Abstract
The isolate Aspergillus versicolor was obtained from an estuary, which is exposed to metal contamination. It was found to have a good metal tolerance and sorption capacity. Further studies revealed that the rate of metal removal from solution is very rapid in the first 5–10 min, and is favoured by a pH of 6.0. The biosorption data obtained was explained by the Freundlich adsorption isotherm model and followed a pseudo-second order kinetics reaction. The fungus showed a higher accumulation of fatty acids when grown in presence of metals as compared to the mycelium grown in absence of the metal; there was also an increase in the saturation index of fatty acids in presence of Cu2+ which serves as a protective mechanism for the fungus. Fourier Transform Infrared, scanning electron microscopy and EDAX analysis indicated that metal removal from solution by A. versicolor occurred by a passive adsorption to the fungal cell surface, involving an ion exchange mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental contamination by improper disposal of industrial, mining, agricultural, municipal, and other residues is known worldwide. Lead is highly hazardous (Volesky 1994), while copper, though not potentially toxic in low concentrations, can pose serious health problems due to extensive and prolonged use and a consequent increase in levels in the environment (Bueno et al. 2008). Thus, release and accumulation of lead and copper in the aquatic environment could result in toxicity to both human and aquatic life.
The use of living and dead biomass of bacteria, algae, fungi, and plants as biosorbents to sequester metal ions in trace level from contaminated effluents forms the foundation of biosorption technology that offers a promising and economical alternative to treat wide variety discharges of metal-containing industrial effluents (Akar et al. 2007; Alluri et al. 2007; Singh 2006). Biomass characteristics, physicochemical properties of the target metals, and factors, such as pH, temperature, initial concentrations of biomass and metal ions significantly affect their biosorption capacities (Al-Garni et al. 2009). The biosorbent, unlike mono functional ion exchange resins, contains a variety of functional sites including carboxyl, imidazole, sulphydryl, amino, phosphate, sulfate, thioether, phenol, carbonyl, amide and hydroxyl moieties.
Biosorbents are cheaper, more effective for the removal of metallic elements, especially heavy metals from aqueous solution (Akhtar et al. 1996; Bairagia et al. 2011). Because of the high surface to volume ratio of microorganisms and their ability to detoxify metals they are considered as potential alternative to synthetic resins for remediation of dilute solutions of metals and solid wastes (Magyarosy et al. 2002).
Fungi are present in aquatic sediments, terrestrial habitats and water surfaces, and play a significant part in natural remediation of metal. Furthermore, fungal hyphae can penetrate contaminated soil and have advantages over bacteria in natural environments (Leitão 2009). The use of non-living fungal biomass in batch treatment of metal waste is of advantage since it does not depend on requirements for growth. The problem of toxicity of metals does not affect this type of biomass, which is seen as one of the major advantages of biosorption.
In this investigation, a composite study has been made on removal of Pb2+ and Cu2+ from solution by A. versicolor, with respect to the factors affecting sorption capacity and rate, together with the isotherm models and kinetics, the mechanism of sorption as determined by FTIR, SEM and EDAX analysis, and the response of the fungus to heavy metals through changes in its fatty acid composition.
Materials and methods
Culture and growth conditions
The isolate A. versicolor EM2wt41, obtained from the top water of Mandovi estuary, Goa, India, showed a high resistance to heavy metals such as lead and copper, as well as a good capacity for removal from aqueous solution. The culture was maintained on Czapek Dox Agar containing 2 % salt (S-CzA).
Spore suspension (106 spores) of a freshly grown agar slant culture was inoculated in 100 mL of Czapek Dox Broth containing 2 % salt (S-CzB) and incubated for 3 days at 30 °C, 150 rpm.
Biosorption experiments
Stock solutions of metals containing 1,000 mg L−1 of Pb2+ as Pb(NO3)2 and of Cu2+ as CuSO4.5H2O were prepared. The stock solutions were appropriately diluted to get the desired concentrations of metal as per the experiment.
The culture was grown as described above; the biomass was harvested by filtering through double layered muslin cloth, washed with deionized water and then treated by boiling in 5 % KOH for 15 min followed by washing with deionized water until the pH of the solution was neutral. The treated biomass was dried by lyophilization and used as biosorbent.
All batch experiments were carried out by incubating 0.1 g of the biosorbent in 20 mL of metal solution under the desired conditions of pH and metal concentration as per the experiment, incubated at 150 rpm, 30 °C. The biosorbent was then removed and the metal remaining in solution was estimated by atomic absorption spectrophotometry (AAS) using Varian AA2402. Metal controls containing only Pb2+ and Cu2+ solution without biosorbent were maintained. Standards of lead and copper solutions (Reagecon) for AAS were prepared in 0.1 N HNO3 in the range of 0–10 ppm Pb2+ or 0–2 ppm Cu2+ for obtaining the standard curve. The samples were diluted so as to obtain metal concentrations within this range and read against the standards. Each experiment was conducted in duplicates and plotted with the standard error bars.
The amount of heavy metal ions removed from solution by the biosorbent was calculated using the equation q e (mg g−1) = (C i − C f ) V/M where q e is the specific metal biosorption (mg metal g−1 biosorbent), V is the volume of metal solution (L), C i and C e are the initial and equilibrium concentration of metal (mg metal L−1) respectively, and M is the dry weight of the biosorbent (g), (Volesky and Holan 1995; Yen and Viraraghavan 2003).
Effect of pH on metal biosorption was studied at pH 4, 5, 6 and 7, for a contact time of 1 h; the pH of the metal solution (20 mL) containing 0.1 g % of Pb2+ or of Cu2+ ions, was adjusted with 0.1N HCl.
Effect of contact time on metal biosorption was determined by incubating the biosorbent in different flasks containing 20 mL of 0.1 g % metal solution, pH 6.0; pH was maintained constant throughout. Flasks in duplicate were removed at specified time intervals of up to 180 min and treated as above to determine the residual metal.
Effect of initial metal ion concentration on biosorption was assessed by incubating the biosorbent with metal ion concentrations ranging from 50 to 300 mg L−1, at pH 6.0 for 6 h.
Equilibrium adsorption isotherms
Freundlich and Langmuir isotherms were employed to analyze the equilibrium distribution between metal ions adsorbed and ions in solution.
The Freundlich method is described by the equation:
log q e = log K F + 1/n log C e , where q e is the amount of metal ions adsorbed onto the biosorbent at equilibrium (mg g−1), C e is the heavy metal ions concentration in the solution (ppm) at equilibrium and K F (L g−1) and n are the Freundlich adsorption isotherm constants.
Langmuir isotherm equation is given by the following equation:
C e /q e = 1/q max K L + C e /q max where q e and q max are the amount of heavy metal ions removed at equilibrium and maximum uptake capacity (mg g−1), respectively, C e is the equilibrium concentration (ppm), and K L is the Langmuir constant.
Kinetic models
The pseudo-first order and pseudo-second order kinetic models were employed to determine the mechanism of biosorption of Pb2+ and Cu2+ (Ho and McKay 1999).
The pseudo-first order rate equation is expressed as log (q e − q t ) = log q e – k 1 /2.303t, where q e and q t are the amounts of lead ions (mg g−1) adsorbed at equilibrium and at time t respectively, and k 1 is the first-order rate constant (min−1). The value of k 1 was calculated from the slope of the plot of log (q e − q t ) versus t.
The pseudo-second-order kinetic model has the linear form of t/q t = 1/k 2 q 2 2 + 1/q 2 t), where q 2 is the maximum adsorption capacity (mg g−1) for the pseudo-second order adsorption and k 2 is the equilibrium rate constant for the pseudo-second order adsorption (gm g−1min−1). The values of k 2 and q 2 were calculated from the plot of t/q t versus t.
FTIR
The functional chemical groups present on the cell walls of the fungal biomass which are involved in heavy metal biosorption was analyzed by Fourier Transform Infrared (FTIR) spectroscopy. The biosorbent before and after exposure to heavy metals, was dried at 60 °C for 12 h; sample disks of finely ground biomass encapsulated with KBr (1:10, w/w) was prepared and analyzed using IR (Prestige-21 FTIR- Shimadzu).
SEM, EDX analysis
The cell-metal interactions were also evaluated for alterations in the cell-surface morphology and confirmation of the presence of the metal ion bound to the cell-surface by scanning electron microscopy (JEOL Model: 15800, LV, Japan) equipped with energy-dispersive X-ray analyzer (SEM-EDX). The samples of biosorbent before and after exposure to heavy metals, were dehydrated by immersion of mycelial strands in acetone of increasing concentrations from 10 to 100 % for 30 min. Samples were then coated with a thin layer of gold–palladium prior to analysis.
Lipid extraction and fatty acids analysis
Spore suspension (106 spores) of a freshly grown agar culture was inoculated in 100 mL of S-CzB containing 2 mM Pb2+ or 1 mM of Cu2+ ions incubated for 3 days at 30 °C, 150 rpm; the control of cultures grown in the absence of the heavy metal was also maintained. The biomass was then harvested, washed and dried as detailed above, then treated using a modification of the method of Bligh and Dyer (1959) as described by Volkman et al. (1989) for fatty acid methyl esters (FAME) analysis. The lyophilized biomass (0.25 g) was saponified in 10 % KOH in methanol: water (80:20) at 80 °C for 2 h in a screw-capped Pyrex tube and cooled. The samples were then extracted thrice with 15 mL of hexane-diethyl ether (9:1). The aqueous layer was acidified to pH 2 by addition of concentrated HCl and extracted thrice with 15 mL of hexane: diethyl ether (9:1). The pooled extracts were dried over anhydrous sodium sulphate, purified by passage through a silica gel column (60–120 mesh) and elution with hexane:ethyl acetate (4:1). The eluate was concentrated under vacuum. FAMEs were analyzed by using a Varian CP-3800 gas chromatograph interfaced with GC/MS Saturn 2200 mass spectrometer. Fungal fatty acids were identified by comparison with the retention times of the authentic standards (Sigma, Supelco) and expressed as μg g−1 dry weight biomass.
Results and discussion
Effect of pH on biosorption of lead and copper ions
The sorption capacity of the biosorbent for both metal ions was markedly influenced by the levels of initial pH in the solution, being enhanced substantially with increase in pH from 4.0 to 6.0 and then decreasing at pH 7.0. The biosorption capacity at pH 6.0 was 17.35 and 12.0 mg g−1 for lead and copper respectively (Fig. 1). Similar results for pH effect on lead or copper biosorption were also reported by earlier investigators (Gabr et al. 2008; Hefne et al. 2008; Hussain et al. 2009; Joo et al. 2011; Mukhopadhyay et al. 2007; Oh et al. 2009; Ozsoy 2010; Say et al. 2001).
At low pH, cell wall ligands would be closely associated with the hydronium ions H3O+, that restrict the binding of metal cations as a result of repulsive forces (Dursun 2006). The increase in the biosorption capacity with increase in pH can be attributed to a change in the charge density of the biosorbent. The pH results in a deprotonation of the metal binding sites of the cell wall, such as carboxyl, phosphate and amino groups, thus increasing the negative charge density of these functional groups. Consequently there is a corresponding increase in attraction of metal cations and biosorption on to the cell wall (Akar et al. 2007; Bairagia et al. 2011; Kapoor et al. 1999; Karaca et al. 2010). This indicated the involvement of ionic attractions between cell wall ligands and the metal ions as a mechanism in metal biosorption by the fungi. At pH greater than 6, metal ions precipitate as hydroxides or hydrated oxides, resulting in decreased amounts of Pb2+ and Cu2+ in the solution, thus limiting the biosorption process at higher pH solutions (Oh et al. 2009).
Effect of contact time on lead and copper ions biosorption
The Pb2+ and Cu2+ removal capacity by A. versicolor biosorbent as a function of time is presented in Fig. 2. It can be seen that sorption occurred rapidly in the first 5–10 min, followed by a slower phase until equilibrium was attained within 30 min, with no further increase in the sorption after 60 min. This could be due to the mechanism of ionic attraction between negatively charged cell wall ligands and the metal cations; at the initial phase, the functional groups are abundantly available for binding by the metal ions, and as the ligands get saturated, the sorption rate decreases. The high initial concentration of heavy metal ions enhances the contact proximity and consequently, the rate of adsorption to the cell wall. This corroborates earlier reports on sorption by A. parasiticus, A. niveus and other species (Akar et al. 2007; Dursun 2006; Karaca et al. 2010; Oh et al. 2009). The rate of metal sorption was seen to be faster than that obtained by A. versicolor MTCC 280 which reached equilibrium after 3 h (Bairagia et al. 2011) and by Rhizopus oligosporus, which required 6 h for maximum sorption (Ozsoy 2010); however, it was slower than that obtained by commercial natural bentonite for Pb2+ sorption (Hefne et al. 2008). The natural econiche from where the isolate A. versicolor EM2wt41 was obtained, and which is exposed to metal contamination, could have caused the isolate to develop a resistance to metals (Ezzouhri et al. 2009) and a mechanism for a speedy passive metal removal as a means of survival.
Effect of initial metal concentration on biosorption
The effect of initial metal concentration on biosorption of Pb2+ and Cu2+ is shown in Fig. 3. As the initial metal ion concentration (C i ) was increased, the biosorption capacity also increased. Similar results were observed in studies on A. niger and A. niveus (Goyal et al. 2003; Karaca et al. 2010; Mukhopadhyay et al. 2007). The effect of initial metal ion concentration on sorption is related to the number of available active sites on the cell surface for binding (Bhatti et al. 2008; Joo et al. 2010). The increase in concentration of the metal ions in the vicinity of the binding sites would also enhance the sorption rate, till a point where the active sites are fully bound to the metal ions. Yalcin et al. (2010) state that the initial metal concentrations provide an important driving force to overcome all mass transfer resistances between the metal solution and the fungal cell wall. Furthermore, the number of collisions between metal ions and the adsorbent are reported to enhance the biosorption process (Wang et al. 2010). The percentage sorption was enhanced a little with increase in initial metal concentrations up to 250 mg L−1, and then showed a slight decrease at 300 mg L−1. This could be due to a competition of increasing metal ions for ligands on the cell surface, coupled with a decrease in number of available sites with increasing metal concentrations (Khambhaty et al. 2009; Ozsoy 2010).
The sorption capacity q e of the isolate was 51.978 mg Pb2+ and 43.66 mg Cu2+ g−1 biosorbent at C i of 300 mg L−1, higher than results obtained with A. niger (Dursan 2006) but less than that obtained by Fusarium solani (Bhatti et al. 2008). It was also observed that q e values were in the order of Pb2+ > Cu2+ for a given initial metal concentration. This preferential type of adsorption may be ascribed to the difference in their ionic radii (Gabr et al. 2008). The ionic radius of Pb2+ is 1.20 Å while that of Cu2+ is 0.73 Å. The smaller the ionic radius, the greater its tendency to be hydrolyzed, leading to reduced biosorption, for this reason the fungal biomass has greater affinity for lead rather than copper. These results were in accord with earlier studies by Akar and Tunali (2006) and Iskandar et al. (2011) on Aspergillus species, and by (Bueno et al. 2008; Oh et al. 2009) on bacteria Rhodococcus opacus and Pseudomonas stutzeri.
Biosorption isotherms
The non-linearized adsorption isotherms of each metal are given in Fig. 4. The biosorption isotherm curve represents the equilibrium distribution of metal ions between the aqueous and solid phase. The isotherms indicate that the biosorption rate increases with an increase in equilibrium concentration of the metal.
The Langmuir and Freundlich isotherms are given in Figs. 5 and 6 respectively. The Langmuir isotherm model is based on monolayer sorption onto a surface containing a given number of identical sorption sites which are homogeneously distributed over the sorbent surface; the Freundlich isotherm equation describes the sorption based on a heterogeneous surface. The constants of Langmuir and Freundlich isotherm models for Pb2+ and Cu2+ biosorption onto lyophilized cells of A. versicolor are presented in Table 1. The equilibrium data fitted the Freundlich adsorption isotherm better than the Langmuir model for Pb2+ and Cu2+ biosorption at various initial concentrations, as seen from the correlation coefficient r2.
As shown in Table 1, the magnitude of the K f intercept in the Freundlich equation showed a high Pb2+ adsorptive capacity of A. versicolor, which was higher than reported values for A. versicolor (Bairagia et al. 2011), while that for Cu2+ was lower. The intensity of sorption, denoted by ‘n’ (Table 1), which is related to the distribution of bound ions on the sorbent surface, was greater than unity, indicating that there is a favourable sorption of lead and copper ions by the biomass of A. versicolor.
The maximum adsorption capacities for Pb2+ and Cu2+ biosorption by A. versicolor calculated from Langmuir adsorption isotherm were 25.25 and 13.15 mg g−1, respectively (Table 1) that for lead being higher than that for copper. The biosorption capacity of A. versicolor was seen to be better than that of other aspergilli such as A. flavus (Akar and Tunali 2006) and A. niger (Dursun et al. 2003), and of clays such as bentonite (Donat et al. 2005) and kaolinite (Gupta and Bhattacharyya 2005), as well as A. nidulans (Gazem and Nazareth 2012) but less than that obtained by A. niveus (Karaca et al. 2010), Fusarium solani (Bhatti et al. 2008), Rhizopus arrhizus (Fourest and Roux 1992), Pseudomonas stutzeri (Oh et al. 2009), Rhodococcus opacus (Bueno et al. 2008), Spirogyra neglecta (Hussain et al. 2009) and a bentonite sample as shown by Hefne et al. (2008).
The Langmuir constant, ‘b’, values obtained for Pb2+ and Cu2+ were found to be 0.070 and 0.035, respectively, which indicate that lyophilized cells of A. versicolor possess a high adsorption affinity for Cu2+ as compared to Pb2+.
Sorption of lead and copper ions by A. flavus and A. niger was also found to follow the Freundlich model (Akar and Tunali 2006; Kapoor et al. 1999; Parvathi et al. 2007) a similar observation was made using the basidiomycetes Phanerochaete chrysosporium, Trametes versicolor, Pleurotus eryngii and compost (Say et al. 2001; Bayramoglu et al. 2003; Joo et al. 2011; Seelsaen et al. 2007).
Biosorption kinetics
The linear form of the pseudo-first order model and the pseudo-second order model for the adsorption of Pb2+ and Cu2+ is given in Figs. 7 and 8. The biosorption kinetics of heavy metals provides the mechanism of the sorption reaction, describing the metal uptake, which in turn controls the time during which the metal remains at the solid-solution interface.
The correlation coefficients (r2) for the linear plots using the pseudo-first order model was 0.4010 for Pb2+ and 0.839 for Cu2+ sorption, while the correlation coefficients for the linear plots from the pseudo-second order model was 0.996 for Pb2+ and 0.999 for Cu2+ sorption (Table 2). The adsorption capacities calculated (q e calc ) by the pseudo-second-order model are also close to those determined by experiments (q e exp ). These results indicated that the pseudo-first order model is less suitable to describe the biosorption process. It was therefore concluded that the pseudo-second order adsorption model is more applicable to describe the adsorption kinetics of Pb2+ and Cu2+ by lyophilized culture of A. versicolor. This was also observed in the biosorption of Pb2+ and Cu2+ by Fusarium solani (Bhatti et al. 2008), Pseudomonas stutzeri (Oh et al. 2009), Rhodococcus opacus (Bueno et al. 2008), Spirogyra neglecta (Hussain et al. 2009), as well as by bentonite (Hefne et al. 2008).
FTIR
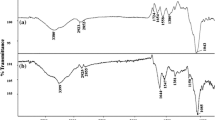
The IR spectrum of the KOH-treated, lyophilized biomass in comparison to the untreated native biomass (Fig. 9), indicated a broadening of the –NH and –OH bands and a shift or an increase in sharpness and intensity of peaks, that could be assigned to functional groups of carbonyl, carboxyl and amide groups of biomolecules of the mycelium, thus indicating that K+ must have adsorbed to the mycelial mass. The IR spectra of the KOH treated biosorbent after metal sorption showed a further broadening of the –NH and –OH band at 3,500–3,200 cm−1, intensified in presence of Cu2+, and an increase in the intensity of the peaks corresponding to the –CH stretching vibrations of CH2 and CH3 groups at 3,000–2,800 cm−1, a shift in peaks of the carbonyl –C=O of amide or carboxyl groups at 1,670–1,650 cm−1 and 1,550–1,540 cm−1, the amide or sulfamide at 1,381 cm−1, the C–O or C–N stretching vibrations of proteins at 1,153 cm−1 and the P–O–C linkage of the organo-phosphorous groups about 1,026 cm−1. This would indicate an ion exchange mechanism, including a replacement of the K+ ions adsorbed on the biosorbent with the Pb2+ and Cu2+ ions. This ion-exchange mechanism also supports the observation that at low pH, H+ ions compete with metal ions for the binding sites on the cell wall, causing low sorption capacity at acidic pH, as seen in the results indicated above. The increase in peak intensity as well as a shift in the stretching vibration of the C–O or C–N of proteins at 1,155 cm−1 and the P–O–C linkage of the organo-phosphorous groups at 1,030 cm−1 were more pronounced when exposed to Cu2+.
This mechanism of ion exchange in Pb2+ and Cu2+ sorption by the A. versicolor isolate is in accordance with reports on metal sorption by aspergilli: A. parasiticus, A. niger and A. versicolor (Akar et al. 2007; Akhtar et al. 1996; Bairagia et al. 2011) as well as earlier results obtained (unpublished data).
Fatty acids composition of fungus biomass
Aspergillus versicolor showed a marked increase in most of the fatty acids when grown in presence of Pb2+ and Cu2+ in comparison to the control grown in absence of the metal, being enhanced more by Cu2+ than by Pb2+ (Table 3). The increase in fatty acids in presence of metals was particularly seen with the saturated stearic acid (C16:0) and the unsaturated oleic acid (C18:1n9c, C18:1n9t) and linoleic acids (C18:2 n6t), the latter being more intense in presence of Pb2+. Similar results were also obtained with Aspergillus terreus in response to Cu2+ (Al Abboud and Alawlaqi 2011) and with Curvularia lunata exposed to Ni2+ (Paraszkiewicz et al. 2010). In addition, mycelia of A. versicolor grown in presence of Cu2+ also showed a high increase in palmitic acid (C18:0), whereas there was a decrease when grown in presence of Pb2+.
The saturation index of fatty acids decreased from 0.626 seen in the control biomass, to 0.177 in biomass cultured with 2 mM of Pb2+ but was increased to 0.96 when cultured with 1 mM of Cu2+. This increase in saturation index in presence of Cu2+, corroborates earlier findings on Stachybotrys chartarum by Hefnawy et al. (2010), who conclude that this results in a decreased membrane fluidity or increased rigidity, thus enabling the organism to tolerate toxic metals up to certain concentrations and might be one of the tolerance mechanisms to heavy metals stress by filamentous fungi. Their observation that the saturation index decreases at higher metal concentration, could be a reason for decreased tolerance of the fungus to high concentrations of metal ions.
SEM and EDX analysis
The mycelium grown in presence of metal ions, showed considerable changes as seen in the SEM micrographs (Fig. 8). The ridges and grooves seen on the pristine biomass (control), were intensified when the culture was exposed to metal and are thought to aid in metal sorption (Khambhaty et al. 2009; Srivastava and Thakur 2006). The mycelium in presence of lead ions became broadened, with a shiny appearance, attributable to the deposition of metal on the surface (Bansal et al. 2009). On exposure to copper ions, the mycelium was greatly thickened and showed a highly wrinkled surface. The thickening of the mycelia in presence of metal ions could be a result of chitin deposition in the cell wall, which contributes ligand molecules for the sorption of metal (Nazareth and Marbaniang 2008; Ram et al. 2004). The sorption of metal ions onto the cell surface ligands has been indicated by the IR analysis.
The EDX spectra of the biomass before and after exposure to Pb2+ and Cu2+ (Fig. 10) showed the presence of a strong signal due to Pb2+ at 2.2 keV (Fig. 10b) and a signal of Cu2+ between 0.9 keV (Fig. 10c), indicating the sorption of Pb2+ and Cu2+ ions respectively on to the mycelial biomass and confirming the results obtained by FTIR and SEM analysis. The phosphorous signal at 2.1 keV disappeared after sorption of metal, indicating the involvement of the phosphate group on the biomass in the complexation with metal ions, as was also seen in FTIR analysis. These signals for Pb2+ and Cu2+ ions on the biomass, indicating the accumulation of metal ions on the fungal cell walls, were also recorded by Akar et al. (2007) and Bairagia et al. (2011) using different species of fungi such as A . parasiticus, A. versicolor and Botrytis cinerea.
Conclusion
The results obtained indicated that metal sorption by A. versicolor occurred rapidly in the first 5–10 min, favoured by a pH of 6.0. The equilibrium data fitted the Freundlich adsorption isotherm model, indicating the involvement of a heterogeneous surface in metal sorption, following a pseudo-second order kinetics reaction. The fungus showed a higher accumulation of fatty acids when grown in presence of metals as compared to the mycelium grown in absence of the metal; there was also an increase in the saturation index of fatty acids in presence of Cu2+ which serves as a protective mechanism for the fungus. Metal removal from solution occurred by a passive adsorption by the fungal cell surface, involving an ion exchange mechanism as seen by FTIR, SEM and EDX analysis, which explains the rapid rate of removal from solution. The isolate A. versicolor EM2wt41 obtained from an estuary that is exposed to metal contamination and has consequently developed a good mechanism of metal sorption, serves as a potential culture in bioremediation measures for removal of metal from aqueous waste.
References
Akhtar MN, Sastry KS, Mohan PM (1996) Mechanism of metal ion biosorption by fungal biomass. Biometals 9:21–28
Akar T, Tunali S, Çabuk A (2007) Study on the characterization of lead (II) biosorption by fungus Aspergillus parasiticus. Appl Biochem Biotechnol 136:389–405
Akar T, Tunali S (2006). Biosorption characteristics of Aspergillus flavus biomass for removal of Pb(II) and Cu(II) ions from an aqueous solution. Bioresour Technol 97:1789–1787
Al Abboud MA, Alawlaqi MM (2011) Biouptake of copper and their impact on fungal fatty acids. Aust J Basic Appl Sci 5(11):283–290
Al-Garni S, Ghanem KM, Bahobail AS (2009) Biosorption characteristics of Aspergillus fumigatus in removal of cadmium from an aqueous solution. Afr J Biotechnol 8:4163–4172
Alluri HK, Ronda SR, Settalluri VS, Singh JB, Suryanarayana V, Venkateshwar P (2007) Biosorption: an eco-friendly alternative for heavy metal removal. Afr J Biotechnol 6(25):2924–2931
Bairagia H, Khan MMR, Raya L, Guhab AK (2011) Adsorption profile of lead on Aspergillus versicolor: a mechanistic probing. J Hazard Mater 186:756–764
Bansal M, Singh D, Garg VK, Rose P (2009) Use of agricultural waste for the removal of nickel ions from aqueous solutions: equilibrium and kinetics studies. Eng Technol 51:431–437
Bayramoglu G, Baktas S, Arica MY (2003) Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J Hazard Mater 101:285–300
Bhatti HN, Samin G, Hanif MA (2008) Enhanced removal of Cu(II) and Pb(II) from aqueous solutions by pretreated biomass of Fusarium solani. J Chin Chem Soc 55:1235–1242
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37: 911–917
Bueno BYM, Torem ML, Molina F, De Mesquita LMS (2008) Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: Equilibrium and kinetic studies. Miner Eng 21:65–75
Donat R, Akdogan A, Erdem E, Cetisli H (2005) Thermodynamics of Pb2 and Ni2 adsorption onto natural bentonite from aqueous solutions. J Colloid Interface Sci 286(1):43–52
Dursun AY, Uslu G, Cuci Y, Aksu Z (2003) Bioaccumulation of copper(II), lead(II) and chromium(VI) by growing Aspergillus niger. Process Biochem 38:1647–1651
Dursun AY (2006) A comparative study on determination of equilibrium, kinetic and thermodynamic parameters of biosorption of copper (II) and lead (II) ions onto pretreated Aspergillus niger Biochem. Eng J 28:187–195
Ezzouhri L, Castro E, Moya M, Espinola F, Lairini K (2009) Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier, Morocco. Afr J Biotechnol 3(2):035–048
Fourest E, Roux JC (1992) Heavy metal biosorption by fungal mycelial by-products: mechanism and influence of pH. Appl Microbiol Biotechnol 37(3):399–403
Gabr RM, Hassan SHA, Shoreit AAM (2008) Biosorption of lead and nickel by living and non-living cells of Pseudomonas aeruginosa ASU 6a. Int Biodeterior Biodegrad 62:195–203
Gazem MAH, Nazareth S (2012) Sorption of lead and copper from an aqueous phase system by marine-derived Aspergillus species. Annals Microbiol (Accepted)
Goyal N, Jain SC, Banerjee UC (2003) Studies on the microbial adsorption of heavy metals. J Adv Environ Res 7:311–318
Gupta SS, Bhattacharyya KG (2005) Interaction of metal ions with clays: I. A case study with Pb(II). Appl Clay Sci 30(3–4):199–208
Hefnawy MA, Ali MI, Abdul-Ghany S (2010) Influence of copper and cobalt stress on membrane fluidity of Stachybotrys chartarum. Can J Pure Appl Sci 4(1):1003–1009
Hefne JA, Mekhemer WK, Alandis NM, Aldayel OA, Alajyan T (2008) Kinetic and thermodynamic study of the adsorption of Pb(II) from aqueous solution to the natural and treated bentonite. Int J Phys Sci 3(11):281–288
Ho YS, McKay G (1999) The sorption of lead(II) ions on peat. Water Res 33:578–584
Hussain MA, Salleh A, Milow P (2009) Charactrization of the adsorption of the lead(II) by the nonliving biomass Spirogera neglecta (Hasall) Kutzing. Am J Biochem Biotechnol 5(2):75–83
Iskandar NL, Zainudin NA, Tan SG (2011) Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J Environ Sci 23(5):824–830
Joo J-H, Hassan SHA, Oh S-E (2010) Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int Biodeterior Biodegrad 64:734–741
Joo J-H, Hussein KA, Hassan SHA (2011) Biosorptive capacity of Cd(II) and Pb(II) by lyophilized cells of Pleurotus eryngii. Korean J Soil Sci Fert 44(4):615–624
Kapoor A, Viraraghavan T, Cullimore DR (1999) Removal of heavy metals using the fungus Aspergillus niger. Bioresour Technol 70:95–104
Karaca H, Tay T, Kıvanç M (2010) Kinetics of lead ion biosorption from aqueous solution onto lyophilized Aspergillus niveus. Water Pract Technol 5(1):1–10
Khambhaty Y, Mody K, Basha S, Jha B (2009) Biosorption of Cr(VI) onto marine Aspergillus niger: experimental studies and pseudo-second order kinetics. World J Microbiol Biotechnol 25:1413–1421
Leitão AL (2009) Potential of Penicillium species in the bioremediation field. Int J Environ Res Public Health 6:1393–1417
Magyarosy A, Laidlaw RD, Kilaas R, Echer C, Clark DS, Keasling JD (2002) Nickel accumulation and nickel oxalate precipitation by Aspergillus niger. Appl Biochem Biotechnol 59:382–388
Mukhopadhyay M, Noronha SB, Suraishkumar GK (2007) Kinetic modeling for the biosorption of copper by pretreated Aspergillus niger biomass. Bioresour Technol 98(9):1781–1787
Nazareth S, Marbaniang T (2008) Effect of heavy metals on cultural and morphological growth characteristics of halotolerant Penicillium morphotypes. J Basic Microbiol 48:363–369
Oh SE, Hassan SHA, Joo JH (2009) Biosorption of heavy metals by lyophilized cells of Pseudomonas stutzeri. World J Microbiol Biotechnol 25:1771–1778
Ozsoy HD (2010) Biosorptive removal of Hg(II) ions by Rhizopus oligosporus produced from corn-processing wastewater. Afr J Biotechnol 9(51):8783–8790
Paraszkiewicz K, Bernat P, Naliwajski M, Długon′ski J (2010) Lipid peroxidation in the fungus Curvularia lunata exposed to nickel. Arch Microbiol 192:135–141
Parvathi K, Narensh Kumar R, Nagendran R (2007) Biosoprtion of manganese by Aspergillus niger and Saccharomyces cerevisiae. World J Microbiol Biotechnol 23(5):671–676
Ram AFJ, Arentshorst M, Damveld RA, VanKuyk PA, Klis FM, Van den Hondel C (2004) The cell wall stress response in Aspergillus niger involves increased expression of the glutamine: fructose-6-phosphate amidotransferase-encoding gene (gfa A) and increased deposition of chitin in the cell wall. Microbiology 4:139–143
Say R, Denizli A, Arıca MY (2001) Biosorption of cadmium(II), lead(II) and copper(II) with the filamentous fungus Phanerochaete chrysosporium. Bioresour Technol 76:67–70
Seelsaen N, Mclaughlan R, Moore S, Stuetz RM (2007) Influence of compost charactristics on heavy metal sorption from synthetic storm water. Water Sci Technol 55(4):219–226
Singh H (2006) Mycoremediation fungal bioremediation. Wiley, Hoboken, NJ, pp 484–532
Srivastava S, Thakur IS (2006) Biosorption potency of Aspergillus niger for removal of chromium (VI). Curr Microbiol 53:232–237
Volesky B (1994) Advances in biosorption of metals: selection of biomass types. FEMS Microbiol Rev 14:291–302
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Volkman JK, Jeffery SW, Nichols PD, Rogers GI, Garland CD (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J Exp Mar Biol Ecol 128:219–240
Wang J-S, Hu X-J, Liu Y-G, Xie S-B, Bao Z-L (2010) Biosorption of uranium(VI) by immobilized Aspergillus fumigatus beads. J Environ Radioact 101:504–508
Yalcin E, Cavusoglu K, Kinalioglu K (2010) Biosorption of Cu2+ and Zn2+ by raw and autoclaved Rocella phycopsis. J Environ Sci 22(3):367–373
Yen G, Viraraghavan T (2003) Heavy metal removal from aquase solution by fungus Mucor rouxii. Water Res 37:4486
Acknowledgments
The authors are grateful to A. Majumdar, P. Naik and S. Mirza, National Institute of Oceanography, Goa, India, for the analysis of GC-MS, and to P. Torney, Goa University for the FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gazem, M.A.H., Nazareth, S. Isotherm and kinetic models and cell surface analysis for determination of the mechanism of metal sorption by Aspergillus versicolor . World J Microbiol Biotechnol 28, 2521–2530 (2012). https://doi.org/10.1007/s11274-012-1060-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1060-z