Broth (PDB). After incubating for seven days at 25 ± 2 °C, the fungal biomass was obtained. Fresh biomass was taken out and added to 200 ml flasks containing PDB and metal concentrations of 50 and 100 ppm per metal. The flasks were kept in a rotatory shaker at 150 rpm and 25 ± 2 °C, pH was adjusted to 5 ± 0.2. Fungi-free metal solutions in PDA media served as the control to rule out any role of media in sequestration. The fungal growth was collected after 7 days and centrifuged at 9000 rpm. Each collected fungal biomass was washed two or three times with double distilled water and dried in a hot air oven at 70 ± 2 °C for three hours followed by filtering through a Whatman filter paper no. 42. The dried fungal biomass was dissolved using a 3:1 mixture of nitric and perchloric acids. The digested fungal biomass was filtered once again using Whatman filter paper no. 42 and the final volume was made upto 50 ml in a volumetric flask. Atomic Absorption Spectroscopy (AAS) (Agilent Technologies GTA 120 Graphite Tube Atomiser 200 Series A) was used to assess the heavy metal absorption (Lekwot et al. 2012). The following formula was used to determine the quantity of heavy metal uptake:
q = [(Ci − Cf)/m] V
where m (g) is the quantity of biomass, q (mg/g) is the mg of metal ions absorption per gram of biomass, Ci (mg Lˉ¹) is the initial metal concentration, Cf (mg Lˉ¹) is the final metal concentration, and V (L) is the final volume of medium.
Statistical analysis
Each experiment was run in triplicates. In this study, the values are presented as mean ± SD. Using SPSS software, one-way ANOVA were used to analyse the trials. When P < 0.05, the difference was deemed significant.
Results
Morphological identification of fungi
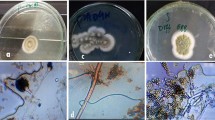
Different fungal strains (26 species) were isolated from the soil, out of which five were found to be highly tolerant to all the seven metals used and were thus taken for further studies. The 5 fungi isolated were first identified on the basis of their morphological characteristics (Fig. 2). The isolates were identified using morphological criteria and microscopic examination with Lactophenol cotton blue (LCB) stain. Different morphological traits were observed on PDA plates. The Aspergillus niger strain initially generated woolly white colonies, but conidial production soon turned those colonies black. Conidial heads were frequently short and loosely columnar under the microscope, with biseriate phialides. Epicoccum mackenzie produced greyish colonies on this medium and staining revealed a spherical uniseriate conidial head, while Fusarium oxysporum and Fusarium verticillioides produced white cottony colonies. Fusarium species yielded both macro- and microconidia from thin phialides. Macroconidia were fusiform to sickle-shaped, hyaline, two to multiple cells, and primarily had an extended apical cell and a pedicellate basal cell. Microconidia were pyriform, fusiform to ovoid, straight or curved, one or two-celled, hyaline, and smaller than macroconidia. Aspergillus fischeri also produced white coloured colonies. Vesicles were found to be globose to clavate, and conidiophores biseriate.
Molecular identification of fungi
Molecular identification was done by the sequencing of their ITS-rDNA region. The ITS sequences of the fungal isolates were submitted to GenBank National Center for Biotechnology Information (NCBI) and following accession numbers were granted (Table 1).
Phylogenetic analysis
After repeated ClustalW alignment of the ITS sequences, a 1000 replicate bootstrap analysis was used to create a neighbour-joining tree using MEGA 7.0 software. The analysis involved 30 nucleotide sequences. All ambiguous positions were removed for each sequence pair. Using phylogeny, commonalities between certain taxa and patterns of metal resistance were investigated (Fig. 3). The strain identities were further supported by similar GenBank sequences and the evolutionary relatedness of the isolates. All the fungi under investigation belonged to the phylum Ascomycota, or sac fungi, which is the most abundant and diverse class of fungi on the planet. Ascomycota fungi are the dominant members of soil fungal communities worldwide. The most characteristic feature of the group is the ascus, a sac-like structure where ascospores are formed, that are the sexual propagules of ascomycetes. They are important for describing most fungi.
The evolutionary history was deduced using the neighbor-joining method. The proportion of duplicate trees, or how many times related taxa clustered together in the bootstrap test (1000 repetitions), is displayed next to the branches. The Maximum Composite Likelihood technique was utilised to compute the evolutionary distances
Determination of minimum inhibitory concentration (MIC) and tolerance index (TI) of fungal strains
The fungal isolates were checked for the heavy metal resistance by calculating their MIC and TI. The MIC of the isolated fungal strains to varied concentrations of Cd, Cu, Pb, Ni, Cr, Zn, and Co differed among the species as presented in Fig. 4. Certain species were sensitive, some were highly tolerant, while as others were moderately tolerant. Aspergillus niger was most tolerant towards Cu and Zn with MIC of 600 ppm and 500 ppm respectively and least tolerant towards Cd and Cr with MIC of 200 ppm. Fusarium oxysporum depicted the highest MIC of 600 ppm with Cr and least MIC of 100 ppm with Zn. Fusarium verticillioides revealed the highest MIC of 500 ppm with Cd and Cu, whereas, the lowest MIC of 100 ppm was shown with Pb. Aspergillus fischeri was most tolerant towards Pb with MIC of 500 ppm and least tolerant with Ni having MIC of 100 ppm. Epicoccum mackenziei exhibited highest MIC of 500 ppm with Ni and lowest MIC of 100 ppm with Cd. The mycelial growth response also varied among the tested species (Fig. 5). The highest tolerance index of 1.13 was shown by Aspergillus niger with Cu, whereas, it exhibited lowest index of 0.6 against Cd. Fusarium oxysporum showed the highest TI of 0.85 with Co and lowest TI of 0.27 with Cd. For Fusarium verticillioides, the highest TI of 0.86 was against Zn and lowest TI of 0.66 was with Cu. Aspergillus fischeri revealed the highest TI of 0.99 with Cd and lowest TI of 0.5 with Zn. Epicoccum mackenziei exhibited the highest TI of 0.54 with Cu and lowest TI of 0.3 with Cr. The current study showed that various fungal species exhibit various patterns of tolerance. The 5 fungi were tolerant against all the metals used but with varying degree of tolerance, thus depicting multi-metal tolerant characteristics.
Heavy metal uptake by fungal strains
It was observed that with the increase in initial metal concentration, the metal uptake capacity of fungi also increased. The highest recorded uptake of heavy metal in this study was 8.31 mg/g of Zn for Fusarium verticillioides at 100 ppm and the lowest uptake of 0.55 mg/g was recorded for Fusarium oxysporum with Cr. The metal uptake by fungal species at both 50 ppm and 100 ppm is shown in the graphs (Fig. 6). With Cd, the highest metal uptake at 100 ppm was observed in Aspergillus fischeri (5.24 mg/g) and lowest (0.7 mg/g) by Fusarium verticillioides. In case of Cu, the uptake was highest in Epicoccum mackenziei (7.65 mg/g), and lowest by Aspergillus fischeri (1.71 mg/g). The Pb uptake value was highest in Aspergillus niger (4.86 mg/g) and lowest in Fusarium verticillioides (2.06 mg/g). The highest Ni uptake was recorded in Aspergillus niger (3.64 mg/g) and lowest in Aspergillus fischeri (1.04 mg/g). For Cr, the uptake capacity was highest in Fusarium verticillioides (4.88 mg/g) and lowest in 0.55 mg/g with Fusarium oxysporum. In case of Zn, the highest metal uptake was observed in Fusarium verticillioides as already mentioned above and lowest uptake of 1.99 mg/g in Aspergillus niger. With Co, the uptake was highest in Aspergillus niger (5.08 mg/g) and lowest in Fusarium oxysporum (1.04 mg/g). The metal sequestration at 100 ppm by different fungi revealed the following order:
Aspergillus niger (IA10): Co˃ Pb˃ Ni˃ Cr˃ Cd˃ Zn˃ Cu.
Fusarium oxysporum (IA16): Cu˃ Cd˃ Zn˃ Ni˃ Pb˃ Co˃ Cr.
Fusarium verticillioides (IA35): Zn˃ Cr˃ Co˃ Ni˃ Cu˃ Pb˃ Cd.
Aspergillus fischeri (IA42): Cd˃ Pb˃ Cr˃ Cu˃ Zn˃ Co˃ Ni.
Epicoccum mackenziei (IA44): Cu˃ Zn˃ Cr˃ Co˃ Cd˃ Pb˃ Ni.
Discussion
The ability of microorganisms to withstand metals can be useful for detoxifying and removing heavy metals from polluted soils, offering a practical option for bioremediation (Nazir et al. 2020). As fungi are primarily found in polluted areas, their use in bioremediation is well documented (Deshmukh et al. 2016).
The main aim of this study was to isolate metal-tolerant fungal strains and simultaneously assess their removal capacities. Five multi-tolerant metal resistant fungi were isolated and characterized (Table 1). The findings of our study revealed that depending on the tested isolate, all of the species exhibited varying degrees of resistance to various metals. This indicated inhibition of some of the fungal isolates at higher concentration of heavy metals. Similar observations about toxic effect of higher concentration of heavy metals on growth of fungi have been reported in other studies (Malik 2004). Fusarium and Aspergillus isolated in the present study showed high metal-tolerance traits and uptake capacities. These species have also been reported from metal stress conditions by previous researchers (Zafar et al. 2007; Iram et al. 2012). It was observed that these fungal species can be effectively utilized for the elimination of toxic heavy metals (Bai and Abraham 2003). Aspergillus niger revealed high tolerance levels and uptake capacity with different heavy metals like Co, Pb, Cu among others. Our findings corroborated with other researchers (Doku et al. 2015; Ahmad and Ansari 2006). Aspergillus and Fusarium species also exhibited high tolerance and sequestration with Ni and Cr. Similar findings were reported from previous research which discovered that most of the strains were resistant to Ni, the majority of which belonged to species in the Aspergillus and Fusarium (Saxena et al. 2006). Some studies found the filamentous fungi completely tolerant to Cr (Zapana-Huarache et al. 2020). Fusarium oxysporum and Fusarium verticillioides exhibited high tolerance with all the metals investigated. Our results draw support from the study of Sanyal et al. (2005) and Mohammadian et al. (2017). Aspergillus fischeri and Epicoccum mackenziei, studied for the first time for their metal resistance properties, have proved to be great options for bioremediation of contaminated soils. Furthermore, fungi exhibited good tolerance and uptake capacities on almost all metals investigated. Cd, Pb, Cr and other harmful metals can cause stress and destabilise membrane structures, which releases mucilaginous binding molecules with a significant affinity for metals and physicochemical properties that facilitate metal absorption (Gadd et al. 2000; Akar and Tunali 2006). Even though Cu is an antifungal agent, most fungi have been demonstrated to thrive in the presence of high concentrations of Cu. It is because the tolerance mechanism is connected to the manner Cu binds to the surface of absorption sites (Goma and Azab, 2013; Price et al. 2001). Zinc resistance in filamentous fungi is ascribed to vacuolar sequestration and conferred by the presence of metallothionein-like peptides (Robinson et al. 2021). As depicted in our study, several fungal species that were resistant to cobalt have been shown to flourish at greater cobalt concentrations (Tripathi and Srivastava 2007). Conversely, a discernible alteration in colony morphology was noted upon exposure to metals, with this alteration being more pronounced in the presence of elevated metal concentrations. This signifies the precipitation of metal ions on the fungal cell wall (Martino et al. 2000).
Fungi may become toxic to these metals if they accumulate to high amounts. The five fungi under investigation were shown to exhibit notable variations in their capacity for absorption of metals as their concentrations changed. Similar results were found by previous researchers (Mishra and Malik 2012). Also, there was an absolute absence of association between metal tolerance limits and removal capability of fungi in our study. Some isolates exhibited low or no tolerance with low uptake capacity, or high tolerance but low or moderate capacity for metal sorption, while others showed moderate tolerance levels but noticeably good metal uptake capacities. Still others had high metal tolerance level and high sorption capacity with regard to a particular metal. This showed that, contrary to expectations that an ability of organism to tolerate metals would result from a genotypic trait, the process of fungal-metal sorption involves cell wall functional groups that are unique to a particular genus and/or species (Gazem and Nazareth, 2012; Zafar et al. 2007). A recent study on the absorption patterns for certain metal accumulations suggests that these processes may result from the oxidation of organic acids, which tend to be absorbed in conjunction with certain harmful metals (Fazli et al. 2015).
Conclusion
The aim of the present study was to find out whether fungi found in the agricultural soil Kashmir Valley were multi-metal tolerant and to what extent. Five identified fungi, Aspergillus niger, Fusarium oxysporum, Fusarium verticillioides, Aspergillus fischeri, Epicoccum mackenziei were screened for their resistance to seven heavy metals (Pb, Cd, Cu, Cr, Zn, Co and Ni) on PDA media containing 50 ppm to 600 ppm metal concentrations. There was decrease in number of fungi for their tolerance to heavy metal with increase in concentration of heavy metal. Majority of the fungal isolates were able to tolerate heavy metals up to 400–500 ppm. Aspergillus niger and Fusarium oxysporum exhibited highest MIC of 600 ppm against Cu and Cr respectively. Growth of Aspergillus niger with copper revealed high tolerance with index values > 1. Data revealed that some of the fungi removed substantial amount of heavy metals particularly Fusarium verticillioides (8.31 mg/g Zn) and Epicoccum mackenziei (7.65 mg/g Cu). The unique characteristics that these fungal species have shown in response to elevated heavy metal levels indicate the potential for bioremediation that these indigenous fungal species possess. The study of tolerance traits of Aspergillus fischeri and Epicoccum mackenziei will add to the knowledge in existing field of mycoremediation by soil-borne fungi. However, some limitations occur as how to make bioremediation technically applicable and economically feasible deserve more attention in future work. Standardizing environmental conditions, genetically understanding the metal uptake mechanisms of fungi and manipulation of cell structure to enhance the metal removal capacities can be undertaken to overcome these limitations.
Data availability
Sequence data that supports the findings of this study have been deposited in the NCBI GenBank with the accession numbers MZ267027, MZ267028, MZ267036, MZ267041, MZ267042.
References
Agarwal M, Rathore RS, Chauhan A (2020) A Rapid and High Throughput MIC determination method to screen uranium resistant microorganisms. Methods Protoc 3(1):21. https://doi.org/10.3390/mps3010021
Ahmad I, Ansari MI (2006) Biosorption of Ni, Cr and Cd by metal tolerant aspergillus Niger and Penicillium sp. using single and multimetal solution. Indian J Exp Biol 44:73–76
Akar T, Tunali S (2006) Biosorption characteristics of aspergillus flavus biomass for removal of pb(II) and Cu(II) ions from an aqueous solution. Bioresour Technol 97:1780–1787
Akpasi SO, Anekwe IMS, Tetteh EK, Amune UO, Shoyiga HO, Mahlangu TP, Kiambi SL (2023) Mycoremediation as a potentially promising technology: current status and Prospects—A. Rev Appl Sci 13:4978. https://doi.org/10.3390/app13084978
Amin I, Nazir R, Rather MA (2021) Nano-bioremediation: an innovative approach for remedying heavy metals using fungi. J bioremediat Biodegrad 12:487
APHA (2017) standard Methods for examination of water and waste water.23rd. american public health association, washington DC
Bai RS, Abraham T (2003) Studies on chromium (VI) adsorption–desorption using immobilized fungal biomass. Bioresour Technol 87:17–26
Balamurugan K, Schaffner W (2006) Copper homeostasis in eukaryotes: teetering on a tight rope. Biochem Biophys Acta 1763:737–746
Birch L, Bachofen R (1990) Complexing agents from microorganisms. Cell Mol Life Sci 46:827–834
Congeevaram S, Dhanarani S, Park J, Dexilin M, Thamaraiselvi K (2007) Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater 146:270–277
Deshmukh R, Khardenavis AA, Purohit HJ (2016) Diverse metabolic capacities of Fungi for Bioremediation. Indian J Microbiol 56(3):247–264. https://doi.org/10.1007/s12088-016-0584-6
Doku TE, Belford EJD (2015) The potential of aspergillus fumigatus and aspergillus Niger in bioaccumulation of heavy metals from the Chemu Lagoon. Ghana J appl Biosci 94:8907–8914
Domsch KH, Gams W, Anderson TH (1980) Compendium of soil fungi, vol 1. Academic Press (London) Ltd
Fazli M, Soleimani N, Mehrasbi M, Darabian S, Mohammadi J, Ramazani A (2015) Highly cadmium tolerant fungi: their tolerance and removal potential. J Environ Health Sci Eng 13:19
Gadd GM, Sayer JA (2000) Fungal transformations of metals and metalloids. In: Lovley DR (ed), Environmental Microbe-Metal Interactions. American Society of Microbiology, Washington 237–256
Gadd GM, White C (1993) Microbial treatment of metal pollution- a working biotechnology? Biotechnol 11:353–359
Gazem MA, Nazareth S (2012) Isotherm and kinetic models and cell surface analysis for determination of the mechanism of metal sorption by Aspergillus Versicolor. World J Microbiol Biotechnol 28:2521–2530
Gomaa OM, Azab KS (2013) Biological indicators, genetic polymorphism and expression in aspergillus flavus under copper mediated stress. J Radiat Res Appl Sci 6:49–55
Gönen F, Aksu Z (2009) Single and binary dye and heavy metal bioaccumulation properties of Candida tropicalis: Use of response surface methodology (RSM) for the estimation of removal yields. J Hazard Mater 172:1512–1519
Iram S, Arooj A, Parveen K (2012) Tolerance potential of fungi isolated from polluted soil of Multan, Pakistan. Int J Biosci 2:27–34
Kavita B, Keharia H (2012) Biosorption potential of Trichoderma Gamsii Biomass for removal of cr(VI) from Electroplating Industrial Effluent. Int J Chem Eng 1–7
Kumar A, Bisht BS, Joshi VD (2011) Bioremediation potential of three acclimated bacteria with reference to heavy metal removal from waste. Int J Environ Sci 2:896–908
Kumar S, Stecher G, Tamura K (2016) Mega 7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger databases. Mol Biol Evol 33(7):1870–1874
Le L, Tang J, Ryan D, Valix M (2006) Bioleaching nickel laterite ores using multi-metal tolerant aspergillus foetidus organism. Min Eng 19:1259–1265
Lekwot VE, Adamu IB, Ayuba KN (2012) Effects of effluent discharge of Kaduna refinery on the water quality of river Romi. Res J Environ Toxicol 1(3):41–46
Liaquat F, Munis MFH, Haroon U, Arif S, Saqib S, Zaman W, Khan AR, Shi J, Che S, Liu Q (2020) Evaluation of metal tolerance of fungal strains isolated from contaminated mining soil of Nanjing, China. Biology 9:469. https://doi.org/10.3390/biology9120469
Malik A (2004) Metal bioremediation through growing cells. J Environ Int 30:271–278
Martino E, Turnau K, Girlanda M, Bonfante P, Perotto S (2000) Ericoid Mycorrhizal fungi from heavy metal polluted soils: their identification and growth in the presence of zinc ions. Mycol Res 104:338–344
Mishra A, Malik A (2012) Simultaneous bioaccumulation of multiple metals from electroplating effluent using aspergillus lentulus. Water Res 46:4991–4998
Mohammadian IE, Ahari AB, Arzanlou M, Oustan S, Khazaei SH (2017) Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province. Chemosphere 185:290–296
Nazir R, Amin I (2021) Role of Green Nanotechnology in alleviating Climate Change. Microbiomes and Global Climate Change. Springer 365–374
Nazir R, Ganai BA, Rahi P, Rehman S, Farooq S, Dar R et al (2020) MALDI-TOF-MS and 16S rRNA characterization of lead tolerant metallophile bacteria isolated from saffron soils of Kashmir for their sequestration potential. Saudi J Biol Sci 27(8):2047–2053
Nguyena TTT, Jeon YJ, Mun HY, Goh J, Chung N, Lee HB (2020) Isolation and characterization of four unrecorded Mucor species in Korea. Mycobiology 29–36. https://doi.org/10.1080/12298093.2019.1703373
Price MS, Classen JJ, Payne GA (2001) Aspergillus Niger absorbs copper and zinc from swine wastewater. Bioresour Technol 77:41–49
Puglisi I, Faedda R, Sanzaro V, Piero ARL, Petrone G, Cacciola SO (2012) Identification of differentially expressed genes in response to mercury I and II stress in Trichoderma Harzianum. Gene 506:325–330
Robinson JR, Isikhuemhen OS, Anike FN (2021) Fungal-metal interactions: a review of toxicity and homeostasis. J Fungi (Basel) 7(3):225
Sanyal A, Rautaray D, Bansal V, Ahmad A, Sastry M (2005) Heavymetal remediation by a fungus as a mean of lead and cadmium carbonate crystals. Langmuir 21:7220–7224
Saxena P, Bhattacharyya AK, Mathur N (2006) Nickel tolerance and accumulation by filamentous fungi from sludge of metal finishing industry. Geomicrobiol J 23(5):333–340
Sheng-cui Z, Jian-xin T, Xiao-xi Z, Ben-jie W, Shao-di Y, Bin H (2015) Isolation of Mucor circinelloides Z4 and Mucor racemosus Z8 from heavy metal-contaminated soil and their potential in promoting phytoextraction with Guizhou oilseed rap. J Cent South Univ 22:88–94. https://doi.org/10.1007/s11771-015-2498-6
Sim CSF, Tan WS, Ting ASY (2016) Endophytes from Phragmites for metal removal: evaluating their metal tolerance, adaptive tolerance behaviour and biosorption efficacy. Desalin Water Treat 57:6959–6966
Simonescu CM, Ferdes M (2012) Fungal biomass for Cu (II) Uptake from Aqueous systems. Pol J Environ Stud 21:1831–1839
Tripathi P, Srivastava S (2007) Mechanism to combat cobalt toxicity in cobalt resistant mutants of aspergillus nidulans. Indian J Microbiol 47:336–344
Uqab B, Mudasir S, Qayoom A, Nazir R (2016) Bioremediation: a management tool. J Bioremediat Biodegrad 7(2)
Uqab B, Nazir R, Ganai BA, Rahi P (2022) In vitro sequestration of Molecular and Mass Spectra Characterized Metallophilic Cadmium tolerant Bacteria for sustainable agriculture. Front Microbiol 13:845853. https://doi.org/10.3389/fmicb.2022.845853
Vaksmaa A, Guerrero-Cruz S, Ghosh P, Zeghal E, Hernando-Morales V, Niemann H (2023) Role of fungi in bioremediation of emerging pollutants. Front Mar Sci Sec Mar Biotechnol Bioprod 10. https://doi.org/10.3389/fmars.2023.1070905
Watanabe T (2002) Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. CRC
White TJ, Bruns T, Lee S et al (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols. Academic, London, pp 315–322
Zafar S, Aqil F, Ahmad I (2007) Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agriculture soil. Bioresour Technol 98:2557–2561
Zapana-Huarache SV, Romero-Sánchez CK, Gonza AD, Torres-Huaco FD, Rivera AL (2020) Chromium (VI) bioremediation potential of filamentous fungi isolated from Peruvian tannery industry effluents. Braz J Microbiol 51:271–278
Acknowledgements
The authors are highly thankful to the Centre of Research for Development (CORD), University of Kashmir and National Institute of Technology (NIT), Srinagar for providing the laboratory facilities.
Funding
This research work was financially supported by the Department of Science and Technology (DST), Government of India, under WOS-A Scheme, vide reference no.: SR/WOS-A/LS-470/2018.
Author information
Authors and Affiliations
Contributions
IA: Concept, experimentation, compilation, interpretation of data, data curation, formal analysis, writing– original draft and editing. RN: Concept, interpretation of data, formal analysis, data curation, validation, visualization, review and editing. MAR: Concept, interpretation of data, formal analysis, data curation, validation, visualization, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amin, I., Nazir, R. & Rather, M.A. Evaluation of multi-heavy metal tolerance traits of soil-borne fungi for simultaneous removal of hazardous metals. World J Microbiol Biotechnol 40, 175 (2024). https://doi.org/10.1007/s11274-024-03987-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-03987-z