Abstract
Lactic acid bacteria, being generally recognized as safe, are the preferred choice among other microbial producers of selenium nanoparticles. For successful production of SeNPs, it is necessary to take into account the physiological properties of the bacterium used as a biotransformer of inorganic forms of selenium in Se0. The antimicrobial and antioxidant activity of SeNPs allows to use them in the form of pure nanoparticles or biomass of lactic acid bacteria enriched with selenium in preparation of food, in agriculture, aquaculture, medicine, veterinary, and manufacturing of packing materials for food products. To attract attention to the promising new directions of lactic acid bacteria applications and to accelerate their implementation, the examples of the use of SeNPs synthesized by lactic acid bacteria in the mentioned above areas of human activity are described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an important microelement in the human nutrition, in particular, it is part of Se-containing enzymes such as glutathione peroxidase, iodothyronine deiodinase, and thioredoxin reductase, which are essential in the processes of antioxidant protection, detoxification, and thyroid function (Brigelius-Flohé 2018).
Selenium was discovered by the Swedish chemist Jons Jakob Berzelius in 1817, but was considered a poisonous element until 1957, when the dependence of its toxic effect on the amount consumed was established. The deficiency of selenium in human nutrition leads to serious health problems (Fordyce 2013). However, a gap between toxic (≥ 400 µg/day) and deficient (≤ 40 µg/day) levels of selenium in human diet (NAS 2000; Monsen 2000; UK EGVM 2003) is narrow, so consumption of Se should be under strict control. Recommended daily allowance (RDA) of selenium in the United States for adult men and women over 19 years of age is 55 µg/day (IMFNB 2000), in the UK, RDA is 75 µg/day for males and 60 µg/day for females over 19 years of age (GDR 2016), and according to the World Health Organization, selenium RDA is from 30 to 40 µg/day (Kieliszek and Blazejak 2016).
There are four natural oxidation states of selenium such as elemental selenium (0) (Se0), selenide (− 2) (Se2−), and soluble salts selenite (+ 4) (SeO23−), and selenate (+ 6) (SeO4−2) (Barceloux 1999). Selenium is found in food in organic form such as selenomethionine and selenocysteine that are the most bioavailable selenium substances for humans. The transformation of inorganic Se into organic form can be achieved by cultivation of yeast in a medium with hydroselenite, or by germinating plants using this substance. Yeast or sprouts enriched with selenium could be used in the manufacturing of dietary bakery products (Stabnikova et al. 2008, 2019, 2022). However, the microbial production of selenium nanoparticles (SeNPs) containing selenium in form of Se0 that compared to its other forms has minor toxicity along with high bioavailability is promising (Ullah et al. 2021).
It was shown that the biogenic Se NPs were less toxic than chemically produced SeNPs and much less toxic than the inorganic selenium (Mal et al. 2017; Shakibaie et al. 2012). Comparison of the toxicity of selenite, elemental selenium synthesized biologically or chemically assessed by a lethal dose LD50 on zebrafish embryos showed that biogenic selenium was 3.2 times less toxic than selenite and 10 times less toxic that chemogenic SeNPs. The authors explained this by the presence of organic layer containing extracellular polymeric substances on the surface of biogenic SeNPs, which determines their surface properties and decreases the negative effect of nanoparticles on embryos (Mal et al. 2017). The fact that biogenic SeNPs are enclosed in an organic material was also shown for selenium nanoparticles synthesized by bacterial strains Stenotrophomonas maltophilia and Ochrobactrum sp., which were surrounded with the layer containing proteins and amphiphilic molecules (Piacenza et al. 2018). It was proved by proteomics analysis that lactic acid bacteria Lactobacillus casei ATCC 393 growing in the medium containing selenite produced protein-capped-SeNPs (Qiao et al. 2023).
Particles with length of 1–1000 nm in at least one dimension are considered as nanoparticles (Gosh et al. 2021; Jeevanandam et al. 2018), however particles with size ranging from 10 to 100 nm have a large surface-to-volume ratio and high surface energy, which possess modified properties for their new applications. Decreasing particle size resulted in increase of surface-to-volume ratio, so smaller SeNPs have greater biological activity and protective properties including their role against DNA oxidation (Shoeibi et al. 2017). The introduction of nanotechnology into nutrition can increase the bioavailability of food supplements, improve taste and odor, absorption and solubility, protection against oxidation and enzymatic degradation, prolong residence time and effective penetration through the gastrointestinal tract (Murugesan et al. 2019), as well as increase the bioavailability of this element and ensure its controlled release in the body (Hosnedlova et al. 2018).
Production of SeNPs using bacteria of the genera Bacillus, Pseudomonas, and Staphylococcus are known (Murugesan et al. 2019; Sasidharan and Balakrishnaraja 2014; Ullah et al. 2021). There are a lot studies in which opportunistic pathogens, which are belonging to the group of Biological safety level 2, are regarded as potential producers of selenium nanoparticles, for example Klebsiella pneumoniae (Sasidharan et al. 2014), Bacillus cereus (Pouri et al. 2018), Pseudomonas aeruginosa, Staphylococcus aureus (Cruzet al. 2018), Proteus mirabilis (Wang et al. 2020), Stenotrophomonas maltophilia (Cremonini et al. 2016) and others. Meanwhile, Klebsiella pneumoniae is a major cause of opportunistic healthcare-associated infections (Gorrie et al. 2022), Bacillus cereus frequently causing gastrointestinal diseases (Allende et al. 2016), Pseudomonas aeruginosa known for its ability to cause chronic lung infections (Vance et al. 2004), Staphylococcus aureus is infection agent for a variety of diseases ranging from boils to sepsis and pneumonia (Fetsch and Johler 2018; Plata et al. 2009), Proteus mirabilis causes various human diseases including the respiratory tract, gastrointestinal tract, and urinary tract (Wasfi et al. 2020), Stenotrophomonas maltophilia known to cause nosocomial infections in immunocompromised patients (Buchovec et al. 2022).
A potential way to produce SeNPs is the use of probiotic microorganisms, in particular lactic acid bacteria (LAB), which are considered as Generally Recognized as Safe (GRAS) by the US Food and Drug Administration and have Qualified presumption of safety (QPS) status by the European Food Safety Authority allowing them to be used in food production and as probiotic cultures in medicine (Colautti et al. 2022; EFSA 2016). However, it should be noted that some lactic acid bacteria could produce toxic substances and show signs of virulence (Kim et al. 2018). That’s why when choosing a producer of selenium nanoparticles even among lactic acid bacteria for their subsequent use in the food production, in the agriculture, aquaculture, medicine, veterinary, and manufacturing of packing materials for food, it is necessary to follow microbial safety standards and evaluate producer’s safety status (EFFCA 2003). The easiest and surest way is to choose a strain of lactic acid bacteria for the biosynthesis of selenium nanoparticles among known proven strains that are already used in the food industry or as probiotics in pharmaceuticals.
The use of lactic acid bacteria for the biotransformation of toxic selenite into non-toxic Se-containing amino acids has been reported. Lactic acid bacteria able to reduce sodium selenite and the strains Lactococcus lactis CRL 2011, Weissella cibaria 10 and 25, Enterococcus casseliflavus 47 and 82, Lactobacillus brevis CRL 2051, L. plantarum CRL 2030, and Fructobacillus tropaeoli CRL 2034 converted more than 80% of selenium from sodium selenite present in the medium and accumulate from 126 to 580 µg of intracellular selenium per gram of cell biomass (Martinez et al. 2020). There are some studies show the ability of different lactic acid bacteria for biotransformation of inorganic form of selenium into selenium nanoparticles (El-Saadony et al. 2021a; Eszenyi et al. 2011; Martínez et al. 2020; Moreno-Martin et al. 2017; Shoeibi et al. 2017; Spyridopoulou et al. 2021).
The aim of the present review was to determine the conditions of selenium nanoparticle biosynthesis by lactic acid bacteria and the areas of their possible applications.
Lactic acid bacteria used for production of selenium nanoparticles
Among the lactic acid bacteria there are a lot of representatives capable to transform inorganic Se into its organic forms and synthesizing nanoparticles of this element. These bacterial strains belong to the genera Lactobacillus, Lactococcus, Enterococcus, Streptococcus, Pediococcus, Levilactobacillus, Leuconostoc, Fructobacillus, Weissela and Bifidobacterium. All lactic bacteria are safe for humans and can be used in food production. Meanwhile, strains of different genera and species vary by the resistance to the toxic effect of inorganic selenium and ability to transform it into organic Se compounds (selenocysteine (SeCys), selenomethionine (SeMet) and methylselenocysteine (MeSeCys) or elemental selenium (Gómez-Gómez et al. 2019).
Different strains of lactic acid bacteria vary by their ability to grow in the presence of inorganic selenium and accumulate it. Dependence of selenium accumulation on the strain was shown in many studies. For instance, it was found that at the concentration 5 mg/l of sodium selenite (Na2SeO3) in medium for cultivation of 96 strains lactic bacteria belonging to genera Lactococcus, Weissella, Leuconostoc, Lactobacillus, Enterococcus, and Fructobacillus, growth of 41 strains was not significantly affected by the presence of sodium selenite, meanwhile growth of 10 strains was strongly inhibited resulting in a reduction in growth rate and amounts of accumulated biomass (Martínez et al. 2020). The ability to accumulate selenium was strain dependent and varied from 8 to 100%, and only Enterococcus casseliflavus (5 strains), Lactococcus lactis subsp. lactis (1 strain), and Lactobacillus plantarum(1 strain) showed 100% removal of selenium from the culture medium (Martínez et al. 2020).
Lactic acid bacteria can assimilate Se from inorganic compounds contained in the culture medium and convert it into organic forms accumulating it intracellularly (Kheradmand et al. 2014; Kousha et al. 2017; Pophaly et al. 2014; Zhang et al. 2009). It has been shown for the bacterium Bifidobacterium animalis that, when it was grown in medium containing sodium selenite, most of the organic Se in the cell was in protein (50.7–63.0%), polysaccharide (9.62–18.7%), nucleic acid (0.273–0.754%) fractions, and 20.8–30.9% remained was in other cell components, such as lipids, and in the form of low molecular weight Se compounds (Zhang et al. 2009). The same selenium distribution was shown to the cells of lactic acid bacteria Lactobacillus paracasei grown in the medium with selenite concentration 6 µg/ml: the highest content of organic Se was found in protein, 864.03 μg/g, the lowest in nucleic acids 295.12 μg/g, and its content in polysaccharides was 588.32 μg/g (Sun et al. 2022). Some lactic acid bacteria can reduce selenium salts to elemental Se by detoxification mechanisms (Andreoni et al. 2000; Lampis et al. 2014). SeNPs can be formed as Se0 aggregates alone or in combination with exopolysaccharides and proteins (Xu et al. 2018a, b).
The largest group of LAB with the ability to transform inorganic Se compounds into Se-containing organic compounds and SeNPs is formed by the strains of Lactobacillus genus. The possibility of using such species as L. acidophillus, L. rhamnosus, L. casei, L. delbrueckii subsp. bulgaricus, L. plantarum, L. fermentum, L. bulgaricus, L. helveticus, L. reureti, and L. brevis, has been reported (Crespo et al. 2021; Jin 2015; Husen et al. 2014; Kurek et al. 2016; Martínez et al. 2020; Pescuma et al. 2017; Pophaly et al. 2014; Spyridopoulou et al. 2021).
Less studied but the promising lactic bacteria strains belonging to Enterococcaceae family, because compared to other LAB, enterococci have a high ability to adapt to extreme conditions such as high temperatures, acidic or alkaline environments, and presence of toxic metals that helps them to reduce inorganic selenite to elemental Se0. Thus, it has been reported the ability of Enterococcus spp. to withstand very high (> 100 mM) selenite SeO32− concentrations (Tendenedzai et al. 2021). This allows to carry out biotransformation at high concentrations of inorganic Se sources, and, accordingly, to obtain a high yield of biosynthesized selenium compounds. For example, it was shown that strain of the bacterium E. faecalis removed 59.70 mg/l of selenite from the medium in 24 h, and the formation of elemental Se by this strain occurred within the first 0.5 h (Shoeibi and Mashreghi 2017).
Streptococcus thermophilus strain showed the ability to grow and accumulate selenium at a concentration of sodium selenite 80 mg per l of the medium (Yang et al. 2018).
Representatives of the genera Bifidobacterium and Pediococcus are the least studied in the bioconversion of inorganic Se compounds. However, several cases of successful use of strains of these genera in the conversion of selenite into Se-containing organic compounds and elemental Se are known (Jin 2015; Prokisch and Zommara 2008).
Thus, lactic acid bacteria, being safe microorganisms, are of interest as possible biotransformers of inorganic selenium both into its organic forms and into elemental selenium as nanoparticles.
Factors influencing biosynthesis of selenium nanoparticles by lactic acid bacteria
Source and concentration of selenium in the medium for cultivation of lactic acid bacteria synthesizing selenium nanoparticles
Among the factors influencing biotransformation of selenium, the most essential are the source of Se and its concentration in the medium (Liao and Wang 2022). The most common forms of Se in Earth’s biosphere are its inorganic salts. In alkaline environments, the thermodynamically stable form of Se is the selenate ion/Se oxyanion (SeO42−). Selenate is more soluble but less absorbable than selenite. Whereas selenite (SeO32−) is stable in an environment with neutral pH. It is less soluble than selenate, and can be biologically reduced to elemental Se (Se0) (Eszenyi et al. 2011). The inorganic selenium substance, which is used to be added to the medium for lactic acid bacteria cultivation, is Na2SeO3 (most often), followed by NaHSeO3, and very rare SeO2.
Numerous studies indicate variable tolerances of different strains of lactic acid bacteria to the presence in the medium of inorganic selenium. The best growth for lactic bacteria strains was observed at sodium selenite concentrations in the medium ranging from 1.0 to 2.5 mg/l, while further increase could inhibit bacterial growth. Thus, in the media with concentrations of Na2SeO3, mg/l: 0, 1, 4, 8, 16, and 32, accumulations of dry bacterial biomass by strain Lactobacillus bulgaricus, counted in g/l, were: 0.73, 0.82, 0.77, 0.74, 0.72, 0.66, and 0.63, respectively (Xia et al. 2007). Concentration 5 mg/l of Na2SeO3 in medium for cultivation is considered to be the maximum at which there are no significant changes in microbial growth and there is no evidence of massive cell death (Pescuma et al. 2017). This selenium concentration was used in the majority of studies of selenium biotransformation using lactic acid bacteria (Lamberti et al. 2011; Martínez et al. 2020; Pescuma et al. 2017; Xia et al. 2007). Comparison of results of lactic acid bacteria Pediococcus acidilactici cultivation in MRS broth with different concentrations of sodium selenite ranging from 0 to 4 mg/l added after 24 h of growth for the next 24 h showed that the presence of Na2SeO3 in concentration 1 and 2 mg/l did not inhibit the bacterial growth and maximum content of biomass, 1.44 g/l, was obtained in the medium with 1 mg/l Na2SeO3. Increasing the concentration of sodium selenite in medium from 0.5 to 4 mg/l resulted in an increase of organoselenium content from 0.14 to 1.45 g of biomass dry weight (Kousha et al. 2017). It was shown for L. bulgaricus that addition of Na2SeO3 in concentration less than 4 mg/l resulted in an increase of the total amino-acids content in bacterial biomass, meanwhile higher concentrations of sodium selenite led to decrease of their synthesis. Thus, at concentration of sodium selenite in the MRS broth 0, 1, 4 and 16 mg/l, the total content of amino acid consists 39.55, 41.76, 41.30, and 38.90 g/100 g of DW (Xia et al. 2007). The same trend for the enrichment of bacterial biomass with such essential elements as P, Mg, Mn, Zn, and Ca was observed (Xia et al. 2007).
At the high concentrations of selenium in the medium detoxification processes were activated resulting in production of elemental selenium detected by the color change to red in case of amorphous Se0 or gray in case of crystalline Se0 synthesis, indicating that toxic and colorless selenite was converted into nontoxic SeNPs (Xia et al. 2007). No elemental selenium deposits in bacterial cells of Lactobacillus. bulgaricus were observed when concentrations of selenium in the medium was lower than 4 mg/l Na2SeO3 testifying that most of selenium in cells is in organic forms, but under higher concentration of sodium selenite red color of deposits indicates on the presence of elemental selenium.
The general dependence of the effect of the concentration of Se compounds on the content of selenite is predictable, increasing the selenite concentration increases the formation of Se-containing compounds. Bioaccumulation of selenium by some strains of lactic acid bacteria growing in the medium with different concentrations of Na2SeO3 is shown in Table 1.
Most lactic bacteria strains reduce inorganic forms of Se into a variety of Se-containing compounds. A major part is occupied by Se-containing amino acids and only a tiny fraction is elemental SeNPs. Analyzing the conditions under which SeNPs are formed, it is possible to find that production of selenium containing nanoparticles is going at concentrations of selenite in medium higher than 4–5 mg/l when a sharp change in the color of the solutions to red is observed (Kurek et al. 2016; Pescuma et al. 2017; Spyridopoulou et al. 2021; Xia et al. 2007). However, the high concentration of selenite affects the viability of Se-synthesizing bacteria. For example, it was found that L. paracei 20241 cells were morphologically intact at inorganic Se concentrations of less than 6 mg/l, while higher values caused membrane rupture (Sun et al. 2022).
Meanwhile, it was shown that high concentrations of Se activate detoxification processes resulting in conversion of selenite to elemental Se0 (Kousha et al. 2017). Some strains of lactic acid bacteria could grow and transform inorganic selenium in medium with high initial concentration of sodium selenite. The concentration of 200 mg/l of sodium selenite has been reported, at which a complete growth of Streptococcus thermophilus inhibition was observed, and the selenite concentration that was determined as the point of inhibition was 140 mg/l (Castañeda-Ovando et al. 2019). The number of colony forming units per milliliter of S. thermophilus increased with the increase of selenite concentration in medium reaching the maximum at 80 mg of sodium selenite per l with the content of accumulated selenium 11.56 mg/g of bacterial biomass (Yang et al. 2018). It was shown for the two strains of Enterococcus genus that larger amounts of selenite were reduced and converted to Se0 faster at higher selenite concentrations, namely, the average reductions rate were 0.61, 1.92 and 3.23 mmol/l·h for SeO32− concentrations 1, 3 and 5 mM, respectively, probably dгe to the mechanism of response to the toxic effects of high selenite concentrations (Tendenedzai et al. 2021).
Chemical composition of the medium for cultivation of lactic acid bacteria synthesizing selenium nanoparticles
An increase in the concentration of selenite creates stressful conditions under which the cell consumes significantly more carbon and energy sources than usual. In particular, such observations were characteristic of Enterococcus strains, for which glucose consumption was 6.1, 6.5, and 7.0 mmol/h at selenite concentrations in the culture medium of 1, 3, and 5 mM, respectively (Tendenedzai et al. 2021).
Considering the high cost of special media for the accumulation of LAB biomass, it is important to find cheaper alternative ones. A cheese whey with sodium selenite was proposed for cultivation of Lactobacillus delbrueckii ssp. bulgaricus ATCC 11842, L. plantarum ATCC 14917, and L. casei ATCC 393 (Calomme et al. 1995). The use of milk permeate allowed strains of Levilactobacillus brevis, Lactiplantibacillus plantarum, and Pediococcus lolii to biotransform sodium selenite into SeNPs to a similar extent (99.0–100%) as using MRS medium, while the selenium nanoparticles had smaller size in the permeate (Zommara et al. 2022). In many studies milk or skimmed milk were used instead of MRS broth as media for the synthesis of SeNPs (Salama et al. 2021; Vicas et al. 2021). Skimmed buffalo's milk containing sodium selenite was used for SeNPs production by cultivation of yogurt starter cultures L. acidophilus CH2, L. plantarum L. plantarum 4496, and L. casei FEGY 9973 (Salama et al. 2021).
Another approach to increase Se biotransformation was proposed by Xu et al. (2020) that consists in application of osmotic stress created by high salt concentration 8% in the medium containing 0.17 mg/ml sodium selenite for Lactobacillus rhamnosus strain ATCC 53103 cultivation. The results indicated that accumulation of Se compared with the unstressed group, increased by 437.63 μg/g of bacterial biomass.
Correlation of selenium nanoparticles formation with lactic bacteria growth
Accumulation of selenium depends on the time of bacteria cultivation. The amounts of accumulated Se increased with the incubation time for Lactobacillus acidophilus CRL 636 and L. reuteri CRL 1101 (Pescuma et al. 2017). Formation of selenium nanoparticles during the cultivation of lactic acid bacteria could be monitored by the appearance of dark red colour. It was found that the time of its appearance varied for different strains and could appear in different stages of bacterial growth. Colour of cultural medium of Lactobacillus casei with initial content of NaHSeO3, 20 μg/ml, turned red only on 96 h of cultivation, which corresponded to late log/early stationary phase of bacterial growth (Spyridopoulou et al. 2021). It was noted that bacterial growth of L. casei was suppressed by the presence of sodium hydrogen selenite confirmed by the fact that concentrations of cells, CFU (colony formed units) in their late log/early stationary phase were (1.037 ± 0.018) × 109 CFUs per ml in control medium, but (0.210 ± 0.014) × 109 CFUs per ml for medium with NaHSeO3, and authors supposed that lactobacilli convert toxic forms of Se to less toxic ones. Cultural liquid became red during the stationary phase of L. bulgaricus growth (Xia et al. 2007). Lactobacillus paracasei HM1 changed the bright yellow medium to red color on 32 h of cultivation (El-Saadony et al. 2021a).
The rate of selenite transformation due to its reduction to elemental selenium also is strain dependent. For example addition of active cultures of some lactic acid bacteria to the medium with sodium selenite, 4 mM, resulted in the appearance of red colour in flask with L. rhamnosus within 12 h, L. acidophilus after 18 h, and L. plantarum after 26 h of incubation (Rajasree and Gayathri 2015).
Time of selenite addition to the medium for cultivation of lactic acid bacteria
The next point of influence on the growth characteristics of LAB strains is the time of selenite addition to the medium. It has been shown that later addition of selenite is better tolerated by bacterial strains (Jin 2015). When 10 μg/mg selenite was added at the beginning of cultivation (0 h), a decrease in cell biomass was observed for L. bulgaricus KCTC 3188, L. acidophilus KCTC 3142, L. casei KFRI 704, L. brevis 353, B. bifidum BGN4 strains by 40.0, 26.9, 41.9, 31.1, and 75.9%, respectively, compared to the control group without selenite. Whereas the addition of selenite after 12 h of cultivation did not cause a decrease in cell biomass. When adding higher concentrations of selenite in the amount of 1 mM for L. brevis 353, a similar pattern was observed, namely, the addition of selenite after 0 h reduced the accumulation of biomass by 59.3%, while the addition after 12 h did not cause a decrease in the final content of cell biomass. Therefore, the addition of selenite 12 h after the start of incubation can be recommended as a way to reduce the sensitivity of bacterial cells to the toxic effect of selenite.
Other parameters essential for selenium nanoparticles biosynthesis by lactic acid bacteria
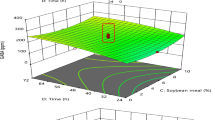
Other parameters that have less effect on the synthesis of selenium nanoparticles and more on the growth of bacterial cells, include temperature and pH, and in most cases are already known from the literature. However, to select the optimal values of a large number of parameters affecting SeNPs synthesis, Yang et al. (2017) proposed the use of response surface design. In this study, these parameters were determined for the strains L. delbrueckii ssp. bulgaricus and S. thermophilus. For L. delbrueckii ssp. bulgaricus, Se enrichment of 94.34% was achieved at pH 5.96, inoculum dose 6.73%, and temperature 33.24 °C. For S. thermophilus, these values were 6.37 units, 6%, and 40 °C, respectively.
Lactic acid bacteria synthesize SeNPs in aerobic or anaerobic conditions depending on their physiological characteristics. The majority of LAB produce SeNPs in aerobic conditions, however, some strains transform sodium selenite to SeNPs under anaerobic conditions, for instance, Lactobacillus casei 393 (Xu et al. 2018a, b).
Place of deposition of selenium nanoparticles synthesized by lactic acid bacteria
According to the place of elemental selenium deposition in cell, there are some observations. Elemental selenium is found to be located mainly inside of the cells of lactic acid bacteria. This was shown for Streptococcus thermophilus, Bifidobacterium sp. (Eszenyi et al. 2011), L. lactis CRL 2011, W. cibaria 10 and 25, E. casseliflavus 47 and 82, L. brevis CRL 2051, L. plantarum CRL 2030, F. tropaeoli CRL 2034 (Martínez et al. 2020), and Lactobacillus casei 393 (Xu et al. 2018a, b).
Amorphous granules of elemental selenium deposited in the cytoplasm and in the extracellular space of Lactobacillus bulgaricus were observed (Xia et al. 2007). The amorphous nature of SeNPs was evident by their red color (Guo et al. 2016; Kumar et al. 2014). It was suggested that reduction of inorganic selenium Se occurred as a result of activity of a membrane-associated reductase(s) and precipitated elemental selenium is deposited near cell membrane (Losi and Frankenberger 1997), meanwhile the presence of selenium particles in surrounding medium maybe result of their following expulsion. However, it is possible that granules of elemental selenium are released from the cells due to cell lysis (Tomei et al. 1995). The location of SeNPs in E. faecalis (Shoeibi and Mashreghi 2017), L. rhamnosus, L. acidophilus and L. plantarum (Rajasree and Gayathri 2015) was mainly extracellular. At a concentration of sodium selenite 64 mg/l, deposits (180 nm in diameter) were found in the cell not far from the cell wall (Xia et al. 2007). It was shown that high concentrations of selenium in the cultural medium activate detoxification processes resulting in conversion of selenite to elemental Se0, which is deposited near the bacterial cell membranes in the cells (Kousha et al. 2017).
So, it looks that biosynthesized by lactic acid bacteria from inorganic selenium compounds SeNPs could be located intracellular, extracellular or be bonded to the membrane.
Characteristics of selenium nanoparticles synthesized by lactic acid bacteria
The shape, structure, and size of SeNPs are dependent on different factors, such as source of selenium, selenite concentration, time of cultivation, temperature, and pH of the cultural medium. There is a significant difference in the size of SeNPs produced by LAB species. The size-dependence of nanoparticle properties is that with decreasing particle size, surface area-to-volume ratio increases, and bioavailability and biological activity against hydroxyl radicals and protective effect against DNA oxidation increase. In addition, the absorption of smaller nanoparticles in the gastrointestinal tract is 15–250 times higher than that of larger ones. It was reported that the size of these nanoparticles decreases in the presence of O2, as it promotes the oxidation of Se, as a result, the redox process becomes slower and smaller SeNPs are formed (Martínez et al. 2020; Spyridopoulou et al. 2021). Particle size depends on the strain as it was shown in Martínez et al (2020). Growing under the same conditions, lactic bacteria formed SeNPs with different size, nm: Fructobacillus tropaeoli and Lactobacillus brevis, 55–65; L. plantarum, 55–70; Weissella cibaria, 50–70; Streptococcus thermophilus, 50–100; Enterococcus casseliflavus, 65–145; Lactococcus lactis,130–155. The same results were obtained in study (Eszenyi et al. 2011), where three lactic acid bacteria strains synthesized selenium nanospheres with size, nm: Lactobacillus sp., 100–200; Bifidobacter sp., 400–500, and Streptococcus thermophilus, 50–100. Most lactic acid bacteria produce selenium nanoparticles of spherical shape, but it was reported about hexagonal SeNPs synthesized by Lactobacillus paracasei HM1 (El-Saadony et al. 2021a). SeNPs could be individual or form aggregated conglomerates (Spyridopoulou et al. 2021). The size of NPs formed by some lactic acid bacteria is shown in Table 2.
To recover SeNPs located inside the bacterial cells, it is needed to digest resistant cell wall. Acid hydrolysis with 37% hydrochloric acid (Eszenyi et al. 2011; Laslo et al. 2022), concentrated HNO3 and 30% (v/v) H2O2 (Martínez et al. 2020; Pescuma et al. 2017; Sasidharan et al. 2014), ultrasonic treatment (Moreno-Martin et al. 2017), sonication (Zhang et al. 2021) to disrupt cell walls, or enzymatic treatment (Alzate et al. 2008; Kousha et al. 2017; Palomo-Siguero et al. 2016; Xu et al. 2018a, b) for selenium recovery from bacterial cells are used. Comparative study of different enzymatic treatments using lysozyme, lipase, protease, a combination of lipase and protease, and lysozyme and protease for selenium recovery from the cells of lactic acid bacteria Pediococcus acidilactici showed that maximum selenium recovery, 98.5%, was obtained using a mixture of lysozyme and protease, meanwhile the lowest, 14.9%, was observed in case when lipase alone was used (Kousha et al. 2017). However, in spite of enzymatic hydrolysis being more efficient, acid hydrolysis is more often used as a cheaper method for selenium release. Meanwhile, for certain strains, for instance, E. faecalis (Shoeibi and Mashreghi 2017), L. rhamnosus, L. acidophilus and L. plantarum (Rajasree and Gayathri 2015), SeNPs are accumulated in cultural liquid and could be separated directly by centrifugation.
Based on the data presented, the range of possible sizes of SeNPs should be limited from 50 to 550 nm (Table 2). The size of SeNPs depends on the time of formation and the type of microorganism that reduces Se oxyanions. The size and form of NPs change such properties as conductivity, color, mechanical strength, magnetic behavior, and melting point. NPs can be found in crystalline and amorphous forms. The amorphous nature of SeNPs is evidenced by their red color. In most cases, for LAB, biogenic reduction of Se oxyanions induces the formation of amorphous spherical nanoparticles (Spyridopoulou et al. 2021). It was reported that the visible spectrum of selenium nanoparticles synthesized by Lactobacillus casei had a peak at 575–585 nm (Spyridopoulou et al. 2021), 601 nm for SeNPs synthesized by Providencia sp. DCX (Zhang et al. 2021), which are matched to results of Shirsat et al. (2015) found a characteristic peak at 590 nm for SeNPs produced by non-lactic bacteria Bacillus cereus strain CM100B. Absorption peak at 300 nm in UV–vis spectra was observed for SeNPs biosynthesized using Lactobacillus acidophilus ML14 (El-Saadony et al. 2021b) and at 263 nm for SeNPs biosynthesized using the free-cell supernatant of Bacillus licheniformis from SeO2 (Khiralla and El-Deeb 2015). These variations depending on the microbial producers of SeNPs might be explained by the presence of various organic materials accumulating on the surface of the nanoparticles during their formation.
Zeta potential is an important characteristic of the colloidal dispersion of nanoparticles, and nanoparticles with zeta potential greater than + 20 mV or less than − 20 mV are considered stable (Bhattacharjee 2016; Laslo et al. 2022). Nanoparticles with higher zeta potential have greater stability due to stronger electrostatic repulsion between them. The surface charge of nanoparticles is characterized by the zeta potential, which is a factor that plays an important role in antimicrobial activity, as the interaction between nanoparticles and the cell membrane is based on electrostatic adhesion. Both Gram-positive and Gram-negative bacteria have negatively charged surfaces that can attract positively charged nanoparticles. So, SeNPs have shown higher antimicrobial activity against Gram-positive bacteria than Gram-negative ones (Escobar-Ramírez 2021).
In addition, the study by Filipović et al. (2021) found the influence of zeta potential on antimicrobial action. Two types of stabilized particles were used: SeNPs-BSA and SeNPs-chitosan showed a positive potential (SeNPs-BSA, + 27 ± 3; SeNPs-Chit, + 24 ± 1), and SeNPs-Glucose showed a negative potential (− 45 ± 1). It was noted that the zeta-positive SeNPs demonstrated significantly greater antibacterial activity than negatively charged SeNPs, except for those that have been in contact with Escherichia coli. This bacterium was inhibited by negatively charged SeNPs at a lower concentration, 290 μg/ml, than SeNPs-BSA, which were positively charged, 400 μg/ml (Filipović 2021).
Properties of selenium nanoparticles synthesized by lactic acid bacteria
Antimicrobial activity of selenium nanoparticles synthesized by lactic acid bacteria
In recent years selenium nanoparticles have been extensively studied as antibacterial agents against multidrug-resistant bacteria (Han et al. 2021). It will be interesting to note, that SeNPs could be synthesized from selenium salts using plant extracts and removed from it by a simple precipitation (Kokila et al. 2017; Fardsadegh and Jafarizadeh-Malmiri 2019; Menon et al. 2019; Ndwandwe et al. 2021a, b, 2022). This is due reducing action of phenols, flavonoids and steroids, which are present in plant extracts on selenium salts producing selenium nanoparticles (Kokila et al. 2017). Biosynthesis of SeNPs using plant extracts is inexpensive, does not require any special conditions and could be easily realized. The antioxidant and antibacterial properties of SeNPs of either plant or microbial origin has been shown in several studies (Table 3).
Effect of inhibition depends on the concentration of SeNPs in the reaction medium. For instance, growth of such opportunistic pathogens as Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, and Vibrio parahemolyticus was strongly inhibited under the presence of 500 mg/l SeNPs synthesized by the Providencia sp. DCX (treatment was under shaking, 150 rpm, at 30 °C), and the most cells of tested bacteria were destroyed within 12 h (Zhang et al. 2021). However, at SeNPs concentration 100 mg/l, the growth rates of P. aeruginosa, E. coli, V. parahemolyticus, S. aureus and B. cereus decreased only by 91.27%, 63.28%, 55.60%, 51.63% and 39.00%, respectively. Interestingly, the inhibiting effect was more obvious for Gram negative bacteria (P. aeruginosa, and E. coli, V. parahemolyticus) than for Gram positive bacteria (S. aureus, B. cereus, and B. subtilis). The authors explained this by the fact that nanoparticles penetrate more easily through the thinner cell wall of Gram-negative bacteria, cause damage to the cell membrane, and assumed that it is possible to expect a higher antimicrobial activity of selenium nanoparticles against Gram-negative microorganisms than Gram-positive. But, selenium nanoparticles synthesized by Enterococcus faecalis possessed the antimicrobial activity against S. aureus (Gram-positive) and did not show inhibition activity against E. coli (Gram-negative) (Shoeibi and Mashreghi 2017).
It should be noted that not only selenium particles themselves possess antimicrobial activity, but also bacterial cells containing these nanoparticles demonstrate an increased ability to inhibit the growth of various microorganisms, including pathogenic bacteria. Thus, the inhibitory activities of lactic acid bacteria Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus containing selenium nanoparticles against pathogens Salmonella typhimurium, Listeria monocytogenes and opportunistic pathogens E. coli, S. aureus, increased in comparison with non-Se-containing strains (Yang et al. 2018), as well as selenium-enriched probiotics Lactobacillus acidophilus, Lactobacillus rhamnosus, and Streptococcus thermophilus strongly inhibited growth of opportunistic pathogen E. coli (Yang et al. 2009). The cells of Lactobacillus pentosus enriched with Se-NPs with average size of 106 nm demonstrated antimicrobial activity against foodborne pathogens Escherichia coli, Salmonella arizonae, Salmonella typhimurium ATCC 14028, and Staphylococcus aureus (Adebayo-Tay et al. 2021). The authors suggested that the addition of selenium nanoparticles to food can be used to control the development of food-borne pathogens.
Antioxidant properties of selenium nanoparticles synthesized by lactic acid bacteria
Cells of lactic acid bacteria strains Streptococcus thermophilus CCDM 144 and Enterococcus faecium CCDM 922A cultivated aerobically in the medium M17 containing 50 mg/l of sodium selenite for 24 h of aerobic cultivation at 37 °C were characterized by increasing of hydrophobic properties and antioxidant capacity compared with non-selenized cells. Hydrophobicity of bacterial cells was determined by their adhesion to hydrocarbons, antioxidant capacity was assessed by the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) method (Krausova et al. 2020) (Table 4).
Content of accumulated selenium in bacterial biomass was 7348 ± 2395 µg/g for S. thermophilus and 6491 ± 1158 µg/g for E. faecium. Authors supposed that LAB concentrated Se intracellularly in organic forms but also as elemental Se. The increased hydrophobicity of the bacterial cells enriched with selenium indicates the potential of strains to colonize the intestinal epithelium, but increase of antioxidant capacity was not significant.
Applications of selenium nanoparticles synthesized by lactic acid bacteria
Selenium nanoparticles can find applications in various fields of human activity (Fig. 1).
The potential use of selenium nanoparticles synthesized by lactic acid bacteria in food packaging materials
SeNPs possess antioxidant and antimicrobial activities, which makes their application in manufacturing of packaging materials for food products very promising due to the ability of these particles to extend the shelf life of food as well as reduce the amounts of chemical preservatives added to products to prolong time of their storage.
Application of different metal nanoparticles such as silver, gold, and metal (titanium, zinc, copper), oxide nanoparticles, and silicon dioxide in s packing materials are proposed to extend time for keeping food fresh (Dash et al. 2022; Peighambardous et al. 2019; Souza and Fernando 2016). One of the fields of application of the antimicrobial properties and antioxidant capacity of SeNPs synthesized by lactic acid bacteria could be also food packing. SeNPs due to their biocidal properties altogether with minimal toxicological effect are perspective bioagents to be used in the development of new packing materials (Ndwandwe et al. 2021b). Currently, the biocidal mechanism of SeNPs in food packaging is still unknown. However, it was assumed that SeNPs interact with the peptidoglycan layer of the bacterial cell wall and further damage the double-stranded DNA structure (Ndwandwe et al. 2021b). Currently, most studies on application of selenium nanoparticles in food packing were done with SeNPs produced chemically or using plant extracts to reduce sodium selenite (Table 5).
The problem with the use of SeNPs lies in their dosage, since at the wrong choice of concentration, these substances can have a toxic effect, but currently, there are no strict recommendations and guidelines for implementation of the appropriate dosage in the packing materials. However, SeNPs in the packing films are not in direct contact with food products. The location of SeNPs in the packaging material is usually in the middle layers of the package, where there is obviously no direct contact with the product or consumer (Garza-García et al. 2021; Ndwandwe et al. 2021b, 2022; Vera et al. 2016).
Addition of chemically synthesized selenium microparticles (SeMPs) with average particle size 900 nm, 1.5%, to polylactic acid (PLA) in the formation of film membrane increased film water resistance, UV resistance, and antibacterial active against E. coli and S. aureus (Lu et al. 2020). Incorporation of spherical SeNPs with diameter 50–60 nm, which were synthesized using the reduction of selenite with ascorbic acid in the presence of different stabilizers agent, in several multilayer laminated structure composed by polyethylene terephthalate (PET)-adhesive–low-density polyethylene (LDPT) ensured the antioxidant capacity to developed packing film (Vera et al. 2016). Because SeNPs were placed between PET and LDP in the adhesive (100 mg/l) there was not direct contact with packing product. This packing film was recommended to be used to protect the packaged product such as fresh meat, ready to eat, dried nuts, snacks, and everything susceptible of oxidation to extend the shelf life. Addition of SeNPs (0.8 wt%) with size in the range of 41–59 nm, produced by reduction of sodium selenite placed in aquatic solution of albumin for 14 h at 30 °C to dissolved in deionized water polyvinyl alcohol (PVA) and carboxymethyl cellulose (CMC) (70/30 wt%) allowed to increase antibacterial activity of produced packing film against up to 98% (S. aureus), 78% (B. cereus), 66% (E. coli), and 16% (P. aeruginosa) (Al-hakimi et al. 2022). Incorporation of SeNPs obtained from sodium selenite using ethanol extract from Moringa oleifera leaf (Ndwandwe et al. 2021a) into potato starch film gave it new antioxidant and antimicrobial properties (Ndwandwe et al. 2022). Coating of orange fruit with polymer NCT/PPE/SeNPs nanocomposite was effective against fungi Penicillium digitatum, which commonly cause postharvest spoilage of citrus fruit (Salem et al. 2022). Sodium selenite was reduced using extract from the peels of pomegranate fruit (PPE) to produce SeNPs and chitosan nanoparticles (NCT) were obtained by ionic gelation of chitosan extracted from white prawn shells. It was proposed to use chemically synthesized SeNPs to coat paper towels to possess them antimicrobial activity resulted in bacterial inhibition after 24 h of treatment against Staphylococcus aureus (90%) (Wang and Webster 2013), S. aureus (91%), E. coli (50–60%), and Staphylococcus epidermidis (91%) Pseudomonas aeruginosa (55%, but 84% after 48 h) (Wang et al. 2015).
So, application of SeNPs synthesized by lactic acid bacteria could find their application of production of packing materials with new useful properties to store food products.
Selenium nanoparticles synthesized by lactic acid bacteria in food products
Enrichment of lactic acid bacteria cells with non-toxic forms of Se and their use in food product manufacturing can be an alternative to the use of dietary supplements with this trace element. Lactic bacteria, which are able to produce selenium nanoparticles, are traditionally used in production of different food products. Thus, LAB enriched with SeNPs as well as SeNPs synthesized by lactic acid bacteria could find a wide range of application in manufacturing of functional food, production of which is one of the main trends in current food technologies (Ivanov et al. 2021). Some examples of food products enriched with selenium nanoparticles are present in Table 6.
In production of yogurt enriched with selenium nanoparticles, the powder of dry biomass of three lactic acid bacteria Lactobacillus acidophilus CH2, L. plantarum 4496, and L. casei FEGY 9973 (individual or mixed) containing SeNPs was used. Fresh buffalo's milk was heat-treated at 90 °C for 3 min, then cooled to 42 °C and inoculated with 2% yoghurt starter (L. bulgaricus and S. thermophilus in ratio 1:1), and then dry powder of SeNPs was added followed by procedure of yogurt preparation (Salama et al. 2021). The addition of the bacterial preparation with SeNPs did not have a noticeable effect on the chemical composition and texture of the final product (Salama 2021).
It was shown in experiment on mice that biomass of LAB Lactobacillus casei LC4P1, grown in skimmed milk with sodium selenite, 200 mg/l, for 48 h at 37 °C, or preparation of pure Se-NPs isolated from LAC biomass have ability to prevent toxic effects of cadmium, which is usually present in grain products, vegetables, and starchy roots like potatoes, on liver (Vicas et al. 2021). This was demonstrated by the restoration of blood hepatic markers and antioxidant enzymes, catalase and glutathione peroxidase. Application of LAC biomass enriched with SeNPs showed the best protective effects. So, the authors suggested that preparation of yogurt that includes both probiotic bacteria and elemental SeNPs will have increased nutritional and health values of the product and should have protective effects against heavy metals.
Strain of lactic acid bacteria Lactobacillus brevis isolated from kefir grains was grown in the MRS containing 5 mM sodium selenite at 30 °C for 36 h under shaking, 150 rpm, until elemental selenium was produced. Biomass of L. brevis enriched with elemental selenium was added to skimmed milk containing the starter culture (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) used for milk fermentation at 43 °C until the pH decreased below 4.5. Elemental selenium was detected in prepared yoghurt, a new functional dairy product (Deng et al. 2015).
Effect of lactic acid bacteria selenization (Lactobacillus brevis CRL 2051 or Fructobacillus tropaeoli CRL 2034) on their growth and survival during gastrointestinal digestion and after storage of fermented mixture of mango-orange juice with reconstituted skim-milk (10%, w/v) in a 1:4 (v/v) ratio was studied (Martínez et al. 2019). It was shown that selenization did not affect LAB growth in the fermented drink, and selenized cells survived after the 28 days of storage and in vitro gastrointestinal digestion. After digestion, Se was detected in the soluble fraction of the drinks fermented by both strains, being higher for L. brevis, 23.6 μg/l.
Fermented fruit drinks from passion fruit and mango were prepared from fresh juices inoculated with lactic acid bacteria (Levilactobacillus brevis CRL 2051 and Fructobacillus tropaeoli CRL 2034), then sodium selenite to achieve concentration of selenium 0.15 mg/l was added and fermentation of juice took place during 24 h at 30 °C (Crespo et al. 2021). Lactic acid bacteria Levilactobacillus brevis CRL 2051 and Fructobacillus tropaeoli CRL 2034 were chosen due to their ability to produce SeNPs from sodium selenite. Selenium accumulation values were in a range of 34–67% of the added Se in fermented fruit drinks, and authors proposed to use LAB strains for preparing functional fermented fruit beverages bio-enriched in Se.
Selenium bio-enriched drinks were prepared from pomegranate and table red grape juices added with sodium selenite and fermented by Levilactobacillus brevis CRL 2051 and Fructobacillus tropaeoli CRL 2034 individually or combined (Gaglio et al. 2021). Authors reported that after fermentation Se was found in the microbial pellets of fermented juices because of accumulation in biomass of lactic acid bacteria, and assumed that such fermented drinks could serve as functional foods especially for lactose intolerant people.
All these studies show the principal possibility of using lactic acid bacteria to enrich certain food products, such as yoghurts or fermented drinks, with selenium. However, when developing these functional products, it is mandatory to strictly adhere to the daily norms of this element consumption and, in accordance with this, recommendations should be developed on the possible amount of the proposed product to be included in the diet. Clinical studies of each such product must be carried out before it is approved in the diet.
Selenium nanoparticles synthesized by lactic acid bacteria in aquaculture
Application of selenium nanoparticles synthesized by lactic acid bacteria in aquaculture are intensively studied. Some examples are shown in Table 7.
Lactic acid bacteria strain Lactiplantibacillus plantarum was isolated from the intestine of healthy rainbow trout (Oncorhynchus mykiss) to be used as probiotic culture (Yanez-Lemus et al. 2022). These bacteria are natural inhabitants of the rainbow trout and are able to grow in its intestine. L. plantarum was enriched with SeNPs because of growth in MRS broth with 1 mM Na2SeO3 under microaerophilic condition (agitation 100 rpm), at 37 °C for 24 h. Sphere-like SeNPs with size 98–245 nm attached to the cell surface were verified by Scanning Electron Microscopy—Energy Dispersive X-ray Spectroscopy. Including in the fish diet lactic acid bacteria biomass with SeNPs resulted in improving fish healthy status.
Addition to diet of Nile tilapia (Oreochromis niloticus) biomass of yogurt lactic acid bacteria Streptococcus thermophilus CNCM I-1670, Lactobacillus delbrueckii subsp. bulgaricus NCAIM B 02206 enriched with spherical intracellular selenium nanoparticles with size 100–500 nm, 1 to 2 mg/kg of fish fed, resulted in significantly increase of body weight, weight gain, and feed efficiency (Dawood et al. 2020).
Selenium nanoparticles synthesized by lactic acid bacteria in agriculture
Selenium nanoparticles produced by lactic acid bacteria could be recommended as functional feed additives alone or with biomass of LAB (Eszenyi et al. 2011; Prokisch and Zommara 2008). SeNPs synthesized by lactic acid bacteria can find wide and diverse applications in agriculture such as production of silage to feed farm animals (Lee et al. 2019a, b) or protect plants against fungal pathogens (El-Saadony et al. 2021b) (Table 8).
Silage of wilted grass was provided with lactic bacteria L. brevis DSMZ, L. plantarum LF1, and L. plantarum SSL MC15, which were able to grow in the medium with sodium selenite and accumulate inside cells predominantly SeNPs (Lee et al. 2019a). Application of LAB enriched with selenium did not change the silage fermentation characteristics in comparison with use of non-selenized bacteria. It was shown the possibility to add sodium selenite directly to the fermented grass instead of medium for LAB cultivation. SeNPs in enriched silage were available to sheep and extended shelf life of the meat (8.05 vs 9.2 days; P < 0.05) (Lee et al. 2019b). The authors supposed that application of silage produced using lactic acid bacteria enriched with selenium will be healthy feed for ruminant animals.
Supplementation of rabbit diets with SeNPs synthesized by lactic acid bacteria isolated from human breast milk, 25 or 50 mg/kg, allowed rabbits to cope with heat stress demonstrated by improved growth, liver and kidney functions, and antioxidant status (Sheiha et al. 2020). This positive effect was greater in comparison with application of selenium nanoparticles synthesized chemically.
SeNPs synthesized extracellularly by L. acidophilus ML1 were proposed to be used instead of toxic pesticide to protect wheat from crown and root rot diseases (CRDs) caused by pathogenic fungi of the genus Fusarium (El-Saadony et al. 2021b). It was shown the ability of SeNPs to suppress the development and propagation of F. culmorum and F. graminearum, the major causal agents of CRDs, the development of which results in the severe loss of wheat yield and quality. The wheat grains were soaked in suspension of SeNPs (100 mg/ml) before being planted in fusarium-infected soil. It was shown that this treatment reduced by 75% the incidence of crown and root rot diseases of wheat, improved plant growth, and increased grain quantity and quality by 5–40%.
Selenium nanoparticles synthesized by lactic acid bacteria in medicine and veterinary
Selenium nanoparticles could be used as dietary supplements due to their lower toxicity and ability to slowly release selenium after ingestion. Their anti-cancer and anti-diabetic properties have also been found, so SeNPs may also serve as a therapeutic agent (Skalickova et al. 2017). Potential application of SeNPs produced intracellularly under anaerobic conditions by probiotic strain Lactobacillus casei ATCC 393 as anticancer and antioxidant agents was shown (Xu et al. 2018a). Se-NPs produced by L. casei ATCC 393 also significantly inhibited the growth of human liver tumor cell line-HepG2, and alleviated diquat-induced IPEC-J2 oxidative damage (Xu et al. 2018b). In vivo and in vitro experiments on mice, it was shown that SeNPs synthesized by L. casei ATCC 393 protected against enterotoxigenic Escherichia coli K88 caused intestinal barrier dysfunction (Xu et al. 2018b). The cells of the same strain Lactobacillus casei ATCC 393 enriched with SeNPs had protective effects against D-galactose/aluminum chloride-induced Alzheimer's disease model mice, and authors suggested that L. casei ATCC 393-SeNPs may be used as a food additive to prevent the neurodegenerative disease (Qiao et al. 2022).
To control delivery applications, spherical selenium nanoparticles with size of 400 nm were produced by using probiotic bacteria Lactobacillus casei and were incorporated into alginate and alginate/chitosan microspheres by cross-linking and ionotropic gelation (Cavalu et al. 2017). The study of cumulative selenium release in simulated gastrointestinal solutions showed the overall effect is the enhancement of total percentage release concomitant with the longer duration of action. Authors proposed to use alginate/chitosan as a matrix for selenium delivery in duodenum, caecum and colon.
It was shown that SeNPs have a great potential in treatment of diabetes and Alzheimer's disease, oxidative stress, inflammation disorders such as rheumatoid arthritis, in anticancer therapy, and serve as a protector against toxic agents including heavy metals (Ferro et al. 2021; Khurana et al. 2019; Rehman et al. 2021). Selenium nanoparticles synthesized by lactic acid bacteria could find their application in medicine and veterinary as safer than ones produced chemically.
In veterinary, probiotics Levilactobacillus brevis 23017 and L. brevis 23017 enriched with Se-NPs improved the immune effect of the alum adjuvants vaccine in mouse and rabbit models, respectively (Liu et al. 2023). Experiments conducted on laboratory animals showed effectiveness of application of different selenium nanoparticles-enriched lactic acid in treatment of some serious organism’s disorders, for example use of SeNPs-enriched Lactobacillus brevis as immunostimulators, and enhanced immune response in cancer-affected mice (Yazdi et al. 2013).
Conclusions
Among the microorganisms, certain lactic acid bacteria (LAB) being generally recognized as safe are preferable choice for biosynthesis of selenium nanoparticles for their use in food, medicine, veterinary, fish farming, and agriculture. Process of selenium nanoparticles biosynthesis by lactic acid bacteria is depend on source and concentration of inorganic selenium, composition of the medium for cultivation, time of inorganic selenium addition to the medium, phase of bacterial growth, and their physiological needs such as aeration, temperature, and pH. It is possible to use biomass of lactic acid bacteria enriched with selenium nanoparticles or pure SeNPs recovered from bacterial cells in case of their intracellular synthesis or from supernatant in case when they are synthesized extracellularly. Selenium nanoparticles synthesized by lactic acid bacteria possess antimicrobial and antioxidant properties, and can be used in different areas of human activities. SeNPs produced by LAB could be used as an element of packing materials to extend shelf life of food products and protect them from microbial spoilage. In production of food lactic acid bacteria enriched with selenium nanoparticles could be used for preparation of functional products bio-enriched with selenium. Including lactic acid bacteria biomass with SeNPs in the fish or farm animal diets resulted in disease prevention, detoxification, and improving their healthy status. SeNPs is proposed to be used in veterinary and medicine. However, there is a lack of medicinal studies and clinical trials are needed to make practical recommendations. Thus, the importance and promise of using lactic acid bacteria for the biosynthesis of selenium nanoparticles are evident and deserve the attention of researchers to ensure their practical use.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adebayo-Tayo BC, Yusuf BO, Alao SO (2021) Antibacterial activity of intracellular greenly fabricated selenium nanoparticle of Lactobacillus pentosus ADET MW861694 against selected food pathogens. Int J Biotechnol 10:39–51. https://doi.org/10.18488/journal.57.2021.101.39.51
Alam H, Khatoon N, Khan MA, Husain SA, Saravanan M, Sardar M (2020) Synthesis of selenium nanoparticles using probiotic bacteria Lactobacillus acidophilus and their enhanced antimicrobial activity against resistant bacteria. J Clust Sci 31:1003–1011. https://doi.org/10.1007/s10876-019-01705-6
Al-hakimi AN, Asnag GM, Alminderej F, Alhagri IA, Al-Hazmy SM, Abdallah EM (2022) Enhanced structural, optical, electrical properties and antibacterial activity of selenium nanoparticles loaded PVA/CMC blend for electrochemical batteries and food packaging applications. Polym Test 116:107794. https://doi.org/10.1016/j.polymertesting.2022.107794
Allende A, Bolton DJ, Chemaly M et al (2016) Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J 14:e04524. https://doi.org/10.2903/j.efsa.2016.4524
Al-Saggaf MS (2021) Prevalence of resistant Staphylococcus aureus strains in frozen meat and their control using phytosynthesized selenium nanoparticles. Kuwait J Sci 48:1–18. https://doi.org/10.48129/kjs.v48i4.10650
Al-Shemmary AJ, Malallah HA, Al-Mashhadi AR, Jaber AH, Shaker ZB (2022) Biosynthesis of selenium nanoparticles using probiotic Bacillus clausii and their antibacterial efficacy against multidrug-resistant bacteria (MDR). J Pharm Negat Results 13:1011–1019. https://doi.org/10.47750/pnr.2022.13.S07.142
Alzate A, Fernández-Fernández A, Pérez-Conde MC, Gutiérrez AM, Cámara C (2008) Comparison of biotransformation of inorganic selenium by Lactobacillus and Saccharomyces in lactic fermentation process of yogurt and kefir. J Agric Food Chem 56:8728–8736. https://doi.org/10.1021/jf8013519
Andreoni V, Luischi MM, Cavalca L, Erba D, Ciappellano S (2000) Selenite tolerance and accumulation in the Lactobacillus species. Ann Microbiol 50:77–88
Barceloux DG (1999) Selenium. J Toxicol Clin Toxicol 37:145–172. https://doi.org/10.1081/clt-100102417
Bhattacharjee S (2016) DLS and zeta potential: what they are and what they are not? J Control Release 235:337–351. https://doi.org/10.1016/j.jconrel.2016.06.017
Brigelius-Flohé R (2018) Selenium in human health and disease: an overview. In: Michalke B (ed) Selenium. Molecular and integrative toxicology. Springer, Cham, pp 3–26
Buchovec I, Klimkaitė L, Sužiedėlienė E, Bagdonas S (2022) Inactivation of opportunistic pathogens Acinetobacter baumannii and Stenotrophomonas maltophilia by antimicrobial photodynamic therapy. Microorganisms 10:506. https://doi.org/10.3390/microorganisms10030506
Calomme MR, Van den Branden K, Vanden Berghe DA (1995) Selenium and Lactobacillus species. J Appl Bacteriol 79:331–340. https://doi.org/10.1111/j.1365-2672.1995.tb03145.x
Castañeda-Ovando A, Segovia-Cruz JA, Flores-Aguilar JF, Rodríguez-Serrano GM, Salazar-Pereda V, Ramírez-Godínez J, Contreras-López E, Jaimez-Ordaz J, González-Olivares LG (2019) Serine-enriched minimal medium enhances conversion of selenium into selenocysteine by Streptococcus thermophilus. J Dairy Sci 102:6781–6789. https://doi.org/10.3168/jds.2019-16365
Cavalu S, Prokisch J, Laslo V, Vicas S (2017) Preparation, structural characterisation and release study of novel hybrid microspheres entrapping nanoselenium, produced by green synthesis. IET Nanobiotechnol 11:426–432. https://doi.org/10.1049/iet-nbt.2016.0107
Colautti A, Arnoldi M, Comi G, Iacumin L (2022) Antibiotic resistance and virulence factors in lactobacilli: something to carefully consider. Food Microbiol 103:103934. https://doi.org/10.1016/j.fm.2021.103934
Cremonini E, Zonaro E, Donini M, Lampis S, Boaretti M, Dusi S, Melotti P, Lleo MM, Vallini G (2016) Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb Biotechnol 9:758–771. https://doi.org/10.1111/1751-7915.12374
Crespo L, Gaglio R, Martínez FG, Martin GM, Franciosi E, Madrid-Albarrán Y, Settanni L, Mozzi F, Pescuma M (2021) Bioaccumulation of selenium by fruit origin lactic acid bacteria in tropical fermented fruit juices. LWT 151:112103. https://doi.org/10.1016/j.lwt.2021.112103
Cruz DM, Mi G, Webster TJ (2018) Synthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa. J Biomed Mater Res A 106:1400–1412. https://doi.org/10.1002/jbm.a.36347
Dash KK, Deka P, Bangar SP, Chaudhary V, Trif M, Rusu A (2022) Applications of inorganic nanoparticles in food packaging: a comprehensive review. Polymers (basel) 143:521. https://doi.org/10.3390/polym14030521
Dawood MA, Zommara M, Eweedah NM, Helal AI (2020) The evaluation of growth performance, blood health, oxidative status and immune-related gene expression in Nile tilapia (Oreochromis niloticus) fed dietary nanoselenium spheres produced by lactic acid bacteria. Aquaculture 515:734571. https://doi.org/10.1016/j.aquaculture.2019.734571
Deng Y, Man C, Fan Y, Wang Z, Li L, Ren H, Cheng W, Jiang Y (2015) Preparation of elemental selenium-enriched fermented milk by newly isolated Lactobacillus brevis from kefir grains. Int Dairy J 44:31–36. https://doi.org/10.1016/J.IDAIRYJ.2014.12.008
EFFCA (2003) European Food and Feed Cultures Association (EFFCA). Definition of Microbial Food Culture (MFC); EFFCA: Brussels, Belgium. http://www.effca.org/content/food-culture . Accessed 3 Apr 2018
EFSA (2016) Update of the List of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 4: suitability of taxonomic units notified to EFSA until March 2016. https://www.efsa.europa.eu/en/efsajournal/pub/4522 . Accessed 3 Apr 2018
El-Saadony MT, Saad AM, Taha TF, Najjar AA, Zabermawi NM, Nader MM, Salama A (2021a) Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J Biol Sci 28:6782–6794. https://doi.org/10.1016/j.sjbs.2021.07.059
El-Saadony MT, Saad AM, Najjar AA, Alzahrani SO, Alkhatib FM, Shafi ME, Selem E, Desoky EM, Fouda SEE, El-Tahan AM, Hassan MA (2021b) The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J Biol Sci 28:4461–4471. https://doi.org/10.1016/j.sjbs.2021.04.043
Escobar-Ramírez MC, Castañeda-Ovando A, Pérez-Escalante E, Rodríguez-Serrano GM, Ramírez-Moreno E, Quintero-Lira A, Contreras-López E, Añorve-Morga J, Jaimez-Ordaz J, González-Olivares LG (2021) Antimicrobial activity of Se-nanoparticles from bacterial biotransformation. Fermentation 7:130. https://doi.org/10.3390/fermentation7030130
Eszenyi P, Sztrik A, Babka B, Prokisch J (2011) Elemental, nano-sized (100–500 nm) selenium production by probiotic lactic acid bacteria. Int J Biosci Biochem Bioinform 1:148–153. https://doi.org/10.7763/IJBBB.2011.V1.27
Fardsadegh B, Jafarizadeh-Malmiri H (2019) Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process Synth 8:399–407. https://doi.org/10.1515/gps-2019-0007
Ferro C, Florindo HF, Santos HA (2021) Selenium nanoparticles for biomedical applications: from development and characterization to therapeutics. Adv Healthc Mater 10:e2100598. https://doi.org/10.1002/adhm.202100598
Fetsch A, Johler S (2018) Staphylococcus aureus as a foodborne pathogen. Curr Clin Microbiol Rep 5:88–96. https://doi.org/10.1007/s40588-018-0094-x
Filipović N, Ušjak D, Milenković MT, Zheng K, Liverani L, Boccaccini AR, Stevanović MM (2021) Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Front Bioeng Biotechnol 8:624621. https://doi.org/10.3389/fbioe.2020.624621
Fordyce FM (2013) Selenium deficiency and toxicity in the environment. In: Selinus O (ed) Essentials of medical geology. Springer, Cham, pp 375–416
Gaglio R, Pescuma M, Madrid-Albarrán Y, Franciosi E, Moschetti G, Francesca N, Mozzi F, Settanni L (2021) Selenium bio-enrichment of Mediterranean fruit juices through lactic acid fermentation. Int J Food Microbiol 354:109248. https://doi.org/10.1016/j.ijfoodmicro.2021.109248
Garza-García JJ, Hernández-Díaz JA, Zamudio-Ojeda A, León-Morales JM, Guerrero-Guzmán A, Sánchez-Chiprés DR, López-Velázquez JC, García-Morales S (2021) The role of selenium nanoparticles in agriculture and food technology. Biol Trace Elem Res 200:2528–2548. https://doi.org/10.1007/s12011-021-02847-3
GDR, Government Dietary Recommendations (2016). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/618167/government_dietary_recommendations.pdf
Ghosh S, Ahmad R, Zeyaullah M, Khare SK (2021) Microbial nano-factories: synthesis and biomedical applications. Front Chem 9:626834. https://doi.org/10.3389/fchem.2021.626834
Gómez-Gómez B, Pérez-Corona T, Mozzi F, Pescuma M, Madrid Y (2019) Silac-based quantitative proteomic analysis of Lactobacillus reuteri CRL 1101 response to the presence of selenite and selenium nanoparticles. J Proteomics 195:53–65. https://doi.org/10.1016/j.jprot.2018.12.025
Gorrie CL, Mirčeta M, Wick RR et al (2022) Genomic dissection of Klebsiella pneumoniae infections in hospital patients reveals insights into an opportunistic pathogen. Nat Commun 13:3017. https://doi.org/10.1038/s41467-022-30717-6
Gunti L, Dass RS, Kalagatur NK (2019) Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: antioxidant, antimicrobial, and biocompatibility. Front Microbiol 10:931. https://doi.org/10.3389/fmicb.2019.00931
Guo L, Huang K, Liu H (2016) Biocompatibility selenium nanoparticles with an intrinsic oxidase-like activity. J Nanopart Res 18:74. https://doi.org/10.1007/s11051-016-3357-6
Han HW, Patel KD, Kwak JH, Jun SK, Jang TS, Lee SH, Knowles JC, Kim HW, Lee HH, Lee JH (2021) Selenium nanoparticles as candidates for antibacterial substitutes and supplements against multidrug-resistant bacteria. Biomolecules 11:1028. https://doi.org/10.3390/biom11071028
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, Baron M, Melcova M, Opatrilova R, Zidkova J, Bjørklund G, Sochor J, Kizek R (2018) Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomed 13:2107–2128. https://doi.org/10.2147/IJN.S157541
Husen A, Siddiqi KS (2014) Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol 12:1–10. https://doi.org/10.1186/s12951-014-0028-6
IMFNB, Institute of Medicine, Food and Nutrition Board (2000) Dietary reference intakes: vitamin C, vitamin E, selenium, and carotenoids. National Academy Press, Washington, DC
Ivanov V, Shevchenko O, Marynin A, Stabnikov V, Gubenia O, Stabnikova O, Shevchenko A, Gavva O, Saliuk A (2021) Trends and expected benefits of the breaking edge food technologies in 2021–2030. Ukr Food J 10:7–36. https://doi.org/10.24263/2304-974X-2021-10-1-3
Jamroz E, Kopel P, Juszczak L, Jamroz E, Kopel P, Juszczak L, Kawecka A, Býtešníková Z, Milosavljević V, Makarewicz M (2019) Development of furcellaran-gelatin films with Se-AgNPs as an active packaging system for extension of mini kiwi shelf life. Food Packag Shelf Life 21:1–9. https://doi.org/10.1016/j.fpsl.2019.100339
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Jin W (2015) Enrichment of selenium and its selenium species analysis in Bifidobacterium bifidum BGN4, Dissertation
Kheradmand E, Rafii F, Yazdi MH, Sepahi AA, Shahverdi AR, Oveisi MR (2014) The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against Candida albicans. DARU J Pharm Sci 22(1):48
Khiralla GM, El-Deeb BA (2015) Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT 63:1001–1007. https://doi.org/10.1016/j.lwt.2015.03.086
Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C (2019) Therapeutic applications of selenium nanoparticles. Biomed Pharmacother 111:802–812. https://doi.org/10.1016/j.biopha.2018.12.146
Kieliszek M, Blazejak S (2016) Current knowledge on the importance of selenium in food for living organisms: a review. Molecules 21:609. https://doi.org/10.3390/molecules21050609
Kim MJ, Ku S, Kim SY, Lee HH, Jin H, Kang S, Li R, Johnston TV, Park MS, Ji GE (2018) Safety evaluations of Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI. Int J Mol Sci 19:1422. https://doi.org/10.3390/ijms19051422
Kokila K, Elavarasan N, Sujath V (2017) Diospyros montana leaf extract-mediated synthesis of selenium nanoparticles and their biological applications. New J Chem 41:7481–7490. https://doi.org/10.1039/C7NJ01124E
Kousha M, Yeganeh S, Keramat AA (2017) Effect of sodium selenite on the bacteria growth, selenium accumulation, and selenium biotransformation in Pediococcus acidilactici. Food Sci Biotechnol 26:1013–1018. https://doi.org/10.1007/s10068-017-0142-y
Krausova G, Kana A, Hyrslova I, Mrvikova I, Kavkova M (2020) Development of selenized lactic acid bacteria and their selenium bioaccummulation capacity. Fermentation 6:91. https://doi.org/10.3390/fermentation6030091
Kumar A, Sevonkaev I, Goia DV (2014) Synthesis of selenium particles with various morphologies. J Colloid Interface Sci 416:119–123. https://doi.org/10.1016/j.jcis.2013.10.046
Kurek E, Ruszczynska A, Wojciechowski M, Luciuk A, Michalska-Kacymirow M, Motyl I, Bulska E (2016) Bio-transformation of selenium in Se-enriched bacterial strains of Lactobacillus casei. Rocz Panstw Zakl Hig 67:253–262
Lamberti C, Mangiapane E, Pessione A, Mazzoli R, Giunta C, Pessione E (2011) Proteomic characterization of a selenium-metabolizing probiotic Lactobacillus reuteri Lb2 BM for nutraceutical applications. Proteomics 11:2212–2221. https://doi.org/10.1002/pmic.201000747
Lampis S, Zonaro E, Bertolini C, Bernardi P, Butler CS, Vallini G (2014) Delayed formation of zero-valent selenium nanoparticles by Bacillus mycoides SeITE01 as a consequence of selenite reduction under aerobic conditions. Microb Cell Fact 13:35. https://doi.org/10.1186/1475-2859-13-35
Laslo V, Pinzaru SC, Zaguła G, Kluz M, Vicas SI, Cavalu S (2022) Synergic effect of selenium nanoparticles and lactic acid bacteria in reduction cadmium toxicity. J Mol Struct 1247:131532. https://doi.org/10.1016/j.molstruc.2021.131325
Lee MRF, Fleming HR, Cogan T, Hodgson C, Davies DR (2019a) Assessing the ability of silage lactic acid bacteria to incorporate and transform inorganic selenium within laboratory scale silos. Anim Feed Sci Technol 253:125–134. https://doi.org/10.1016/j.anifeedsci.2019.05
Lee MRF, Fleming HR, Whittington F, Hodgson C, Suraj PT, Davies DR (2019b) The potential of silage lactic acid bacteria-derived nano-selenium as a dietary supplement in sheep. Anim Prod Sci 59:1999. https://doi.org/10.1071/an19258
Liao J, Wang C (2022) Factors affecting selenium-enrichment efficiency, metabolic mechanisms and physiological functions of selenium-enriched lactic acid bacteria. J Future Foods 2:285–293. https://doi.org/10.1016/j.jfutfo.2022.08.001
Liu R, Sun W, Sun T, Zhang W, Nan Y, Zhang Z, Xiang K, Yang H, Wang F, Ge J (2023) Nano selenium-enriched probiotic Lactobacillus enhances alum adjuvanticity and promotes antigen-specific systemic and mucosal immunity. Front Immunol 14:1116223. https://doi.org/10.3389/fimmu.2023
Losi M, Frankenberger WT (1997) Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: isolation and growth of the bacterium and its expulsion of selenium particles. Appl Environ Microbiol 63:3079–3084. https://doi.org/10.1128/aem.63.8.3079-3084.1997
Lu R, Sameen DE, Qin W, Wu D, Dai J, Li S, Liu Y (2020) Development of polylactic acid films with selenium microparticles and its application for food packaging. Coatings 10:280. https://doi.org/10.3390/coatings10030280
Mal J, Veneman WJ, Nancharaiah YV, van Hullebusch ED, Peijnenburg WJGM, Vijver MG, Lens PNL (2017) A comparison of fate and toxicity of selenite, biogenically, and chemically synthesized selenium nanoparticles to zebrafish (Danio rerio) embryogenesis. Nanotoxicology 11:87–97. https://doi.org/10.1080/17435390.2016.1275866
Martínez FG, Barrientos MEC, Mozzi F, Pescuma M (2019) Survival of selenium-enriched lactic acid bacteria in a fermented drink under storage and simulated gastro-intestinal digestion. Food Res Int 123:115–124. https://doi.org/10.1016/j.foodres.2019.04.057
Martínez FG, Moreno-Martin G, Pescuma M, Madrid-Albarrán Y, Mozzi F (2020) Biotransformation of selenium by lactic acid bacteria: formation of seleno-nanoparticles and seleno-amino acids. Front Bioeng Biotechnol 8:506. https://doi.org/10.3389/fbioe.2020.00506
Menon S, Shrudhi S, Agarwal H, Venkat Kumar S (2019) Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloids Interface Sci Commun 29:8. https://doi.org/10.1016/j.colcom.2018.12.004
Monsen ER (2000) Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc 100:637–640. https://doi.org/10.1016/S0002-8223(00)00189-9
Moreno-Martin G, Pescuma M, Pérez-Corona T, Mozzi F, Madrid Y (2017) Determination of size and mass-and number-based concentration of biogenic SeNPs synthesized by lactic acid bacteria by using a multimethod approach. Anal Chim Acta 992:34–41. https://doi.org/10.1016/j.aca.2017.09.033
Mörschbächer AP, Dullius A, Dullius CH, Brandt CR, Kuhn D, Brietzke DT, José Malmann Kuffel F, Etgeton HP, Altmayer T, Gonçalves TE, Schweizer YA, Oreste EQ, Ribeiro AS, Lehn DN, Volken de Souza CF, Hoehne L (2019) Assessment of selenium bioaccumulation in lactic acid bacteria. J Dairy Sci 101:10626–10635. https://doi.org/10.3168/jds.2018-14852
Murugesan G, Nagaraj K, Sunmathi D, Subramani K (2019) Methods involved in the synthesis of selenium nanoparticles and their different applications: a review. Eur J Biomed Pharm Sci 6:189–194
NAS (2000) Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. A report of the panel on dietary antioxidants and related compounds, subcommittees on upper reference levels of nutrients and interpretation and uses of dietary reference intakes, and the standing committee on the scientific evaluation of dietary reference intakes. National Academy of Sciences, Institute of Medicine, Food and Nutrition Board, Washington, DC, pp 284–324
Ndwandwe BK, Malinga SP, Kayitesi E, Dlamini BC (2021a) Solvothermal synthesis of selenium nanoparticles with polygonal-like nanostructure and antibacterial potential. Mater Lett 304:130619. https://doi.org/10.1016/j.matlet.2021.130619
Ndwandwe BK, Malinga SP, Kayitesi E, Dlamini BC (2021b) Advances in green synthesis of selenium nanoparticles and their application in food packaging. Int J Food Sci Technol 56:2640–2650. https://doi.org/10.1111/ijfs.14916
Ndwandwe BK, Malinga SP, Kayitesi E, Dlamini BC (2022) Selenium nanoparticles–enhanced potato starch film for active food packaging application. Int J Food Sci Technol 57:6512–6521. https://doi.org/10.1111/ijfs.15990
Palomo-Siguero M, Gutiérrez AM, Pérez-Conde C, Madrid Y (2016) Effect of selenite and selenium nanoparticles on lactic bacteria: a multi-analytical study. Microchem J 126:488–495. https://doi.org/10.1016/j.microc.2016.01.010
Peighambardoust SJ, Peighambardoust SH, Pournasir N, Pakdel PM (2019) Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag Shelf Life 22:100420. https://doi.org/10.1016/j.fpsl.2019.100420
Pescuma M, Gomez-Gomez B, Perez-Corona T, Font G, Madrid Y, Mozzi F (2017) Food prospects of selenium enriched-Lactobacillus acidophilus CRL 636 and Lactobacillus reuteri CRL 1101. J Funct Foods 35:466–473. https://doi.org/10.1016/j.jff.2017.06.009
Piacenza E, Presentato A, Ambrosi E, Speghini A, Turner RJ, Vallini G, Lampis S (2018) Physical-chemical properties of biogenic selenium nanostructures produced by Stenotrophomonas maltophilia SeITE02 and Ochrobactrum sp. MPV1. Front Microbiol 9:3178. https://doi.org/10.3389/fmicb.2018.03178
Plata K, Rosato AE, Wegrzyn G (2009) Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochim Pol 56:597–612
Pophaly SD, Singh P, Kumar H, Tomar SK, Singh R (2014) Selenium enrichment of lactic acid bacteria and bifidobacteria: a functional food perspective. Trends Food Sci Technol 39:135–145. https://doi.org/10.1016/j.tifs.2014.07.006
Pouri S, Motamedi H, Honary S, Kazeminezhad I (2018) Biological synthesis of selenium nanoparticles and evaluation of their bioavailability. Braz Arch Biol Technol 60:17160452. https://doi.org/10.1590/1678-4324-2017160452
Prokisch J, Zommara MA (2008) US8003071B2. Process for producing elemental selenium nanospheres. https://patents.google.com/patent/US8003071B2/en
Qiao L, Chen Y, Song X, Dou X, Xu C (2022) Selenium nanoparticles-enriched Lactobacillus casei ATCC 393 prevents cognitive dysfunction in mice through modulating microbiota-gut-brain axis. Int J Nanomed 17:4807–4827. https://doi.org/10.2147/IJN.S374024
Qiao L, Dou X, Song X, Chang J, Zeng X, Zhu L, Xu C (2023) Selenite Bioremediation by food-grade probiotic Lactobacillus casei ATCC 393: insights from proteomics analysis. Microbiol Spectr 11:e0065923. https://doi.org/10.1128/spectrum.00659-23
Rajasree RSR, Gayathri S (2015) Extracellular biosynthesis of Selenium nanoparticles using some species of Lactobacillus. Indian J Mar Sci 43:766–775
Rehman A, John P, Bhatti A (2021) Biogenic selenium nanoparticles: potential solution to oxidative stress mediated inflammation in rheumatoid arthritis and associated complications. Nanomaterials 11:2005. https://doi.org/10.3390/nano11082005
Salama HH, El-Sayed HS, Abd-Rabou NS, Hassan ZM (2021) Production and use of eco-friendly selenium nanoparticles in the fortification of yoghurt. J Food Process Preserv 45:e15510. https://doi.org/10.1111/jfpp.15510
Salem MF, Abd-Elraoof WA, Tayel AA, Alzuaibr FM, Abonama OM (2022) Antifungal application of biosynthesized selenium nanoparticles with pomegranate peels and nanochitosan as edible coatings for citrus green mold protection. J Nanobiotechnol 20:182. https://doi.org/10.1186/s12951-022-01393-x
Sasidharan S, Balakrishnaraja R (2014) Comparison studies on the synthesis of selenium nanoparticles by various microorganisms. Int J Pure Appl Biosci 2:112–117
Shakibaie M, Shahverdi AR, Faramarzi MA, Hassanzadeh GR, Rahimi HR, Sabzevari O (2012) Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm Biol 51:58–63. https://doi.org/10.3109/13880209.2012.710241
Sheiha AM, Abdelnour SA, Abd El-Hack ME, Khafaga AF, Metwally KA, Ajarem JS, Maodaa SN, Allam AA, El-Saadony MT (2020) Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals 10:430. https://doi.org/10.3390/ani10030430
Shirsat S, Kadam A, Naushad M, Mane RS (2015) Selenium nanostructures: microbial synthesis and applications. RSC Adv 5:92799–92811. https://doi.org/10.1039/C5RA17921A
Shoeibi S, Mashreghi M (2017) Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J Trace Elem Med Biol 39:135–139. https://doi.org/10.1016/j.jtemb.2016.09.003
Skalickova S, Milosavljevic V, Cihalova K, Horky P, Richtera L, Adam V (2017) Selenium nanoparticles as a nutritional supplement. Nutrition 33:83–90. https://doi.org/10.1016/j.nut.2016.05.001
Souza VGL, Fernando AL (2016) Nanoparticles in food packaging: biodegradability and potential migration to food—a review. Food Packag Shelf Life 8:63–70. https://doi.org/10.1016/j.fpsl.2016.04.001
Spyridopoulou K, Tryfonopoulou E, Aindelis G, Ypsilantis P, Sarafidis C, Kalogirou O, Chlichlia K (2021) Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo. Nanoscale Adv 3:2516–2528. https://doi.org/10.1039/D0NA00984A
Stabnikova O, Ivanov V, Larionova I, Stabnikov V, Bryszewska MA, Lewis J (2008) Ukrainian dietary bakery product with selenium-enriched yeast. LWT 41:890–895. https://doi.org/10.1016/j.lwt.2007.05.021
Stabnikova O, Antonuk M, Stabnikov V, Arsenieva L (2019) Ukrainian dietary bread with selenium-enriched malt. Plant Foods Hum Nutr 74:157–163. https://doi.org/10.1007/s11130-019-00731-z
Stabnikova O, Stabnikov V, Antoniuk M, Arsenieva L, Ivanov V (2022) Bakery products enriched with organoselenium compounds. In: Paredes-Lopez O, Stabnikov V, Shevchenko O, Ivanov V (eds) Bioenhancement and fortification of foods for a healthy diet. CRC Press, Boca Raton, London, pp 89–111
Sun Y, Wang H, Zhou L, Chang M, Yue T, Yuan Y, Shi Y (2022) Distribution characteristics of organic selenium in Se-enriched Lactobacillus (Lactobacillus paracasei). LWT 165:113699. https://doi.org/10.1016/j.lwt.2022.113699
Tendenedzai JT, Chirwa EM, Brink HG (2021) Performance evaluation of selenite (SeO32−) reduction by Enterococcus spp. Catalysts 11:1024. https://doi.org/10.3390/catal11091024
Tomei A, Barton LL, Lemanski CL, Zocco TG, Fink NH, Sillerud LO (1995) Transformation of selenate and selenite to elemental selenium by Desulfovibrio desulfuricans. J Ind Microbiol 14:329–336. https://doi.org/10.1007/BF01569947
Ullah A, Yin X, Wang F, Xu B, Mirani ZA, Xu B, Chan MW, Ali A, Usman M, Ali N, Naveed M (2021) Biosynthesis of selenium nanoparticles (via Bacillus subtilis 313), and their isolation, characterization, and bioactivities. Molecules 26:5559. https://doi.org/10.3390/molecules26185559
Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ (2004) The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci USA 101:2135–2139. https://doi.org/10.1073/pnas.0307308101
Vera P, Echegoyen Y, Canellas E, Nerín C, Palomo M, Madrid Y, Cámara C (2016) Nano selenium as antioxidant agent in a multilayer food packaging material. Anal Bioanal Chem 408:6659–6670. https://doi.org/10.1007/s00216-16-9780-9
Vicas SI, Laslo V, Timar AV, Balta C, Herman H, Ciceu A, Gharbia S, Rosu M, Mladin B, Chiana L, Prokisch J, Puschita M, Miutescu E, Cavalu S, Cotoraci C, Hermenean A (2021) Nano selenium-enriched probiotics as functional food products against cadmium liver toxicity. Materials 14:225. https://doi.org/10.3390/ma14092257
Vyas J, Rana S (2018) Synthesis of selenium nanoparticles using Allium sativum extract and analysis of their antimicrobial property against gram positive bacteria. J Pharm Innov 7:262–266
Wang Q, Webster TJ (2013) Inhibiting biofilm formation on paper towels through the use of selenium nanoparticles coatings. Int J Nanomed 8:407–411. https://doi.org/10.2147/IJN.S38777
Wang Q, Larese-Casanova P, Webster TJ (2015) Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int J Nanomed 10:2885. https://doi.org/10.2147/IJN.S78466
Wang Y, Shu X, Hou J, Lu W, Zhao W, Huang S, Wu L (2020) Selenium nanoparticle synthesized by Proteus mirabilis YC801: an efficacious pathway for selenite biotransformation and detoxification. Int J Mol Sci 21:3809. https://doi.org/10.3390/ijms21072638
Wasfi R, Hamed SM, Amer MA, Fahmy LI (2020) Proteus mirabilis biofilm: development and therapeutic strategies. Front Cell Infect Microbiol 10:414. https://doi.org/10.3389/fcimb.2020.00414
Xia SK, Chen L, Liang JQ (2007) Enriched selenium and its effects on growth and biochemical composition in Lactobacillus bulgaricus. J Agric Food Chem 55:2413–2417. https://doi.org/10.1021/jf062946j
Xu C, Qiao L, Guo Y, Ma L, Cheng Y (2018a) Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr Polym 195:576–585. https://doi.org/10.1016/j.carbpol.2018.04.110
Xu C, Guo Y, Qiao L, Ma L, Cheng Y, Roman A (2018b) Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by enterotoxigenic Escherichia coli K88. Front Microbiol 9:1129. https://doi.org/10.3389/fmicb.2018.01129
Xu Y, Wu S, He J, He C, Wang P, Zeng Q, Yang F (2020) Salt-induced osmotic stress stimulates selenium biotransformation in Lactobacillus rhamnosus ATCC 53103. LWT 131:109763. https://doi.org/10.1016/j.lwt.2020.109763
Yanez-Lemus F, Moraga R, Smith CT, Aguayo P, Sánchez-Alonzo K, García-Cancino A, Valenzuela A, Campos VL (2022) Selenium nanoparticle-enriched and potential probiotic, Lactiplantibacillus plantarum S14 strain, a diet supplement beneficial for rainbow trout. Biology 11:1523. https://doi.org/10.3390/biology11101523
Yang J, Huang K, Qin S, Wu X, Zhao Z, Chen F (2009) Antibacterial action of selenium-enriched probiotics against pathogenic Escherichia coli. Dig Dis Sci 54:246–254. https://doi.org/10.1007/s10620-008-0361-4
Yang J, Li Y, Zhang L, Fan M, Wei X (2017) Response surface design for accumulation of selenium by different lactic acid bacteria. 3 Biotech 7:52. https://doi.org/10.1007/s13205-017-0709-6
Yang J, Wang J, Yang K, Liu M, Qi Y, Zhang T, Fan M, Wei X (2018) Antibacterial activity of selenium-enriched lactic acid bacteria against common food-borne pathogens in vitro. J Dairy Sci 101:1930–1942. https://doi.org/10.3168/jds.2017-13430
Yazdi MH, Mahdavi M, Setayesh N et al (2013) Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. DARU J Pharm Sci 21:33. https://doi.org/10.1186/2008-2231-21-33
Zhang B, Zhou K, Zhang J, Chen Q, Liu G, Shang N, Qin W, Li P, Lin F (2009) Accumulation and species distribution of selenium in Se-enriched bacterial cells of the Bifidobacterium animalis 01. Food Chem 115:727–734. https://doi.org/10.1016/j.foodchem.2008.12.006
Zhang H, Li Z, Dai C, Wang P, Fan S, Yu B, Qu Y (2021) Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environ Res 194:110630. https://doi.org/10.1016/j.envres.2020.110630
Zommara M, Omran M, Ghanimah M (2022) Milk permeate medium for the production of selenium nanoparticles by lactic acid bacteria. Int J Dairy Technol 75:603–610. https://doi.org/10.1111/1471-0307.12875
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
OS, MK, IK, VS wrote the mail manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stabnikova, O., Khonkiv, M., Kovshar, I. et al. Biosynthesis of selenium nanoparticles by lactic acid bacteria and areas of their possible applications. World J Microbiol Biotechnol 39, 230 (2023). https://doi.org/10.1007/s11274-023-03673-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03673-6