Abstract
Selenium (Se) is one of the essential trace elements in the human body, and Se-enriched lactic acid bacteria (LAB) can improve the biological utilization value of inorganic Se. The aim of this study was to isolate Se-enriched LAB and study their effects on antioxidant activity and nitrite degradation. The Se-enriched LAB L.P2, which was nitrite-tolerant and could grow in 30 µg/mL sodium selenite (Na2SeO3) medium, was isolated from the traditional fermented Chinese sauerkraut. L.P2 belonged to Lactobacillus plantarum according to the 16S rDNA analysis. The biomass and lactic acid production of L.P2 reached to a maximum (9.52 log CFU/mL and 16.99 mg/mL) when 2.0 µg/mL Na2SeO3 was supplemented in the medium. Additionally, the nitrite degradation rate reached 85.76% when the initial concentration of Na2SeO3 was 2.0 µg/mL. The Se-enriched LAB enhanced the scavenging capacity of hydroxyl radical and superoxide free radical of L.P2 and improved the lipid peroxidation and ion-chelating abilities. Moreover, the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in Se 4 group (4.0 µg/mL Na2SeO3 was added) reached 48.49 and 50.35 U/mg, respectively. Thus, Se 4 concentration was significantly higher than that of Se 0 group (with no Se added). In particular, SOD and GSH-Px enzymes correlated with nitrite degradation (P < 0.01). Collectively, our results indicate that Se supplementation can enhance the antioxidant capacity of LAB, contribute to its nitrite degradation, and thus may have potential applications in functional foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se), a component of selenoamino acids and selenoproteins, possesses important physiological functions such as maintaining redox homeostasis (Behne et al. 2010). This element has a mineral nature and antioxidant effect (Steinbrenner and Siesa 2013; Zeng et al. 2013), can prevent heart diseases, and helps in the treatment of some other diseases, such as cancer (Hatfield et al. 2014; Mashmouli 2013; Hurst et al. 2012). Inorganic Se, which usually refers to sodium selenite (Na2SeO3), is toxic even if in low amount (Lopes et al. 2017). By contrast, organic Se, which mainly includes selenocysteine and selenomethionine (SeMet), are easily absorb and metabolize and has no toxic side effects (Abdulah et al. 2005; Kiełczykowska et al. 2018). Thus, the application of inorganic Se in chemoprevention is limited (Fairweather-Tait SJ 2010). However, some studies showed that lactic acid bacteria (LAB) can biotransform inorganic Se into organic Se compounds such as selenocysteine (Alzate et al. 2007, 2010). According to Kamna Saini’s study, lactobacillus reuteri NCDC77 has the greatest ability to exhibit Se uptake (28.8% of inorganic Se in medium) (Saini et al. 2014). In Yu Deng’s study, Se-enriched Lactobacillus brevis was used to co-ferment a skimmed milk; consequently, L. brevis enhanced Se concentration in yoghurt (Deng et al. 2015). Thus, if Se is used as a fermentation substrate, then LAB may convert toxic inorganic Se into a nontoxic organic Se, and the food fermented by Se-enriched LAB may supplement human dietary Se. Thus, the isolation of Se-enriched LAB is necessary to improve the utilization of Se and human’s health.
Nitrite widely exists in food, especially in fermented vegetables (Bozkurt and Bayram 2006). In the fermentation process of sauerkraut, nitrate can be reduced into nitrite (Chan 2011), which can decrease the oxygen-transport ability that leads to methemoglobinemia (Oldfield et al. 2013; Sohn et al. 2014). Nitrite can also react with secondary amines in the human body, forming a strong cancerogenic nitrosamine in the gastral cavity and resulting in cancer of the digestive system (Lin et al. 2013; DellaValle et al. 2014). Furthermore, zebrafish embryos that were treated with nitrite led to yolk sac edema and craniofacial and axial malformations (Simmons et al. 2012; Keshari et al. 2016). Therefore, nitrite seriously harms the health of humans and animals. Hence, how to reduce the nitrite in fermented foods has become a key problem.
LAB are the dominant species during vegetable fermentation. It is more effective in lowering nitrite concentration by using pure starter cultures compared with spontaneous fermentation in recent years (Oh et al. 2004; Yan et al. 2008; Jagannath et al. 2012). Wang et al. (2013) showed that the nitrite content of sausages fermented by Lactobacillus sakei dropped faster from 100 ppm to 9.6 ppm, whereas the nitrite in spontaneous fermentation dropped slower from 100 ppm to 32.1 ppm (Wang et al. 2013). Liu et al. (2014) found that Lactobacillus casei can effectively degrade nitrites both in the pickle fermentation system and in MRS medium (Liu et al. 2014). According to Fang et al. (2016), Lactobacillus coryniformis BBE-H3, which was isolated from naturally fermented pickled vegetables, showed a high level of activity in degrading sodium nitrite (Fang et al. 2016). Therefore, degrading nitrite using LAB is important.
Antioxidant supplements or foods containing antioxidants may be used to help the human body reduce the oxidative damage. However, chemical antioxidant can damage the health of an organism, including liver damage and carcinogenicity (Simic 1988). Therefore, replacing synthetic antioxidants by exploiting natural antioxidants has become a cardinal direction in recent years. LAB can scavenge accumulated free radicals to prevent oxidative damage (Li et al. 2012) and also have positive synergy effects on antioxidants. Thus, LAB have become the first choice of antioxidants. Furthermore, different forms of Se improve the ability of antioxidation in humans and can be used to develop functional foods (Pophaly et al. 2014). Se was added to sauerkraut before fermentation, leading to a 1.75-fold increase in its anti-oxidative activity (Peñas et al. 2012). Notably, antioxidant enzymes that rely on some of Se and other metal elements play a role in the body defense system. Shakibaie et al. (2017) isolated Se-rich LAB from Iranian traditional products and studied their antioxidant properties such as DPPH· clearance and power reduction. To date, the mechanism on the effect of Se on the antioxidant activity of LAB has been poorly studied (Ren et al. 2011). Thus, the effect of Se-rich LAB on the antioxidant properties of fermented vegetables is a new and interesting topic to study.
In this study, Lactobacillus plantarum was isolated from traditional fermented Chinese sauerkraut, and the antioxidant ability of the strain was investigated. The mechanism of antioxidant properties was further studied in terms of power reduction and certain antioxidant activities of enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). Our study may offer an alternative method to control nitrite production and to improve the antioxidant properties of food. Se-enriched LAB can enhance the antioxidant activity of fermented food and effectively reduce the nitrite content. Thus, it may make fermented foods more nutritious and healthy.
Materials and methods
Sample collection and bacterial screening
The MRS medium was produced according to Guo et al. (2015), and the LAB-selective medium contained 2% CaCO3 and 2% agar. Three samples of homemade fermented Chinese pickles were collected from the three regions (Wuhan, EnShi, and HuangShi) of Hubei Province in China. All samples were obtained when pickle pastes had been fermented at the later stage. Samples were continuously diluted with normal saline (10−5, 10−6, 10−7, and 10−8). An appropriately diluted sample (0.1 mL) was spread on the surface of the LAB-selective medium for cultivation. The single colony was picked up, further separated, and purified (Cui et al. 2012) under microscopic observation until completely purified. To further identify the LAB, we evaluated whether the strains produced lactic acid through thin-layer chromatography (TLC) (Hansen 1976). The purified strains were stored at − 80 °C.
Identification and isolation of nitrite-tolerant strains and Se-resistant LAB
NaNO2 at 0.2, 0.4, 0.6, and 0.8 g was added into 1 L of MRS solid medium to be used for screening nitrite-tolerant strains (Paik and Lee 2014). Then, Na2SeO3 at 5, 10, 20, 25, and 30 mg was added into 1 L of MRS solid medium to be used for screening Na2SeO3-tolerant strains (Zhang et al. 2009). To ensure all the preparations started with the same number of cells, we used aliquots containing 108 CFU/mL bacteria for inoculation. Then, the LAB were cultured at 37 °C for 24 h.
DNA extraction, 16S rDNA PCR amplification, and phylogenetic analysis
DNA extraction was performed according to the method of Ruiz et al. (2000). The bacteria were cultured in MRS medium at 37 °C for 24 h. The cells were then collected, and DNA was extracted according to the instructions of TIANamp Bacteria DNA Kit (Tiangen Biochemical Technology Co., Ltd., Beijing, China). PCR amplification of 16S rDNA was performed using the primers 27f (5′-AGAGTTTGATCCTGGCAG-3′) and 1492r (5′-GGCTACCTTGTTACGACTT-3′). The PCR method was performed according to a previous study (Chen et al. 2016).
The 16S rDNA amplification was sequenced in Sangon Biological Engineering Co., Ltd. (Shanghai, China). Multiple-sequence alignments and phylogenetic analysis were performed using MEGA version 5.0 software (Tamura et al. 2011). The phylogenetic tree based on 16S rDNA was constructed according to the neighbor-joining (NJ) approach (Gonzalez and Mas 2011). The homology of all isolates to other LAB species was analyzed according to their evolutionary divergence.
Effect of Na2SeO3 on the Se-rich ability, biomass, and lactic acid content of LAB
In order to evaluate the effect of Na2SeO3 on the selenium-rich ability, biomass, and lactic acid production of LAB, 0, 2, 4, 6, and 8 mg of Na2SeO3 were added separately into the 1 L medium. The inoculation amount of LAB was 1%. Then, it was cultured at 37 °C for 24 h under anaerobic condition by using Anaerobi/Microaerobic System A30 (Jiang Xue Technology Co., Ltd., Chongqing, China).
Afterward, an hemocytometry counting method was used to detect the biomass of LAB. The Se content in the sample was measured using the method of Pilarczyk et al. (2016) with some modifications. Then, 0.3 g of LAB were extracted, added with mixed acid (HNO3:HClO4 = 1:9), and digested at 230 °C for 180 min. The samples were hydrolyzed with 9% HCl. Se was derivatized with 2,3-diaminonaphthalene, and the complex was extracted into cyclohexane. The samples were then examined by fluorescence spectrophotometry F-700 (Hitachi High-Technologies Corp., Shanghai, China). The excitation wavelength was 376 nm, and the fluorescence emission wavelength was 520 nm.
The concentration of lactic acid was examined according to the method of Taylor (1996), with some modification. The concentrations of lactic acid standard solutions were 1, 2, 3, 4, 5, and 6 µg/mL. Furthermore, 1 mL of lactic acid standard solution was added to the colorimetric tube with 0.05 mL 40% copper sulfate solution. Then, 6 mL of concentrated sulfuric acid was added and reacted in boiling water bath for 5 min. After cooling below 20 °C, 0.05 mL of 1% alkali-soluble hydroxybiphenyl solution was added and mixed, incubating for 6–8 h at room temperature. Then, the absorbance at 570 nm for plotting standard curve was recorded. After centrifugation, 1 mL of supernatant was used to determine the content of lactic acid in the sample.
Effect of Na2SeO3 on the nitrite degradation rate of LAB
NaNO2 at 150, 250, and 350 mg was added into 1 L medium. The inoculation amount of LAB was 1%, then bacteria were cultured at 37 °C for 24 h under anaerobic condition by using Anaerobi/Microaerobic System A30 (Jiang Xue Technology Co., Ltd., Chongqing, China). The LAB’s capability of reducing nitrite was evaluated by analyzing their residue amount in each model-medium (Paik and Lee 2014). Degradation efficiency of nitrite is usually expressed as percentage.
Determination of the antioxidant capacity of Se-enriched LAB
Intracellular extracts
L. plantarum was inoculated in 100 mL MRS medium, which was supplemented with Se (0, 0.2, 0.4, 0.6, and 0.8 mg of Na2SeO3) to obtain a Se-enriched LAB (Xia et al. 2007). The bacterium was cultured at 37 °C for 24 h under anaerobic condition by using Anaerobi/Microaerobic System A30 (Jiang Xue Technology Co., Ltd., Chongqing, China). At the end of the cultivation, bacterial cells were harvested by centrifugation at 5,000 rpm for 15 min and washed thrice with phosphate buffer solution (PBS, pH 7.4). The washed strains were resuspended in PBS. The intracellular cell-free extracts were prepared by the method of Lin and Yen (1999), with minor modification. The cells were lysed with 1 mg/L of lysozyme at 37 °C for 30 min, followed by ultrasonic disruption for 50 s at 60% of ultrasound amplitude (Ningbo Xinzhi Biotechnology Co., Ltd.). Ultrasonic treatment in the ice bath at 200 W was performed every 2 s and rested for 10 s, with a total working time of 50 s and a total resting time of 5 min (Chang 2013; Li et al. 2012; Pescuma et al. 2017). The cell debris was removed by centrifugation at 10,000 rpm for 10 min at 4 °C. Finally, the supernatant was obtained as the intracellular extract of LAB.
Determination of hydroxyl radical scavenging activity
Hydroxyl radical (OH) scavenging activity was analyzed by measuring the hydroxyl radical scavenging activity produced by Fenton’s reaction (He et al. 2004). Furthermore, 1 mL of PBS (0.02 mol/L) was mixed with o-phenanthroline (2.5 mmol/L), then added with deionized water, H2O2 (20 mmol/L), FeSO4 (2.5 mmol/L), and intracellular extracts, each at 1 mL. This mixture was incubated at 37 °C for 1.5 h. Thereafter, absorption at 536 nm was recorded, and the scavenging activity (%) was calculated as: [(As − A0)/(A − A0)] × 100%, where As is the absorbance with the sample and H2O2, Ao is the absorbance of the control without the sample, and A is the absorbance without the H2O2.
Determination of superoxide free radical scavenging activity
The scavenging of superoxide free radical (O−2·) was determined by using the pyrogallol autoxidation method (Wang et al. 2015). Briefly, the reaction mixture containing 150 mmol/L Tris–HCl (8.2), 3 mmol/L diethylenetriamine pentaacetic acid, 1.2 mmol/L pyrogallol, and intracellular cell-free extract at 1 mL each was incubated at 25 °C for 10 min. The scavenging of the superoxide free radical was monitored by measuring the absorbance at 325 nm, and its ratio (%) was calculated as: [1 − (A11 − A10)/(A01 − A00)] × 100%, where A00 is the absorbance of the reaction mixture without the sample and pyrogallol, A01 is the absorbance of the reaction mixture without the sample, A10 is the absorbance of the reaction mixture without pyrogallol, and A11 is the absorbance of the reaction mixture containing the sample and pyrogallol.
Determination of DPPH radical scavenging activity
The DPPH radical-scavenging capacity was determined according to a previous method (Zhang et al. 2013) with some modifications. Briefly, 1 mL of the sample solution was added to 2.0 mL of ethanolic DPPH radical solution (0.05 mmol/L). The mixture was mixed and incubated in the dark for 30 min. The controls included 95% ethanol, instead of the sample. The blanks contained only ethanol and the cells. The absorbance of the resulting solution was measured thrice at 517 nm after centrifugation at 10000 rpm for 10 min. The DPPH radical scavenging ratio (%) was calculated as: [1 − (Asample − Ablank)/Acontrol] × 100%, where Asample is the absorbance of the sample, Ablank is the absorbance of the blank, and Acontrol is the absorbance of the control.
Determination of the inhibition of lipid peroxidation
Inhibition of lipid peroxidation was determined as described by a previous study (Raffaella and Minervini 2011) with some modification. Briefly, 0.5 mL of PBS (0.02 mmol/L, pH 7.4), 1 mL of linoleic acid emulsion, 1 mL of FeSO4, and 0.5 mL of the sample were mixed and incubated at 37 °C in a water bath for 1.5 h. Moreover, 0.2 mL of 4% trichloroacetic and 2 mL of 0.8% thiobarbituric acid were added into the mixture, incubated at 100 °C for 30 min, and then rapidly cooled. The supernatant was collected by centrifugation at 4000 rpm for 10 min, and the absorbance was measured at 532 nm. The inhibition of lipid peroxidation was calculated as: (1 − Asample/Acontrol) × 100%, where Asample is the absorbance of the sample, and Acontrol is the absorbance of the control.
Determination of iron ion chelating ability
The chelating ability of ferrous ion (Fe2+) was measured by the method of Lin and Yen (1999). Herein, 0.1 mL of 4% FeSO4 solution and 0.1 mL of 1% ascorbate were added into 1 mL of 0.2 mol/L NaOH solutions. Then, 0.5 mL of the sample was added into the above mixture, which was subsequently incubated for 20 min at 37 °C. Afterward, 0.2 mL of 10% trichloroacetic acid was added to the reaction medium, and 2 mL of o-phenanthroline (1%) was added into 0.2 mL of supernatant, which was obtained by centrifugation at 4000 rpm for 10 min. The absorbance was measured at 510 nm after 10 min of reaction. The chelate Fe2+ ability of the sample was calculated as: (A0 − AS/A0) × 100%, where Ao is the blank absorbance, and As is the sample absorbance.
Total reducing power
The method developed by Wang et al. (2015) was used to evaluate the reducing power of LAB. In brief, 0.5 mL of PBS (pH 6.6), 0.5 mL of 1% potassium ferricyanide, and 0.5 mL of the sample were mixed and incubated at 50 °C in a water bath for 20 min. The supernatant was collected by centrifugation at 4000 rpm for 5 min. Then, 1 mL of distilled water and 1 mL of ferric chloride (0.1%) were added into 1 mL of supernatant. After mixing, the absorbance was measured at 700 nm. The blank was prepared in the same way, except that deionized water was used, instead of the sample.
Determination of SOD activities
The SOD activities of LAB were assayed by the method of Bayer and Fridovich (Jr and Fridovich 1987) with slight modifications. Herein, 9 mL of Tris-HCl-EDTA (pH 8.2) was incubated at 25 °C for 10 min. Then, 20 mL of intracellular cell-free extract (10 mmol/L HCl as the contrast of the test) was added. The mixture was added with 20 µL of 45 mmol/L pyrogallol. Then, the absorbance was measured immediately. Every 30 s, light absorption was tested for 4 min. SOD activities were expressed as follows: \(\Delta {\text{A}}_{0} - \Delta {\text{A}}_{{{\text{SOD}}}}\)
where ∆AO is the change of absorbance of the control group at 4 min, ∆ASOD is the change of absorbance of the test sample at 4 min, V1 is the volume of reaction liquid, V2 is the total volume of the sample, and n is the multiple of the diluted sample.
Determination of GSH-Px activities
GSH-Px activity was measured based on Paglia and Valentine (Paglia and Valentine 1967). One unit of GSH-Px is defined as the amount of enzyme that catalyzes the oxidation of 1 nmol NADPH per minute. Herein, 0.4 mL of intracellular cell-free extract from each of the sample was mixed with 0.4 mL of 1.0 mmol/L GSH and then incubated at 37 °C for 5 min. H2O2 (37 °C) was then added into the mixture. This mixture was subsequently exposed to a warm-water bath at 37 °C for 3 min to initiate the enzymatic reaction. Then, 4 mL of metaphosphoric acid precipitant was added. Supernatant was collected by centrifugation at 4000 rpm for 10 min. Thereafter, 2 mL of supernatant was mixed with 2.5 mL of 0.32 mol/L Na2HPO4 and 0.5 mL of DTNB. After 1 min, the absorbance of the mixture was measured at 423 nm. The control group included 0.4 mL of inactivated intracellular extracts, instead of the sample. GSH standard curve was generated by 0, 0.2, 0.4, 0.8, and 1.0 µmol/L GSH. The active unit (U/mg) was calculated as: [(ODsample − ODcontrol) × A × 5 min]/(Q × 3 min), where A is the slope of the calibration curve, Q is the dry weight of the sample, ODsample is the absorbance of the sample, and ODcontrol is the absorbance of the control.
Effects of SOD and GSH-Px on nitrite degradation
SOD (≥ 3000 U/mg) and GSH-Px (≥ 300 U/mg) lyophilized powder were the standard substances (Sigma-Aldrich). Different contents of SOD (0, 0.005, 0.01, 0.015, and 0.02 mg) and GSH-Px (0, 0.05, 0.1, 0.15, and 0.2 mg) were individually added into 100 mL of PBS (0.02 mol/L, pH 7.8) to ensure that the corresponding enzyme activity was 0, 15, 30, 45, and 60 U/mg. Then, 15 mg of sodium nitrite was added into the different enzyme solutions (the concentration of sodium nitrite was 150 µg/mL) and reacted at 37 °C for 4 h. The nitrite content was determined according to the method of Paik and Lee (2014). The degradation efficiency was expressed in percentage.
Statistical analysis
One-way analysis of variance (ANOVA) and Duncan’s multiple range test were performed to identify the differences between means by using SPSS software version 20.0 (SPSS-IBM Chicago, IL, USA). Differences were considered statistically significant when P < 0.05.
Results
Screening and identification of LAB with high nitrite and Se tolerance
As listed in Table 1, a total of 11 strains were screened. After staining with the Gram strain, the colony morphology was observed under a microscope. Among these strains, 7 (L.P1, L.P2, L.P4, L.P5, L.P6, L.P7, and L.P9) were nitrite tolerant, and 6 could grow in 5 µg/mL Se. However, only L.P2 could grow in 30 µg/mL Na2SeO3 medium, indicating a high Se-resistant LAB.
Moreover, 16S rDNA of the isolated strain L.P2 was sequenced and subjected to phylogenetic analysis. Multiple sequence alignments showed > 99% similarity between L.P2 and several L. plantarum strains by NJ methods. Phylogenetic tree was then constructed. L.P2 exhibited to be the closest to L. plantarum JL28, with 99.27% similarity (Fig. 1). Thus, L.P2 was suggested to belong to L. plantarum.
Effect of Na2SeO3 on the Se-enrichment ability, biomass, and lactic acid production of L.P2
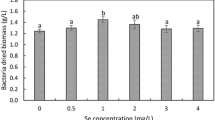
To study the effect of Na2SeO3 on the Se-enrichment ability, biomass, and lactic acid production of L.P2, we used a basic medium appended with different concentrations of Se. The Se-enrichment ability of L.P2 is illustrated in Fig. 2. When the concentration of Na2SeO3 was 4 µg/mL, the Se-enrichment rate of inorganic Se was the highest (52.73%). Followed by 6 and 2 µg/mL, the Se-enrichment rate was 37.62% and 26.43%, respectively. However, the Se-enrichment rate was only 6.95% when the concentration of Na2SeO3 was 8 µg/mL.
As seen in Fig. 2, the lactic acid production increased to 16.99 mg/mL when 2.0 µg/mL Na2SeO3 was added. However, when the concentration of Na2SeO3 increased to 4.0 µg/mL, the lactic acid production of LAB was lower (14.97 mg/mL) than that of 2.0 µg/mL Na2SeO3. The Na2SeO3 content increased the cell amount in the control group at 8.89 log CFU/mL. At 2.0 µg/mL Na2SeO3, the cell counts were 9.52 log CFU/mL, which was 7.09% higher than that of the control, whereas the number rapidly decreased to 4.04 log CFU/mL at 8 µg/mL Na2SeO3.
Effect of Se on the nitrite degradation rate of L.P2
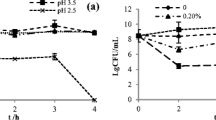
The effect of different Na2SeO3 concentrations on the nitrite degradation was assayed. The results were calculated as a percentage of the starting concentration as presented in Fig. 3. When the nitrite concentration was 125 mg/L, the highest degrading efficiency reached 85.76%, which was observed in the batch with 2.0 µg/mL Na2SeO3 medium. The degradation efficiency values of other groups were 80.48% (4.0 µg/mL Na2SeO3), 80.33% (6.0 µg/mL Na2SeO3), and 70.09% (8.0 µg/mL Na2SeO3), better than the control group (with no Se added, 54.22%). The initial concentration of nitrite had a certain effect on the degradation rate of nitrite, but the degradation trend was consistent with different initial concentrations of nitrite. Thus, the nitrite degradation rate reached the highest value when the initial concentration of Na2SeO3 was 2.0 µg/mL.
Effect of Se on the antioxidant activity of L.P2
The most efficient way to avoid oxidation induced by oxygen radicals is to scavenge the reactive oxygen species. The scavenging ability of LAB for two of the most important reactive oxygen species (hydroxyl radical and superoxide free radical) was investigated.
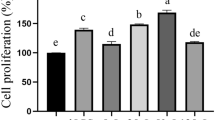
Scavenging of hydroxyl radical and superoxide free radical is shown in Fig. 4a. In the intracellular extracts, the hydroxyl radical scavenging activity of four Se intervention groups was more than that of the control (Se 0). Se 4 (4.0 µg/mL Na2SeO3 was added) had the highest scavenging ability with an inhibition of 47.60%. Specifically, 30.02%, 34.57%, 47.21%, and 44.88% hydroxyl radicals were scavenged in Se 0, Se 2 (2.0 µg/mL Na2SeO3 was added), Se 6 (6.0 µg/mL Na2SeO3 was added), and Se 8 (8.0 µg/mL Na2SeO3 was added) (Fig. 4a). The super-oxide anion radical scavenging activity of Se intervention groups in the intracellular extracts did not increase significantly (Fig. 4a). The scavenging activity of super-oxide anion radicals of Se 2 was better than that of the other groups. The scavenging rate of Se 2 was 37.99%, whereas the others were 33.20% (Se 0), 37.01% (Se 4), 32.84% (Se 6), and 28.68% (Se 8).
Free radicals attacked the polyunsaturated fatty acid and triggered a chain reaction of lipid peroxidation. In this study, the inhibitory effects of the intracellular extracts on lipid peroxidation were determined (Fig. 4b). Se 0 has a low inhibition activity (7.34%). Se intervention was significantly higher than that of Se 0. The other inhibition activities were 17.34% (Se 2), 22.15% (Se 4), 16.56% (Se 6) and 15.06% (Se 8). Thus, adding Se helped in inhibiting lipid peroxidation. Transition metal ions can initiate lipid peroxidation and start a chain reaction through the decomposition of hydroperoxides (Halliwell et al. 1995). Chelators can capture metal ions and prevent metal ions from catalyzing the oxidation. Therefore, the chelating ability of LAB toward iron ions was investigated. The chelating ability results of intracellular cell-free extract of L.P2 for Fe2+ are shown in Fig. 4a. The Fe2+ chelating rates in different Se concentrations were 19.38% (Se 0), 24.92% (Se 2), 24.69% (Se 4), 21.18% (Se 6) and 18.15% (Se 8). Se 2 demonstrated the best chelating ability for Fe2+ among the five tested groups. Meanwhile, the chelating ability of Se 8 was lower than that of the control group (Se 0).
The DPPH radical scavenging activity of the intracellular extracts is depicted in Fig. 4b. Se 0 group had the highest radical-scavenging activity (32.91%) followed by Se 2 (28.12%), Se 4 (27.79%), Se 6 (27.07%) and Se 8 (25.03%). As the Se density increased, the ability of eliminating the DPPH decreased. The reducing power of L.P2 was determined, as shown in Fig. 4b. The Se 6 showed the highest reducing power (24.22%). The reducing power of other groups was higher (20.22%, Se 2; 20.67%, Se 4; 22.67%, Se 8) than that of Se 0.
Effect of Se on the SOD and GSH-Px activities of L.P2
Antioxidant enzymes are regarded as an important enzymatic antioxidant defense system in LAB (Li et al. 2012). SOD and GSH-Px are intracellular antioxidant enzymes that protect against oxidative stress (Manda and Bhatia 2003). The SOD activity in the Se 2 and Se 4 groups was 30.17 and 48.49 U/mg (Fig. 5), which increased significantly compared with that in the Se 0 group. Additionally, the SOD activity in 8 µg/mL Na2SeO3 still maintained at 30.16 U/mg. In Fig. 5, the GSH-Px activity in the cell-free extract of Se-enriched L.P2 increased significantly from Se 0 to Se 2. The GSH-Px activity was highest in 4 µg/mL Na2SeO3. Thus, Se improved the activity of SOD and GSH-Px.
Effects of SOD and GSH-Px on nitrite degradation
The two enzymes SOD and GSH-Px had a certain ability in degrading nitrite. The effect of SOD on nitrite degradation is shown in Fig. 6. When the activity of SOD was 15 U/mg, the nitrite degradation ratio was 2.97%. With the increase of SOD activity, the degradation of nitrite also increased. Figure 6 shows that GSH-Px also had a great effect on nitrite degradation. When the GSH-Px activity was 60 U/mg, the nitrite degradation rate was more than 14.81%, which was significantly higher than without GSH-Px (0.19%). As shown in Table 2, the correlation between the biomass, lactic acid, enzyme activity, and nitrite degradation rate was analyzed. The results showed that the activity of SOD and GSH-Px significantly correlated with the degradation of nitrite (P < 0.01).
Discussion
Certain LAB can convert inorganic Se (Se4+) into organic Se (Se-amino acid) that are beneficial to humans (Alzate et al. 2008; Palomo et al. 2014). Se can be concentrated in the biomass by various LAB, either as an organic form such as selenoproteins, or as an elemental form when inorganic Se is added to the culture medium (Andreoni et al. 2000; Eszenyi et al. 2011). When the selenite exceeds a certain concentration, LAB detoxify selenite into elemental Se. Thus, the strain that can grow in 30 µg/mL Na2SeO3 medium was chosen and identified as L. plantarum (Table 1; Fig. 1).
As shown in Fig. 2, when the concentration of Na2SeO3 was 4 µg/mL, the Se-enrichment percentage of LAB was 52.73%, which was higher than that of 2 µg/mL Na2SeO3. To date, Se-enriched yeast is a common form of Se used to supplement the dietary intake of this important trace mineral. Se-enriched yeasts are commercially available to improve the Se status, but yeasts mainly produce SeMet, which is nonspecifically incorporated into proteins (Korhola et al. 1986). LAB species could concentrate Se as selenocysteine in biomass and can be used as an organic Se source for dietary supplementation (Calomme et al. 1995; Alzate et al. 2010, 2007). The amount of LAB increased when the added content of Na2SeO3 was 2.0 µg/mL (Fig. 2), thus suggesting that Se can improve the metabolism of LAB; adding Se can produce some particular essential nutrients to promote the growth of LAB. Se stimulates the growth of prokaryotic cells, but at high concentrations can also cause toxicity (Sarang et al. 2014). Incorporation of high concentrations of Se salts in the nutrient substrate used to cultivate the LAB can inhibit the growth of bacterial cells. This conclusion agrees with the results found by other researchers to some extent (Xia et al. 2007).
The accumulation of nitrite in fermented vegetables has become a growing concern. LAB, which are commonly used in fermentation industry, can reduce nitrite production, primarily because the metabolism of LAB produces acid and nitrite reductase (Con and Gokalp 2000; Hugas and Monfort 1997; Wolf and Hammes 1988; Ammor and Mayo 2007). Low concentration of inorganic Se has certain biological utilization value and can promote the growth and metabolism of an organism. However, the amount of nitrite degradation maintained high when the added amount of Se was more than 2 µg/mL (Fig. 3); thus, lactic acid and biomass are not the main factors to promote the degradation of nitrite. Furthermore, nitrite degradation and lactic acid content had no significant correlation (Table 2). Numerous research showed that antioxidants such as polyphenols, can degrade nitrite (Wu et al. 2015). Therefore, we evaluated the effects of antioxidant on nitrite degradation.
Hydroxyl radical is the strongest oxidizing free radicals, which can damage biological cells and have strong binding capacity with DNA, proteins, and lipids (Li et al. 2012). Superoxide anions are precursors of active free radicals that can induce tissue damage by reacting with biological macromolecules (Halliwell and Gutteridge 1984). In both Se 2 and Se 4 groups, the hydroxyl radical scavenging activity and super-oxide anion radical scavenging activity increased (Fig. 4a). Proper concentration of Se improved the activity of scavenging free radicals. The lipid peroxidation of polyunsaturated fatty acids in the cellular membrane was associated with cell membrane stability (Lucy 1972). Therefore, the inhibition rate of lipid peroxidation was investigated. Among the five tested groups, Se-containing group had a great reducing power on lipid peroxidation. Thus, Se (< 8 µg/mL) effectively improved the inhibition of lipid peroxidation of LAB. DPPH can be removed by LAB, showing that it can depress hydroxyl free radical and interrupt the lipid peroxidation chain reaction (He et al. 2004). Adding Se negatively affected the DPPH radical scavenging activity of LAB. The reducing activity of LAB was probably from the intracellular antioxidants and proteins (Manda and Bhatia 2003). Shakibaie et al. (2017) also found that the reducing power of Se-enriched LAB significantly improved when it was studied on its antioxidation characteristics. The reducing power of the intracellular extracts of the Se 0 was considerably lower than that of the other groups (Fig. 4b) possibly because the accumulation and biotransformation of Se by LAB enhanced the activity of the intracellular antioxidants.
The cell-free extracts of LAB have an antioxidant activity, considering the antioxidant enzymes released into the extracellular after cell disruption (Kullisaar et al. 2002). SOD, an important enzyme that can eradicate the oxygen free radicals, has a great influence on the stress resistance of strains (Farahnak et al. 2013). In this study, the SOD and GSH-Px activities in the Se 2 and Se 4 groups were improved greatly (Fig. 5), possibly associated with the Se metabolic pathway. Se is an integral part of the catalytic site of GSH-Px, which catalyzes the reduction of hydroperoxides and hydrogen peroxide by glutathione reduction (Wang and Fu 2012).
In the Fig. 6, 60 U/mg SOD activity can degrade approximately 11% nitrite, probably because of the strong oxidation ability of SOD enzyme. This finding also indicated that a certain relationship existed between the antioxidant activity and the nitrite degradation of LAB (Table 2). In Fig. 5, the GSH-Px activity in the Se 2 and Se 4 groups was 45.321 and 50.353 U/mg, respectively. However, with the increase of the GSH-Px activity, the degradation of nitrite increased (Fig. 6). Moreover, the significant correlation between GSH-Px activity and nitrite degradation is shown in Table 2. Therefore, low concentrations of Se could promote the growth and metabolism of LAB. Furthermore, the nitrite degradation greatly improved in Se-enriched LAB, which enhanced the antioxidant capacity, especially the activity of SOD and GSH-Px.
The reduction of nitrite in the food matrix is a detoxification mechanism and can be an important strategy for producers. The L.P2 is considered as the most potential strain in Se-enriched fermented pickles due to its favorable nitrite reduction ability and antioxidant capacity. As a starter culture, L.P2 can also be potentially utilized for the production of fermented foods and thus offers new opportunities for designing novel probiotic functional foods.
References
Abdulah R, Miyazaki K, Nakazawa M, Koyama H (2005) Chemical forms of selenium for cancer prevention. J Trace Elem Med Bio 19(2–3):141
Alzate A, Cañas B, Pérezmunguía S, Hernándezmendoza H, Pérezconde C, Gutiérrez AM, Cámara C (2007) Evaluation of the inorganic selenium biotransformation in selenium-enriched yogurt by HPLC-ICP-MS. J Agric Food Chem 55(24):9776–9783
Alzate A, Ferna´ndez-Ferna´ndez A, Perez-Conde M, Gutie´rrez A, Ca´mara C (2008) Comparison of biotransformation of inorganic selenium by Lactobacillus and Saccharomyces in lactic fermentation process of yogurt and kefir. J Agric Food Chem 56(18):8728–8736
Alzate A, Pérezconde MC, Gutiérrez AM, Cámara C (2010) Selenium-enriched fermented milk: a suitable dairy product to improve selenium intake in humans. Int Dairy J 20(11):761–769
Ammor MS, Mayo B (2007) Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: an update. Meat Sci 76(1):138–146
Andreoni V, Moro LM, Cavalca L, Erba D, Ciappellano S (2000) Selenite tolerance and accumulation in the Lactobacillus species. Ann Microbiol 50(1):77–88
Behne D, Alber D, Kyriakopoulos A (2010) Long-term selenium supplementation of humans: selenium status and relationships between selenium concentrations in skeletal muscle and indicator materials. J Trace Elem Med Bio 24(2):99
Bozkurt H, Bayram M (2006) Colour and textural attributes of sucuk during ripening. Meat Sci 73(2):344–350
Calomme M, Hu J, Van DBK, Vanden Berghe DA (1995) Seleno-lactobacillus. An organic selenium source. Biol Trace Elem Res 47(1–3):379
Chan TYK (2011) Vegetable-borne nitrate and nitrite and the risk of methaemoglobinaemia. Toxicol Lett 200(1–2):107–108
Chang MW Edirisinghe M, Stride E (2013) Ultrasound mediated release from stimuli-responsive core–shell capsules. J Mater Chem B 1(32):3962–3971
Chen Y, Bai Y, Li D, Wang C, Xu N, Hu Y (2016) Screening and characterization of ethanol-tolerant and thermotolerant acetic acid bacteria from Chinese vinegar Pei. World J Microb Biot 32(1):14
Con AH, Gokalp HY (2000) Production of bacteriocin-like metabolites by lactic acid cultures isolated from sucuk samples. Meat Sci 55(1):89–96
Cui Y, Qu X, Li H, He S, Liang H, Zhang H, Ma Y (2012) Isolation of halophilic lactic acid bacteria from traditional Chinese fermented soybean paste and assessment of the isolates for industrial potential. Eur Food Res Technol 234(5):797–806
Dellavalle CT, Xiao Q, Yang G, Shu XO, Aschebrookkilfoy B, Zheng W, Chow WH (2014) Dietary nitrate and nitrite intake and risk of colorectal cancer in the Shanghai Women’s Health Study. Int J Gynecol Cancer 134(12):2917–2926
Deng Y, Man C, Fan Y, Wang Z, Li L, Ren H, Jiang Y (2015) Preparation of elemental selenium-enriched fermented milk by newly isolated Lactobacillus brevis from kefir grains. Int Dairy J 44:31–36
Di CR, Surico RF, Minervini G, Rizzello CG, Lovino R, Servili M (2011) Exploitation of sweet cherry (prunus avium l.) puree added of stem infusion through fermentation by selected autochthonous lactic acid bacteria. Food Microbiol 28(5):900–909
Eszenyi P, Sztrik A, Babka B, Prokisch J (2011) Elemental, nano-sized (100–500 nm) selenium production by probiotic lactic acid bacteria. Inter J Biosci Biochem Bioinform 1(2):148–152
Fairweathertait SJ, Collings R, Hurst R (2010) Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr 2010, 91(5):1484
Fang F, Feng T, Du G, Chen J (2016) Evaluation of the impact on food safety of a Lactobacillus coryniformis strain from pickled vegetables with degradation activity against nitrite and other undesirable compounds. Food Addit Contam 33(4):623–630
Farahnak A, Golestani A, Eshraghian MR (2013) Activity of superoxide dismutase (SOD) enzyme in the excretory-secretory products of Fasciola hepatica and F. gigantica parasites. Iran J Parasitol 8(1):167
Jr Fridovich BW I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161(2):559–566
González A, Ngel Mas A (2011) Differentiation of acetic acid bacteria based on sequence analysis of 16S-23S rRNA gene internal transcribed spacer sequences. Int J Food Microbiol 147(3):217–222
Guo Y, Pan D, Li H, Sun Y, Zeng X, Yan B (2015) Antioxidant and immunomodulatory activity of selenium exopolysaccharide produced by lactococcus lactis subsp. lactis. Food Chem 26(2):248–259
Halliwell B, Gutteridge JM (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219(1):1–14
Halliwell B, Murcia MA, Chirico S, Aruoma OI (1995) Free radicals and antioxidants in food and in vivo: what they do and how they work. Crit Rev Food Sci Nutr 35(1–2):7–20
Hansen SA (1976) Thin-layer chromatographic method for the identification of organic acids. J Chromatogr A 124(1):123–126
Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN (2014) Selenium and selenocysteine: roles in cancer, health and development. Trends Biochem Sci 39(3):112–120
He ZS, Cao ZH, Jian D (2004) Photometric determination of hydroxyl free radical in Fenton system by toluidine blue. Chin J Health Lab Technol 6:236–237
Hugas M, Monfort JM (1997) Bacterial starter cultures for meat fermentation. Food Chem 59(4):547–554
Hurst R, Hooper L, Norat T, Lau R, Aune D, Greenwood DC, Sterne JA (2012) Selenium and prostate cancer: systematic review and meta-analysis. Am J Clin Nutr 96(1):1111–1122
Jagannath A, Raju PS, Bawa AS (2012) A two-step controlled lactic fermentation of cabbage for improved chemical and microbiological qualities. J Food Quality 35(1):13–20
Keshari V, Adeeb B, Simmons AE, Simmons TW, Diep CQ (2016) Zebrafish as a model to assess the teratogenic potential of nitrite. J Vis Exp 108(108):53615
Kiełczykowska M, Kocot J, Paździor M, Musik I (2018) Selenium-a fascinating antioxidant of protective properties. Adv Clin Exp Med 27(2):245–255
Korhola M, Vainio A, Edelmann K (1986) Selenium yeast. Ann Clin Res 18(1):65
Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, Kilk A (2002) Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 72(3):215–224
Li S, Zhao Y, Zhang L, Zhang X, Huang L, Li D, Wang Q (2012) Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem 135(3):1914–1919
Lin MY, Yen CL (1999) Antioxidative ability of lactic acid bacteria. J Agric Food Chem 47(4):1460–1466
Lin Y, Totsuka Y, He Y (2013) Epidemiology of esophageal cancer in Japan and China. J Epidemiol 23(4):233
Liu DM, Wang P, Zhang XY, Xu XL, Wu H, Li L (2014) Characterization of nitrite degradation by Lactobacillus casei subsp. rhamnosus LCR 6013. PLoS ONE, 9(4), e93308
Lopes G, Ávila FW, Guilherme LRG (2017) Selenium behavior in the soil environment and its implication for human health. Ciênc Agrotecnol 41(6):605–615
Lucy JA (1972) Functional and structural aspects of biological membranes: a suggested structural role for vitamin E in the control of membrane permeability and stability. Ann NY Acad Sci 203(1):4–11
Manda K, Bhatia AL (2003) Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol 19(6):367
Mashmouli B (2013) Selenium as an effective element for lung cancer prevention and treatment. KUMS J 16(7):693–694
Oh CK, Oh MC, Kim SH (2004) The depletion of sodium nitrite by lactic acid bacteria isolated from kimchi. J Med Food 7(1):38–44
Oldfield EH, Loomba JJ, Monteith SJ, Crowley RW, Medel R, Gress DR (2013) Safety and pharmacokinetics of sodium nitrite in patients with subarachnoid hemorrhage: a phase iia study. J Neurosurg 119(3):634–641
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Paik HD, Lee JY (2014) Investigation of reduction and tolerance capability of lactic acid bacteria isolated from kimchi against nitrate and nitrite in fermented sausage condition. Meat Sci 97(4):609–614
Palomo M, Gutiérrez AM, Pérez-Conde MC, Cámara C, Madrid Y (2014) Se metallomics during lactic fermentation of Se-enriched yogurt. Food Chem 164:371–379
Peñas E, Martinezvillaluenga C, Frias J, Sánchezmartínez MJ, Pérezcorona MT, Madrid Y, Vidalvalverde C (2012) Se improves indole glucosinolate hydrolysis products content, Se-methylselenocysteine content, antioxidant capacity and potential anti-inflammatory properties of sauerkraut. Food Chem 132(2):907–914
Pescuma M, Gomez-Gomez B, Perez-Corona T, Font G, Madrid Y, Mozzi F (2017) Food prospects of selenium enriched-Lactobacillus acidophilus CRL 636 and Lactobacillus reuteri CRL 1101. J Funct Foods 35:466–473
Pilarczyk B, Szewczuk M, Pilarczyk R, Tomza-Marciniak A, Marciniak A, Dobrzanski Z, Bakowska M (2016) Effect of supplementing selenized yeast to ewes from an organic farm on serum Se concentration in lambs. J Elementol 21(4):1093–1101
Pophaly SD, Poonam Singh P, Kumar H, Tomar SK, Singh R (2014) Selenium enrichment of lactic acid bacteria and bifidobacteria: a functional food perspective. Trends Food Sci Technol 39(2):135–145
Ren Z, Zhao Z, Wang Y, Huang K (2011) Preparation of selenium/zinc-enriched probiotics and their effect on blood selenium and zinc concentrations, antioxidant capacities, and intestinal microflora in canine. Biol Trace Elem Res 141(1–3):170–183
Ruiz A, Poblet M, Mas A, Guillamón JM (2000) Identification of acetic acid bacteria by RFLP of PCR-amplified 16S rDNA and 16S-23S rDNA intergenic spacer. Int J Syst Evol Microbiol 50(6):1981–1987
Saini K, Tomar SK, Sangwan V, Bhushan B (2014) Evaluation of lactobacilli from human sources for uptake and accumulation of selenium. Biol Trace Elem Res 160(3):433–436
Samelis J, Maurogenakis F, Metaxopoulos J (1994) Characterisation of lactic acid bacteria isolated from naturally fermented Greek dry salami. Int J Food Microbiol 23(2):179
Sarang DP, Poonam PS, Hitesh K, Sudhir KT, Rameshwar S (2014) Selenium enrichment of lactic acid bacteria and bifidobacteria: a functional food perspective. Trends Food Sci Technol 39:135–145
Shakibaie M, Mohammadi-Khorsand T, Adeli-Sardou M, Jafari M, Amirpour-Rostami S, Ameri A, Forootanfar H (2017) Probiotic and antioxidant properties of selenium-enriched Lactobacillus brevis LSe isolated from an Iranian traditional dairy product. J Trace Elem Med Bio 40:1–9
Simic MG (1988) Mechanisms of inhibition of free-radical processes in mutagenesis and carcinogenesis. Mutat Res 202(2):377–386
Simmons AE, Karimi I, Talwar M, Simmons TW (2012) Effects of nitrite on development of embryos and early larval stages of the Zebrafish (Danio rerio). Zebrafish 9(4):200–206
Sohn CH, Seo DW, Ryoo SM, Lee JH, Kim WY, Lim KS, Oh BJ (2014) Life-threatening methemoglobinemia after unintentional ingestion of antifreeze admixtures containing sodium nitrite in the construction sites. Clin Toxicol 52(1):44–47
Steinbrenner H, Sies H (2013) Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch Biochem Biophys 536(2):152–157
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731
Taylor KACC (1996) A simple colorimetric assay for muramic acid and lactic acid. Appl Biochem Biotech 56(1):49–58
Wang Y, Fu L (2012) Forms of selenium affect its transport, uptake and glutathione peroxidase activity in the Caco-2 cell model. Biol Trace Elem Res 149(1):110
Wang XH, Ren HY, Liu DY, Zhu WY, Wang W (2013) Effects of inoculating Lactobacillus sakei starter cultures on the microbiological quality and nitrite depletion of Chinese fermented sausages. Food Control 32(2):591–596
Wang YQ, Wu YY, Li LH, Wang XC, Cai QX, Yang XQ (2015) Comparative study of eight strains of lactic acid bacteria in vitro antioxidant activity. Adv Mater Res 1073–1076:183–188
Wolf G, Hammes WP (1988) Effect of hematin on the activities of nitrite reductase and catalase in lactobacilli. Arch Microbiol 149(3):220–224
Wu ZQ, Yue GZ, Zhu QP, Jiang YJ, Tang KY, Chen HP (2015) Purification, dynamic changes and antioxidant activities of oleuropein in olive (olea europaea l.) leaves. J Food Biochem 39(5):566–574
Xia SK, Chen L, Liang JQ (2007) Enriched selenium and its effects on growth and biochemical composition in Lactobacillus bulgaricus. J Agr Food Chem 55(6):2413–2417
Yan PM, Xue WT, Tan SS, Zhang H, Chang XH (2008) Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control 19(1):50–55
Zeng H, Cao JJ Jr CGF (2013) Selenium in bone health: roles in antioxidant protection and cell proliferation. Nutrients 5(1):97
Zhang BW, Kang Z, Zhang JL, Qian C, Liu GR, Nan S, Lin FX (2009) Accumulation and species distribution of selenium in Se-enriched bacterial cells of the Bifidobacterium animalis 01. Food Chem 115(2):727–734
Zhang L, Liu C, Li D, Zhao Y, Zhang X, Zeng X, Li S (2013) Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int J Biol Macromol 54(1):270
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No: 31601455).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Li, Q., Xia, C. et al. Effect of selenium supplements on the antioxidant activity and nitrite degradation of lactic acid bacteria. World J Microbiol Biotechnol 35, 61 (2019). https://doi.org/10.1007/s11274-019-2609-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2609-x