Abstract
Selenium (Se) is an essential micronutrient for diverse organisms such as mammals, bacteria, some insects and nematodes, archaea, and algae, as it is involved in a large number of physiological and metabolic processes and is part of approximately 25 selenoproteins in mammals. In plants, Se has no essential metabolic role, high concentrations of inorganic Se can lead to the formation of Se-amino acids, and its incorporation into selenoproteins can generate toxicity. Conversely, low doses of Se can trigger a variety of beneficial effects as an antioxidant, antimicrobial, or stress-modulating agent without being an essential element. Therefore, Se can generate toxicity depending on the dose and the chemical form in which it is supplied. Selenium nanoparticles (SeNPs) have emerged as an approach to reduce this negative effect and improve its biological properties. In turn, SeNPs have a wide range of potential advantages, making them an alternative for areas such as agriculture and food technology. This review focuses on the use of SeNPs and their different applications as antimicrobial agents, growth promoters, crop biofortification, and nutraceuticals in agriculture. In addition, the utilization of SeNPs in the generation of packaging with antioxidant and antimicrobial traits and Se enrichment of animal source foods for human consumption as part of food technology is addressed. Additionally, possible action mechanisms and potential adverse effects are discussed. The concentration, size, and synthesis method of SeNPs are determining factors of their biological properties.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium is an element that belongs to group 16 of the periodic table, has semimetallic properties, and is found in the soil in inorganic forms, such as elemental Se (Se0), selenide (Se2−), selenate (SeO42−), or selenite (SeO32−). Selenium can also be obtained in organic forms such as selenomethionine (SeMet) and selenocysteine (SeCys). This element differs from others in the same group because of its ease of transition to other oxidation states [1].
In the past, Se was considered a toxic element; however, it is currently classified as an element that performs vital functions in diverse organisms, such as mammals, bacteria, some insects and nematodes, archaea, and algae [2,3,4,5]. The applications of Se have been accentuated in different areas of science, such as agriculture and food technology, and more frequently in the medical/pharmaceutical area due to its physical, chemical, and biological properties [6].
For the specific case of plants, Se is considered a beneficial element, depending on its concentration, available form, method of application, and type and growth stage of the plant. Among the main properties of Se in plants are the promotion of growth, improvement of crop quality, and reduction of negative effects by biotic and abiotic stress [7]. Simultaneously, Se shows a dual effect, acting as a pro-oxidant and antioxidant depending on its concentration [8].
In mammals, Se is considered an essential trace element because it performs several biological functions. Se is a cofactor of antioxidant enzymes such as glutathione peroxidase (GPX), which protects the human body by catalyzing the reduction of reactive oxygen species (ROS). Additionally, in mammals, Se forms at least 25 selenoproteins that fulfill antioxidant, catalytic, anti-inflammatory, antiviral, and antitumor functions [9].
In contrast, in plants, Se is not considered essential due to the lack of evidence for any essential metabolic function of this element [7]. However, plants subjected to high concentrations of inorganic Se can assimilate it through its incorporation into the Se-amino acid selenocysteine (SeCys) and selenomethionine (SeMet) by the sulfur (S) metabolic pathway, interfering with molecular folding and proper functioning of proteins, leading to plant toxicity [7, 10]. However, low doses of Se can trigger a variety of beneficial effects for plants as an antioxidant agent, growth biostimulator, stress modulator, or inducer for the accumulation of nutraceutical compounds in the edible parts [10].
The body of an adult person needs approximately 0.070 mg Se/day for men and 0.060 mg Se/day for women [11]. Foods with high Se contents include nuts, salmon, garlic, onions, and chicken eggs [12], which contribute a high percentage of Se to the required daily intake. A deficiency of this element causes a large number of health problems, including Keshan disease (an endemic cardiomyopathy), Kashin-Beck disease (endemic deforming osteoarthropathy) [13], acceleration of carcinogenic processes in the prostate [14], fertility problems, and weakening of the immune defense system against viral infectious diseases such as influenza, HIV, muscular dystrophy and cystic fibrosis [15, 16]. Conversely, doses above 1.2 mg Se/day can be toxic, causing selenosis (hair or nail loss, stained teeth, skin lesions, and changes in the peripheral nerves) [17].
Since there is a fine line between essential and toxic levels of organic and inorganic Se, different options for its supplementation and intake have been proposed, such as the use of nanotechnology, a field of science that addresses the study, design, manufacture, manipulation, and characterization of matter, and controlling the shape and size at the nanometer scale (1–100 nm) [18]. The physical, chemical, and biological properties of materials at the nanoscale are fundamentally different from the properties of materials at the atomic, molecular, or bulk level. Nanomaterials can be classified according to their morphology (nanoparticles, nanowires, nanowires, nanowires, nanotubes, nanobelts, nanofibers, nanospheres, and quantum dots) or by their composition (organic and inorganic) [19]. In particular, NPs have specific characteristics, including their size, shape, structure, and morphology, which give them unique biological properties for a wide range of applications. NPs can be classified into organic and inorganic NPs; in turn, the latter can be grouped into semiconducting, metallic, and magnetic NPs, whereas organic NPs include carbon nanoparticles [20].
The synthesis of NPs is mediated by two methods: “top-down” and “bottom-up.” The first of these methods is based on the reduction of the size of a macroscopic material to finer particles by physical processes (such as lithography, pulse laser ablation, ultrasound, microwaves, and electrodeposition) to subsequently produce agglomerates that will form NPs [21]. In the second case, the synthesis of NPs occurs by the self-assembly of atoms forming a new nucleus that grows to obtain nanometric structures by both chemical and biological processes. The chemical synthesis of NPs requires the use of products such as sodium borohydride, sodium citrate, and ascorbic acid, which exert a reducing function on the metal salts. In addition, the incorporation of stabilizers, including polyvinyl alcohol, polyvinylpyrrolidone, and polymers such as chitosan, is required. Biological synthesis, also known as green synthesis, involves the use of secondary metabolites of plant, bacterial, or fungal origin as well as other microbial compounds, which simultaneously act as reducing and stabilizing agents [21].
In recent years, the green synthesis of NPs has gained popularity over physical and chemical methods because it is cost-effective, environmentally friendly, and nontoxic and can be effectively scaled up to large-scale production. Regardless of the NP synthetic approach, it is important to consider the preparation method, origin of the starting material, nature of the organic compounds and solvents, concentration, strength of the reducing agent, and temperature since the size, morphology, and stability of the NPs depend on these factors [19].
Specifically, selenium nanoparticles (SeNPs) are generating interest in many areas of science due to their high bioavailability and lower toxicity than inorganic (Se2−, SeO42−, and SeO32−) and organic (SeMet and SeCys) forms. In general, the biological properties of SeNPs are a function of their high surface area, solubility, surface chemistry, and surface charge and size, where smaller NPs have higher activity. The physicochemical characteristics of SeNPs make them unique and are determinants of their stability, bioavailability, biocompatibility, multifunctionality, and controlled Se release [21, 22].
Although many studies have described the positive effect of SeNPs, there is some uncertainty regarding their toxicity and use, especially in medical applications. The toxic effect of SeNPs is largely related to the concentration, size, chemical form of Se, and method of synthesis of SeNPs, which seems to indicate that biological synthesis is less toxic than chemical or physical processes [22]. The biological activity of SeNPs is closely related to their pro-oxidant and antioxidant properties.
Therefore, the aim of this review is to provide an overview of the state of the art on biological activity of SeNPs in two major areas of application: in agriculture, during plant growth, pest and disease control, abiotic stress, and crop quality; and in food technology, during food packaging, bacterial growth in food, and animal source food production. The effects, applied doses, and possible mechanisms of action of SeNPs are presented.
SeNPs in Agriculture
Nanotechnology is a rapidly advancing discipline in research and influences all areas of science; in particular, in agriculture, it represents a major innovation in research, generating more effective and cost-effective products that can be applied to crops and thus transform the agricultural industry. SeNPs can be applied mainly to (1) the controlled release of agrochemicals and their delivery to the different organs of the plant, which would increase the efficiency of the products used; (2) the control of pests and diseases caused by pathogenic microorganisms such as fungi and bacteria [23, 24]; (3) as quasi-essential micronutrients by enhancing plant physiological and biochemical processes, improving crop growth, yield and quality [25]; (4) biofortifying crops with Se to increase their content; (5) mitigating abiotic stress; and (6) enhance the nutraceutical value of edible foods (Table 1) [26].
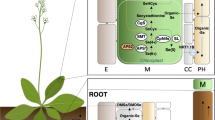
The main SeNP-related mechanisms of action in agriculture are shown in Fig. 1. The antimicrobial activity of SeNPs involves the positive regulation of the transcription factor WRKY1 [27] and the genes 13-LOX2, 13-LOX6, and LOX3 (involved in the synthesis of the phytohormone JA), the upregulation of the PAL1 and PAL3 genes (elements of the SA biosynthetic pathway) [28], and the increase in the generation of ROS [29]. The insecticidal effect is associated with JA synthesis. The biostimulant activity is related to primary metabolism, increasing the enzymatic activity of nitrate reductase [30], which could be linked to a higher uptake of Mo and Fe [31], resulting in a higher content of amino acids and proteins, as reflected in higher plant growth and yield. Another pathway is an increase in the level of the bZIP transcription factor involved in flower development and initial root differentiation [27]. Biofortification increases the concentration of Se in the edible parts of plants (roots, stems, leaves, fruits, or seeds) [30, 32]. The alleviation of biotic and abiotic stresses is related to an increase in the Pro content via the Glu pathway [28], a reduction in ROS/RNS levels and MDA generation [33], and ABA synthesis (induction of the NCDE1/NCDE2 genes by an increased photosynthetic rate) [28]. The nutraceutical value of crops can be achieved by increasing the chlorophyll content, photosynthetic rate, accumulation of soluble sugars, and expression of Kas and Acl genes of the fatty acid pathway [28]. Another pathway is the induction of the genes HCT1 and HQT1 that contribute to the synthesis of secondary metabolites [30], and an alternative pathway involves the phenylpropanoid pathway that includes PAL and 4CL up to the synthesis of flavonoids and phenolic compounds [28].
SeNPs as Antimicrobial Agents
The use of chemicals for the eradication of pathogenic microorganisms in plants is one of the most common methods, although such methods can generate toxicity in the population, resistance to pathogens, loss of biodiversity, and contamination of nearby crops with potentially toxic products and soil infertility. Therefore, SeNPs have been suggested for the protection of various crops against a large number of phytopathogens, such as Alternaria solani [25], Aspergillus brasiliensis, Fusarium anthophilum [34], Bacillus subtilis, Staphylococcus aureus [35], Penicillium digitatum, and Colletotrichum coccodes [36]. One such case was described by Nandini et al. [37]. Here, SeNPs were obtained with extracts of fungi of the genus Trichoderma spp. These fungi are commonly used as biological control agents since they secrete lytic enzymes capable of lysing the cell membrane of phytopathogenic fungi. In this case, SeNPs were used at a concentration of 1000 mg/L, and the combination of Se with the fungal extract was observed to enhance the antimicrobial effect of SeNPs, inhibiting the growth, proliferation, and sporulation of Sclerospora graminicola, which causes powdery mildew disease in pearl millet.
SeNPs have also been reported to be synthesized in combination with other elements, including organic and inorganic molecules such as metabolites and drugs, which is known as functionalization. One example is described by Hu et al. [38], who functionalized SeNPs with various metabolites of the fungus Trichoderma harzianum. This interaction between T. harzianum biomolecules and SeNPs enhanced the antifungal effects and inhibited the growth of hyphae of F. verticillioide, F. graminearum, and A. alternata; in addition, a decrease in fungal toxin production was observed.
This type of functionalization has been carried out not only with fungal extracts but also with plant extracts that have shown great efficacy since they possess important antimicrobial activities that could be potentiated with NP synthesis. An example of this type of NP is that obtained with the extract of Emblica officinalis, which is recognized for its antimicrobial activity. In this research, SeNPs showed in vitro effectiveness against phytopathogenic fungi such as A. brasiliensis, A. flavus, A. oryzae, A. ochraceus, F. anthophilum, and Rhizopus stolonifera, and bacteria such as Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, and Enterococcus faecalis. They exhibited more antifungal than antibacterial activity, which could be due to the previously reported antifungal properties of Se, although the mechanism by which Se exerts this activity is still unknown [34]. Similarly, we evaluated the functionalization of SeNPs coated with different types of polymers, such as poly-L-lysine (PLL+), polyacrylic acid (PAA−), and polyvinylpyrrolidone (PVP). When the effect of SeNPs was compared against silver nanoparticles (AgNPs), it was found that SeNPs functionalized with the neutrally charged polymer (PVP) had a greater antifungal effect against Diaporthe longicolla than AgNPs functionalized with the same polymer and at the same concentration (100 mg/L), potentially indicating that the antifungal activity of functionalized SeNPs did not depend directly on the charge of the compound used for functionalization but on the type of pathogenic microorganism [39]. Therefore, the effectiveness of SeNPs could be due, in part, to the antifungal activity of Se. Quiterio-Gutiérrez et al. [25] tested the functionalization of SeNPs with CuNP copper nanoparticles as an alternative for the control of A. solani in tomato (Solanum lycopersicum) plants, observing a 6% decrease in the progress of early blight disease after the application of SeNPs.
The antibacterial activity of SeNPs has been shown to be an alternative against multidrug-resistant bacteria such as Enterobacter cloacae, S. aureus, and Pseudomonas aeruginosa, which have been considered clinically and are also pathogenic to plants. This phenomenon might be due to chemical conjugation between bioactive compounds such as quercetin. This compound has been shown to have broad-spectrum antibacterial and antifungal properties; in addition, acetylcholine serves as a receptor in bacterial cells, allowing SeNPs functionalized with these compounds to increase the permeability of the bacterial membrane, causing leakage of the cytoplasmic content and allowing SeNPs to penetrate and alter the DNA structure of bacterial cells [40].
SeNPs with Insecticide and Nematicidal Activity
Some insects, including mosquitoes, are important vectors for the transmission of diseases such as chikungunya, malaria, dengue, and Zika. A method for eradicating these insects is the application of organophosphate products, insect growth regulators, and microbial control agents. Regardless, many of these chemicals have negative effects on human health and the environment [41]. In this regard, SeNPs have been demonstrated to be a good option for these treatments due to their good biocompatibility and efficacy at lower doses. An example of this is SeNPs biosynthesized with Clausena dentata leaf extracts, which are known repellents and insecticides, in addition to the known insecticidal properties of Se. The obtained SeNPs showed a potentiated effect, producing 50% mortality (median lethal concentration, LC50) against the larvae of Culex quinquefasciatus, Aedes aegypti, and A. stephensi, using concentrations of 99.60, 104.13, and 240.71 mg/L SeNPs for each larval type. This activity of SeNPs could be due, in large part, to the denaturation of proteins and DNA of the larvae, reducing the permeability of the cell membrane and decreasing the synthesis of ATP, which eventually leads to the loss of cell function and cell death [42].
In another example of green synthesis of SeNPs, the fungal extract of Penicillium corylophilum was used to obtain NPs, and a potent larvicidal effect was observed with 100 mg/mL SeNPs against the malaria vector A. stephensi, achieving 100% mortality of the larvae. An increase in larvicidal activity was reported as the dose of SeNPs increased, since the application of 75, 50, 25, and 20 mg/mL caused larval mortality of 90.6, 70.3, 50.3, and 43.3%, respectively. Additionally, the LC50 value (25 mg/mL) [43] was found to be lower than that of SeNPs obtained with plant extracts (240.71 mg/mL) [42].
In turn, Ramya et al. [44] found a similar trend with the application of SeNPs biosynthesized from Streptomyces sp., which achieved 50% mortality at doses of 7.66 and 11.94 mg/L SeNPs against A. aegypti and C. quinquefasciatus, respectively. Based on these results, it could be suggested that the effectiveness of SeNPs is related to their concentration. One possible mechanism shown for controlling the development of mosquito larvae could be through cell lysis and rupture of their peritrophic membrane and epithelial cells. In addition, histopathological changes were observed in the intestinal regions of A. aegypti and C. quinquefasciatus larvae treated with SeNPs [45].
For nematodes, parasites of the root system of plants that cause the generation of ROS in plant tissue, the application of SeNPs (with a size range from 10 to 70 nm obtained by a physical process such as ablation) suggested an increase in the plant content of protease inhibitors (PIs), which could cause a reduction in the vital activities of these organisms because this type of enzyme is involved in different primary processes, such as nutrition, reproduction, and embryogenesis. This finding indicates that 0.68 μg/mL SeNPs were capable of inducing nonspecific defense reactions in plants, reducing the number of female Meloidogyne incognita nematode in SeNP-treated plants with respect to control plants and decreasing nematode reproduction by reducing the number of eggs in the ootheca [29].
SeNPs as Biostimulants in Plant Growth Promotion
Plant growth promoters improve plant development and yield, resulting in their wide management. However, some data have established that they can cause toxicity at high concentrations. In the specific case of SeNPs, their biological effect on plant growth, compared with commercial products and organic and inorganic forms of Se, is becoming more relevant both in vitro and in vivo in different crops, such as chicory (Cichorium intybus) [30], eggplant (Solanum melongena), tomato, cucumber (Cucumis sativus) [46], peppermint (Mentha piperita) [47], and guar gum (Cyamopsis tetragonoloba) [48].
Some of the first studies on SeNPs as plant growth promoters were conducted by Domokos-Szabolcsy et al. [3], in which the application of 265–530 µM SeNPs in tobacco plants (N. tabacum) stimulated organogenesis and increased root system growth by 40% compared with other forms of inorganic Se such as SeO42−; the latter was unable to induce the same effects at the same concentrations, although the biostimulant effect of Se has been established despite not being an essential plant element. A similar behavior was observed in another investigation under in vitro conditions, where different doses of SeNPs (synthesized by chemical reduction and having a size from 10 to 45 nm) and SeO42− were tested in chili seeds, producing variations in morphology and growth in a SeNP dose-dependent manner. Concentrations of 0.5 and 1.0 mg/L SeNPs favored growth and increased the leaf fresh weight by 65.5%, whereas the same doses of SeO42− increased the fresh weight by 19%. Conversely, doses of 10 and 30 mg/L caused severe toxicity and abnormalities in the development of leaves and roots, as well as an inhibition in the differentiation of xylem tissues, potentially due to DNA hypermethylation for both sources of Se. The differential effect observed between SeNPs and SeO42− in both tobacco and chili plants might be attributable to the mechanism of SeO42− uptake (active transport through sulfate/phosphate transporters) compared with SeNP uptake (passive diffusion through membrane-localized aquaporin channels), causing changes in plant uptake mechanisms [49]. These data contrast with those of tomato, where the application of 10 and 20 mg/L SeNPs, synthesized by chemical reduction with sizes from 2 to 20 nm, did not show symptoms of toxicity and generated an increased plant yield by 21% and 25%, respectively [50]. This finding indicates that the size of SeNPs can play an important role in the beneficial or toxic effect of SeNPs, establishing that the larger the size of SeNPs, the greater is the toxicity of chili plants. Based on these results, it could also be established that the response of plants to SeNPs depends on the dose used and the size and surface composition of the NPs. These characteristics can produce different responses in plants, from biostimulation to toxicity. The effect of the surface composition of SeNPs and their interaction with molecules, such as cytoplasmic proteins, internal membranes, or organelles, also depends on the mode of application, with foliar spraying being the mode of application that notably decreases the toxic effects in plants due to chemical interactions between cuticle components and NPs [51]. In turn, Domokos-Szabolcsy et al. [3] established that the plant species used is another factor for consideration during the application of NPs, indicating a lower degree of toxicity with the use of SeNPs compared with other forms of Se such as SeO32− or SeO42−, potentially because in plant tissue Se ions can be gradually released from NPs. Therefore, Se release occurs systematically at a low but stable concentration, promoting physiological effects such as the stimulation of organogenesis and shoot and root growth.
A similar trend was found in three varieties of peanuts (Arachis hypogaea) by applying different concentrations of 0, 20, and 40 mg/L SeNPs, synthesized by chemical processes, with a size of 10–30 nm, and causing no signs of toxicity. It was observed that 20 mg/L increased the number of pods/plant by 200%, the fresh weight of pods/plant by 129%, and the weight of seeds by 204%. This phenomenon might be due to maintenance of the ionic and osmotic cell balance, improving photosynthesis, causing an improvement in photosynthetic pigments, producing a high photochemical efficiency and decreasing ROS levels. Similarly, it was established that at 40 mg/L, the Giza 6 variety was the most effective treatment for improving yield parameters, such as the number of pods/plant (106.4%), weight of pods/plant (47.0%), and number of seeds/plant (55.5%) compared with the control (without SeNP application) [52]. SeNPs have been shown to promote greater plant growth than inorganic forms of Se (SeO32− or SeO42−), suggesting a possible involvement of SeNPs in the accumulation of signaling hormones, such as ethylene, auxin (AUX), salicylic acid, gibberellins (GA), and cytokinin (CK), which are responsible for plant growth and tissue differentiation [49, 53]. In turn, it has been suggested that SeNP-mediated phytohormones trigger particular signaling by which plant nuclear transcription can be modified. In this regard, Zahedi et al. [54] emphasized the changes triggered by SeNPs in AUX biosynthesis, describing the overexpression of genes involved in the biosynthesis pathway, thus enhancing root biomass and water and nutrient uptake. This finding is consistent with those of El Lateef et al. [55], who reported that SeNPs increased the levels of the growth hormones indol-acetic acid (IAA), GA and CK in cowpea leaves (Vigna unguiculata) and decreased the content of abscisic acid (ABA), which could explain the positive effect of SeNP application on growth and seed weight.
Conversely, treatments including the combination of SeNPs with zinc (Zn) and silver (Ag) NPs have also been tested. For the combination of 300 mg/L ZnNPs and 10 mg/L SeNPs, an increase in biomass, activation of lateral buds, and stimulation of lateral roots were recorded in lemon balm (Melissa officinalis) plants. However, at doses of 300 mg/L ZnNPs and 50 mg/L SeNPs, severe toxicity occurred, with a 45.5% reduction in plant growth. In addition, 50 mg/L SeNPs exhibited higher toxicity than the bulk Se source, which could be due to the absorption rate of SeNPs compared with the bulk source due to the difference in their sizes and the method of Se uptake in bulk form (energy-dependent transport through sulfate and phosphate transporters) [56]. In potato (Solanum tuberosum), the combination of 0.5 mg/L SeNPs, ascorbic acid (AcAsc), AgNPs, and K increased the fresh weight by 25% and the dry weight by 50% compared with the established treatment consisting of AgNPs and K. Thus, the addition of SeNPs and AcAsc provided a potentiated effect [27].
SeNPs in Plant Biofortification
With the advancement of new, vanguard, and emerging technologies such as nanotechnology, the application of SeNPs has arisen as an alternative to conventional Se fertilizers to enrich crops. The interest in SeNPs for the biofortification of plant foods lies in the potential for their slow Se release, avoiding possible losses in agroecosystems when commercial fertilizers are used [10]. For this reason, SeNPs might be used for biofortification, which attempts to increase the Se content in the edible organs of plants and in turn prevent Se deficiency in humans and animals.
In addition, SeNPs have been shown to be less toxic to plants than ionic selenium salts (SeO42− and SeO32−), as verified in studies of tobacco (N. tabacum) and garlic (Allium sativum) [3, 57]. Likewise, it has been possible to establish differences between the treatments of SeNPs, SeO42−, and SeO32− in the same plant species, as observed in onion (Astragalus nutans) plants. It was found that applying the same concentration of the three forms of Se resulted in different total Se contents (μg/kg dry weight), demonstrating a decrease in Se uptake on the order of SeO42− > SeNPs > SeO32− [58]. Similar results were found in male and female spinach (Spinacia oleracea) plants, in which the accumulation of Se between both genders was different using SeNPs [53], as a similar order of uptake to that observed in onion [58] plants was found (SeO42− > SeNPs > SeO32−). Concurrently, a greater difference between Se accumulation was observed for male and female plants using SeNPs. Highlighting a higher uptake of Se by male plants, this phenomenon is in agreement with the differences found in CK and GB content in male and female spinach plants [53].
In rice (Oryza sativa) crops, the Se content of the shoots varied with respect to the different Se doses; for example, 30 µmol SeNPs caused the Se content of the shoots to be 1.4 times higher than that in plants treated with SeNPs at a dose of 10 µmol SeNPs. Similarly, it was reported that the uptake rate of SeNPs was 1.7 times slower than the uptake of SeO42− and SeO32− and then assimilated into organic forms, including SeMet, accumulating mainly in the root cell walls. An opposite trend was observed with SeO42− treatment, as it accumulated mainly in the shoots in the form of SeO42− [59]. A similar case was identified in wheat (Triticum aestivum) plants treated with SeNPs obtained by chemical (140 ± 10 nm NP size) and biological (140 ± 40 nm NP size) reduction, as the particle size and method of SeNP synthesis affected the uptake rates of Se. The uptake rate of SeNPs was slower than that of SeO32−. In addition, SeNPs obtained by chemical methods were absorbed more efficiently than SeNPs synthesized by biological methods. Regarding the size of the NPs, it was reported that wheat roots treated with 40 nm SeNPs, obtained by chemical synthesis, were able to take up 1.8 and 2.2 times more Se than roots treated with 140 and 240 nm SeNPs, respectively, indicating the importance of particle size and the type of NP synthesis for this type of application [60].
There is currently a growing interest in using SeNPs for the biofortification of crops due to their potential to improve the quality, nutritional attributes, and amount of Se available in the edible parts of plants. However, factors such as the size of the NPs, the synthesis method, and the surface composition may cause the intake of plant foods biofortified with SeNPs to have a different effect within the human body compared with other sources of Se that are conventionally used. Therefore, it is necessary to perform a large number of in vivo tests to establish possible toxic effects due to the intake of plant products biofortified with SeNPs. Concomitantly, a generalized recommendation concerning the suggested doses for the consumption of foods biofortified with SeNPs is not possible.
SeNPs in Abiotic Stress Alleviation in Plants
Plants are sessile organisms that are constantly exposed to different physical and chemical factors that generate stress, causing a decrease in their growth and development. Therefore, it is of vital importance to provide crops with various tools to activate their tolerance mechanisms and actively respond to abiotic stress [61].
In this respect, it has been reported that Se is able to reduce stress in plants, since it induces the synthesis of secondary metabolites and stimulates the activity of antioxidant enzymes [31]; therefore, the use of SeNPs plays an important role in mitigating some types of abiotic stress, such as high and low temperatures, drought, heavy metal accumulation, and salinity. For example, in barley (Hordeum vulgare) plants grown under saline stress, treatment with SeNPs at a concentration of 100 mg/L resulted in a direct accumulation of Se in leaves, an increase in the level of total phenolic compounds and a reduction of the content of ROS-mediated cell membrane damage markers, such as malondialdehyde (MDA), which might influence metabolism and be responsible for the higher dry weight yield of shoots [62].
Similarly, the effect of SeNPs on strawberry (Fragaria x ananassa) plants grown under salinity stress (17–273 mmol NaCl) was examined, and a higher percentage of calcium (Ca) and of K levels were observed. In addition, treatment with 10 μM SeNPs improved photosystem II (PSII) performance and enhanced the activity of the antioxidant system, which was also related to the reduction of ROS generation and content. In contrast, exposure to a concentration of 100 μM SeNPs exhibited moderate stress, as determined by an elevation of the hydrogen peroxide (H2O2) concentration and lipid peroxidation rate (LPO) [63]. This phenomenon is related to the findings of Zahedi et al. [54] in the same crop, who attributed a protective effect to SeNPs (10 and 20 mg/L) for their ability to improve the salinity tolerance of strawberry plants by protecting the photosynthetic pigments, reducing the LPO, and decreasing the H2O2 content induced by salt stress. The highest dose of SeNPs (20 mg/L) was observed to decrease MDA levels by 19.6% and H2O2 levels by 7.6% compared with the application of 10 mg/L SeNPs. A trend toward ROS reduction was observed due to the increase in the capacity of antioxidant enzymes such as SOD and POD at low concentrations of SeNPs. In addition, foliar application of SeNPs increased proline and total soluble carbohydrate content, which favored the osmoprotection of strawberry plants exposed to salt stress, stabilizing membranes, and protecting proteins from denaturation. The increase in carbohydrate content could serve as a source of energy, helping to overcome the damage caused to metabolism and thus maintain plant growth.
Another abiotic factor that causes damage to crops is the indiscriminate application of pesticides and herbicides, which causes disorders in plants and in human and animal health. One example is atrazine, which is recognized as one of the most widely used herbicides to prevent weeds. The application of atrazine to fava bean plants (Vicia faba) was associated with an inhibition of the genes encoding the antioxidant enzymes CAT and SOD, which could explain the associated increase in H2O2 and MDA contents in atrazine-treated seedlings, as well as the reduced expression level of the PSII-D1 gene caused by atrazine-induced oxidative stress, which could also explain the low percentages of germination and a reduced length of shoots and roots. To mitigate these negative effects, SeNPs were tested at a concentration of 10 mg/L SeNPs, which improved the germination percentage and reduced the damage caused by atrazine [64].
Similarly, high exposure to gamma radiation can cause stress in plants, inhibiting cell division due to the generation of ROS and causing DNA damage, which is closely related to the decrease in the growth and development of plants. In response to this type of stress, the application of 50 mg/L SeNPs reduced the detrimental effects of radiation, increasing the percentage of germination by 30%, the length of the stem by 52.5%, and the diameter of the stem by 37% in acid lime seedlings (Citrus aurantifolia) compared with seeds that were not treated with SeNPs [32].
Simultaneously, the effect of SeNPs was tested on plants under high-temperature stress, such as sorghum (Sorghum bicolor), in which foliar concentrations of 10 mg/L SeNPs stimulated the defense system of the plant and thus increased the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), proline oxidase (POX) and GPX. The application of SeNPs reduced the generation of O2•−, (25%), H2O2 (25%), MDA (30%), and membrane damage (18%), which could be due to activation of the antioxidant defense system [65].
Another point to highlight is that SeNPs were found to decrease the accumulation of heavy metals in plant tissues, which are highly toxic. In most of the examined studies, Se was shown to be an antagonist of heavy metals such as lead (Pb) and cadmium (Cd); thus, the application of SeNPs tends to significantly decrease the concentration of both elements, reducing the adverse effects of these heavy metals on the plant. As observed in female spinach plants, Cd and Pb concentrations decreased 66% and 19%, respectively, following the application of SeNPs. The protective effect of female spinach plants toward Cd increases with a decreasing oxidation state on the order of SeNPs > SeO32− > SeO42− [53].
SeNPs Increase the Nutraceutical Properties of Crops
In agriculture, nanotechnology offers a wide range of benefits, either replacing toxic chemicals or adding nutritional value to food. An example of this phenomenon can be observed with the well-known nutraceuticals, foods among which some provide health benefits for the prevention and treatment of diseases. They incorporate compounds such as vitamins, minerals, amino acids, essential fatty acids, and proteins and other phytochemical compounds such as phytosterols, lycopene, and β-carotenes [66].
As described above, several studies have indicated that SeNPs may be a viable option for obtaining nutraceutical foods, since they qualify for consideration under this category. The use of SeNPs has increased the nutraceutical content of bell pepper (Capsicum annuum) [28], cress (Lepidium sativum) [33], cowpea (V. unguiculata) [67], and coriander (Coriandrum sativum) [68].
A large number of studies have been conducted on tomatoes; this plant was tested in combination with CuNPs. The application of 10 mg/L SeNPs increased the content of vitamin C compared with 20 mg/L SeNPs, the latter of which produced a 20% reduction in vitamin C content in fruits. However, in leaves, 20 mg/L SeNPs increased the vitamin C content by 30% compared with 10 mg/L SeNPs. In addition, fruit treatments with 20 mg/L SeNPs were found to be superior for the control of parameters such as vitamin C, phenols, and flavonoids [25]. In another study in tomato plants, the application of SeNP concentrations of 1, 10, and 20 mg/L was tested. The treatments with 1 mg/L increased the content of vitamin C in fruits with respect to the higher doses. Conversely, the 20 mg/L dose caused a loss of glutathione levels but increased the content of flavonoids in fruits with respect to the other doses [50]. These data highlight the advantage of applying SeNPs in tomato production to obtain better quality fruits with greater benefits to human health.
The lack of information about the risks of SeNPs to plants and human health often limits their use in agriculture. However, in recent years, it has been confirmed that low concentrations of SeNPs have practically no toxic effect. In this regard, Hussein et al. [52] applied 0, 20, and 40 mg/L SeNPs to Egyptian peanut (A. hypogaea) plants and found that 20 mg/L SeNPs decreased the contents of palmitic, stearic, arachidic, and lignoceric acids by 10.8%, 15.8%, 8.4%, and 10.1%, respectively, but increased the content of behenic acid compared with the control. The opposite was true for linoleic acid, which was reduced with both treatments by 13.2% (20 mg/L) and 23.5% (40 mg/L), as compared to the control. Similarly, 20 and 40 mg/L SeNPs increased oleic acid by 15.5% and 21.0%, respectively, demonstrating that SeNPs could be used in crop production to increase quality and nutraceutical properties.
Conversely, SeNPs (0–500 mg/L) improved the biochemical characteristics of guar gum in a concentration-dependent manner, increasing the content of chlorophyll a, b, and total chlorophyll with 400 mg/L SeNPs. It has also been reported that 400 mg/L was the best concentration for obtaining anthocyanins, L-prolins, free amino acids, and foliar nitrate without signs of toxicity [48]. Similar data were obtained in strawberry crops treated with SeNPs (0, 10, and 100 µM), where 10 µM SeNPs increased the content of phenolic compounds in leaves as well as PAL activity, which could be due to the increase in transcription of genes involved in secondary metabolism. Similarly, 10 µM SeNPs produced an increase in activation of the enzymatic antioxidant CAT, as well as a 27% increase in the content of salicylic acid, 37% increase in catechin, and 20.7% increase in caffeic acid. In addition, doses of 10 and 100 μM SeNPs increased Ca and K but decreased Na contents in leaves [63]. In pomegranate (Punica granatum), in addition to the finding that the denominated nutraceuticals were capable of contributing a greater amount of minerals, the application of SeNPs (1 and 2 μM) and SeO42− was evaluated during two consecutive crop cycles, indicating that 2 µM increased the concentrations of N, P, K, Ca, Fe, and Zn in leaves. Additionally, 2 μM increased the contents of total sugars, phenolic compounds, and anthocyanins. These results suggest that SeNPs stimulate the capacity of plants for mineral uptake; in turn, a greater nutraceutical effect than that obtained with the application of SeO42− was observed at the same concentrations, although several investigations have reported that SeO42− has nutraceutical effects [69].
To develop and commercialize new nutraceutical formulations, evaluations of their efficacy and safety are required. As previously described, SeNPs can be used to improve the bioavailability of active ingredients, increase Se content and add nutritional value to foods. Therefore, consumers will be directly exposed to these nanomaterials, and their effect on human health should be carefully evaluated. In 2011, the Scientific Committee of the European Food Safety Authority (EFSA) proposed an approach to assess the potential risks arising from the use of nanomaterials used in food. This approach focuses on the proper characterization of NPs, which ideally should be determined in five steps: (a) mode of manufacture; (b) mode of delivery for use in food; (c) presence in the food matrix; (d) assessments of toxicity; and (d) presence in biological fluids and tissues. In addition, studies of the physicochemical transformations that take place during the digestive process are required to understand the conversion of NPs to other types of molecules. Thus, appropriate in vitro and in vivo studies should be performed to identify possible harmful effects and to identify dose–response rates [70]. In turn, Zanella et al. [71] proposed the evaluation of a combination of an in vitro model for digestion, mimicking the biological composition of digestive tracts, and an in vitro model for absorbing tissues, where the stability and controlled release of active ingredients during the digestive process, mucoadhesion, mucopenetration, and toxicity should be evaluated.
Potential Adverse Effects of SeNPs in Agriculture
In general, in plants, the toxicity of SeNPs is due to a high concentration of Se, above the appropriate dose for each plant species. Symptoms associated with damage caused by SeNP toxicity consist of arrest in root and area growth [30, 48, 55, 56, 64, 67, 68]; a reduction in fresh and dry biomass production [25, 47, 49, 55, 68]; a decrease in the number of leaves, fruits, and flowers [30]; and a decrease in yield [61] both by fruit size [25] and seed weight [55]. In addition, high concentrations of SeNPs can affect other physiological parameters, such as a reduction in the chlorophyll content [28, 47, 50, 55] and photosynthetic efficiency [64] as well as a lower content of essential elements, such as N, P, K, Fe, and Zn [56, 68]. Biochemical parameters that were affected by high doses of SeNPs consist of increased MDA content and lipid peroxidation [28, 59]; altered levels of phytohormones such as Jasmonic acid, IAA, and ABA [28, 54]; and decreased activity of enzymes such as nitrate reductase (NR) [30, 47, 49, 56], leading to the accumulation of nitrate in leaves [48], increased free amino acid content [48], and altered fatty acid ratio [52], crude protein, and total carbohydrate contents in seeds [55, 67] as well as a lower vitamin C content in fruits [25, 50] and capsaicin content in peppers [28]. The adverse effect of SeNPs at the molecular level is reflected in the increased expression of genes encoding transcription factors of the bZIP, WRKY1, and HSP179 families [49, 64] as well as other genes (DREB1A, PAL, HCT1, and HQT1) associated with the response of plants to stress [28, 30].
To determine the optimal concentration of SeNPs in plants, several in vitro and in vivo studies have been performed evaluating different concentrations. Generally, beneficial effects are associated with low concentrations, and adverse effects are associated with high doses of SeNPs, although this condition depends on the plant species, stage of development, form and frequency of application, exposure time, cropping system, and size of the NPs. However, little has been discussed about the importance of the type of synthesis of NPs for agricultural use. Toxic concentrations of SeNPs vary widely; for example, a dose of approximately 20–25 mg/L caused adverse effects on tomato [25, 46, 50], eggplant and cucumber [46], peppermint [47], and strawberry plants [54], while at 30 and 40 mg/L, chili [49] and peanut [52] plants were adversely affected, respectively. At concentrations equal to or greater than 50 mg/L, toxic effects were reported on cluster bean [48], cowpea [55], lemon balm [56], strawberry [64], chili [28], coriander [68], and cress plants [24]. Although noteworthy, it was found that 100 mg/L SeNPs had no adverse effects on pearl millet [37] and barley [62] plants. It is important to note that the SeNPs used in these two assays were obtained by biological synthesis, while SeNPs with toxic effects at concentrations of 20–40 mg/L were synthesized by chemical methods, and only one case was synthesized by a physical method [46], finding a relationship between the concentration, the synthetic method, and the potentially adverse effect of SeNPs.
The toxicity of SeNPs could be explained by two mechanisms: the first by the induction of oxidative stress and the second by the formation of nonspecific selenoproteins. In the first case, the toxicity of SeNPs is mainly due to their pro-oxidant capacity, generating an imbalance in the production of ROS and reactive nitrogen species (RNS), which can damage cellular components. In turn, one of the negative effects of ROS can be observed in the decrease in the contents of Chl a, Chl b, and total Chl. High doses of SeNPs significantly reduced the levels of these photosynthetic pigments as well as the activation of enzymes involved in the removal of ROS (APX, SOD, POD, CAT, GPX, and GSH) along with a higher content of compounds with antioxidant activity (vitamin C, phenolics, flavonoids). The second mechanism of SeNP toxicity involves an excess of Se in plant cells, which leads to the substitution of S in the amino acid cysteine/methionine by Se during protein synthesis. The incorporation of selenoamino acids (SeCys/SeMet) to give rise to selenoproteins causes structural and functional alterations in proteins; thus, their accumulation causes toxicity in plants [72].
In addition, in one group of studies, a reduction in the activity of the NR enzyme, a key player in the assimilation of nitrate in plants, was found. For this particular case, high concentrations of SeNPs could affect the thiol group (-SH) of the NR enzyme, which would cause a decrease in its activity. Additionally, high doses of SeNPs altered the uptake of some microelements, such as Fe [56], a metal cofactor of NR, which could interfere with efficient N assimilation, decreasing the products of primary metabolism dependent on N assimilation. Thus, high concentrations of SeNPs negatively influence N assimilation, affecting the metabolic functions of plants with adverse implications on the growth; biomass production of leaf, flower, and fruit yields; and, in general, plant development. With high concentrations of SeNPs, adverse effects were also identified on plant secondary metabolism, mainly related to disease resistance, abiotic stress, and senescence [49].
SeNPs in Food Technology
Food technology is constantly changing, with the aim of improving the food consumed on a daily basis. However, the current context is complex due to new consumption trends, where there is a rising interest of consumers about the quality of food and the benefits it brings to health. At the same time, the goal is to improve food quality without altering its nutritional value [73]. In view of this, the demand for materials with NPs has increased, particularly the use of elements such as Se, which are essential for human health. Other advantages offered by-products based on SeNPs are related to their chemical stability, biocompatibility, low toxicity, and improved properties, such as texture, flavor, aroma, color, and other attributes, such as resistance, processability, and stability during shelf life, opening up the possibility of generating a large number of new products [74, 75]. In food technology, SeNPs can be used for food packaging [76], to control the growth of pathogens in food [77, 78], and to increase the Se content in animal source foods [11] (Table 2).
The biological properties of SeNPs in food technology are related to their role as an antimicrobial agent, their use for food packaging, and their application in animal diets to improve the content of Se in animal source foods (Fig. 2). The antimicrobial effect of SeNPs is due to (1) depolarization of the cell membrane; (2) generation of ROS; (3) membrane rupture; (4) leakage of intracellular material; (5) DNA damage; (6) protein dysfunction; (7) ATP depletion; (8) loss of enzymatic activity; and (9) inhibition of biofilms [19, 40, 77]. In food packaging, SeNPs prevent free radicals from coming into contact with food, decreasing lipid peroxidation and the generation of MDA and reducing the loss of the nutritional value of lipids, pigments, vitamins, and rancidity, thus extending the shelf life of food [79]. In animal food sources, supplementation of SeNPs in the animal diet increases the Se content, raising the levels of selenoproteins GPX1, GPX2, GPX4, TXNRD2, TXNRD3, SelW, SelT, SelK, and SelF [80] and improving inflammatory, antiviral and antitumor properties, thus favoring the health status of the animals and enhancing the levels of immunoglobulins IgG, IgM, and IgA [81], resulting in a better immune system. The addition of SeNPs to the animal feed diet increases the enzymatic activity of GSH-Px, SOD, POD, CAT, APX, and GSH [82], which has two effects. The first is a decrease in ROS, which in turn causes a reduction in MDA levels, thus reducing the lipid peroxidation of cell membranes, decreasing the ratios of C18:2n6/C18:3n3 and SFA/USFA fatty acids and resulting in increased freshness and quality of table eggs [83]. The second involves maintaining cell membrane integrity and decreasing drip loss (loss of myofibers, water, iron, and protein), resulting in improved meat quality (Fig. 2) [81].
SeNPs with Antimicrobial Activity in Food Preservation
Antimicrobial agents are one of the most studied active components in food technology since the growth of pathogenic microorganisms is one of the main causes of food deterioration [84]. SeNPs possess antimicrobial activity against different pathogenic microorganisms that cause food contamination.
SeNPs showed efficacy in inhibiting the generation of microbial biofilms, as the presence of these biofilms can increase the resistance of bacteria to antibiotics. From this viewpoint, different concentrations of SeNPs biosynthesized with B. licheniformis extracts (0–40 µg/mL) and 40 µg/mL selenium dioxide (SeO2) were evaluated against six pathogenic bacteria. The concentration of 10 µg/mL SeNPs only showed a significant effect on the bacterial growth of B. cereus and S. aureus. Increasing the concentration of SeNPs increased the inhibitory effect against the growth of all strains evaluated. In contrast, 40 µg/mL SeO2 showed no antibacterial effect against all tested bacteria. It was confirmed that low concentrations of SeNPs have no antibacterial effect, and it was highlighted those high concentrations of SeNPs have antibacterial activity [74].
Some bacterial infections are transmitted through paper towels and other products used in the food industry. In this situation, the bactericidal effects SeNPs coating the surface of these materials was tested, which showed growth inhibition of S. aureus by 90% after 24, 48, and 72 h compared with the untreated paper towels, indicating the stability of SeNPs for this type of application [85] (Table 2). It was also possible to add SeNPs (0–15%) to furcellaran and gelatin biofilms, which increased their thickness, water content, elasticity, and resistance. In relation to antibacterial activity, a greater inhibitory activity was found as the concentration of SeNPs increased, while it was reported that SeNPs showed higher antimicrobial activity against E. coli and the multidrug-resistant strain S. aureus (MRSA) than films containing AgNPs at the same concentrations [93]. This result suggests the possible use of these biofilms for the protection of foods such as kiwi, where the combination of furcellaran/gelatin and SeNPs/AgNPs (5–15%), provided an antimicrobial action on fruit compared with the control films, which showed no antimicrobial activity [87].
SeNPs in Food Packaging
Common food preservation methods include the use of packaging that ensures the quality and safety of food products throughout the food supply chain. During this process, chemicals from nonrenewable fossil resources are required for preservation. However, implementation represents environmental damage, high investment and energy, and the loss of food quality. The application of NPs in the development of packaging was conceived as an alternative to this problem by (1) direct incorporation into food products, (2) incorporation into packaging material, and (3) application during food processing [88] (Table 2).
A critical aspect of the use of SeNPs is the approval of various organizations, which ensures that their use does not involve risks to human health. Such is the case of the European Food Safety Authority (EFSA) that evaluated the utilization of SeNPs in multilayer packaging, which consisted of an exterior composed of polyethylene terephthalate (PET) and an interior layer of polyolefin, which was in contact with food. Through this type of packaging, it was possible to demonstrate that SeNPs do not represent a risk to consumer safety if they are used in multilayered films and are not in direct contact with food [89].
Currently, one of the most relevant examples of the implementation of nanotechnology to innovate food technology is in the production of active packaging, which offers antioxidant and antimicrobial properties [73] (Fig. 2). One such case was proposed by Vera et al. [79], who used SeNPs in a laboratory-scale antioxidant package for hazelnuts (Corylus avellana), walnuts (Juglans regia), and French potato fries. This research attempted to determine the degree of oxidation of lipids, which are factors that reduce the shelf life of foods and in turn are associated with an unpleasant taste due to the generation of aldehydes and ketones such MDA. It was found that with SeNPs, the amount of MDA was reduced by 20% for hazelnuts, 25% for nuts, and 22% for French potato fries. It was found that with SeNPs, the amount of MDA was reduced by 20% for hazelnuts, 25% for nuts, and 22% for French potato fries, while fatty acid values were approximately 50% higher in packages containing SeNPs at both days 21 and 42 than in the control. The greatest difference was found in the nut packaging, where an improvement in the amount of fatty acids present was observed with values ranging from 50 to 66% at day 42. In the case of hazelnut packaging, the amount of fatty acids ranged from 24 to 53% at day 42. This result demonstrates that active packaging containing SeNPs prevents food oxidation and extends shelf life. A similar effect was found at the industrial level in cooked ham, chicken, and a vegetable mixture, achieving reductions of more than 25% in MDA levels with respect to the control. This phenomenon can be attributed to better control of gas and vapor exchange with the outside, avoiding problems related to diseases transmitted by microorganisms, and extending the shelf life [90].
Simultaneously, the incorporation of SeNPs/AgNPs (5–15%) generates changes in the physical and mechanical properties of the films used for kiwi (Actinidia arguta) packing, increasing the elasticity and resistance of the package. In addition, packaging films with 15% SeNPs/AgNPs reduces kiwifruit weight loss [87]. Because of these properties, the use of SeNPs can be extended to a wide variety of foods; however, this is a novel technology, and studies on the possible effects of this type of nanomaterial are ongoing, mainly in terms of toxicity. Some studies have indicated that NPs have the potential to migrate into packaged food products; however, migration assays and assessments of adverse effects remain inconclusive. Thus, in vivo toxicological assays using human and rodent cell lines are necessary, as these are the most commonly used experimental models [91]. However, due to the small number of studies, contradictory results have been reported on the properties and effects of NPs used in food packaging, indicating the possible diffusion of NPs into food in some cases but not others. Therefore, more studies are needed to establish adequate regulations so that consumers can be more certain about the safety of consuming products with smart packaging that incorporates NPs.
SeNPs in Animal Source Foods for Human Consumption
The demand for animal protein and animal source foods (meat, milk, eggs, cheese, and yogurt) has increased progressively in recent years. This phenomenon has brought with it the need to improve the quality of animal feeds, with the aim of increasing their productivity and the quality of the products obtained from them [92]. In animal nutrition, nanotechnology is primarily required for the synthesis of NPs containing minerals, especially trace elements such as Se, which is commonly used in the industry as a feed additive to improve immunity and general animal health, since conventional feeds have a low bioavailability. Additionally, with NPs, it is possible to reduce intestinal mineral antagonism, achieving a reduced excretion of these elements, which suggests improved digestive efficiency, immunity, and productivity of animals and their products [93].
The application of doses less than 1.0 mg/kg SeNPs as a feed supplement in farm animals has resulted in significant growth [94]. For example, 0.3–1.5 mg/L SeNPs in chicken feed improved the Se supply without causing toxicity, demonstrating that the use of this type of NP in broiler feed improves the absorption and diffusion of Se in organs and tissues of the animal and increases the antioxidant capacity. Although the mechanism by which SeNPs are absorbed is unknown, it is believed that the intestinal microbiota could play an important role in this process [95]. Similar results were obtained by Cai et al. [81], who supplied 0.3 mg/kg SeNPs in broiler chicken (Gallus gallus domesticus) feed and obtained higher glutathione peroxidase activity, inhibition of free radical and MDA formation, and better serum IgM and glutathione contents; furthermore, the optimal level of SeNP supplementation ranged from 0.3 to 0.5 mg/kg. In contrast, doses higher than 1.0 mg/kg were toxic. In fish, a concentration of 1 mg/kg SeNPs improved the feeding efficiency, blood health, fish defense system, and absorption of Se without causing toxicity [96]. This phenomenon is consistent with results reported in goat (Capra aegagrus hircus) feeding; when SeNPs were administered as a dietary supplement, it was possible to observe a 13% increase in goat weight and a 244% increase in the blood Se level compared with the control, as well as an increase in the activity of serum antioxidant enzymes at concentrations below 1 mg/kg [82].
Eggs are another food for which a greater number of studies on the use of SeNPs have been conducted. Regardless, this poultry product can present changes in taste and nutritional value depending on the animal feeding. One solution to this problem could be the enrichment of the feed consumed by the animals during their farming using micro- and macroelements [92]. Radwan et al. [83] evaluated the effect of 0.25 mg/kg SeNP supplementation in the diet of hens on table egg production. This procedure resulted in a 4% increase in egg production and a 3% increase in egg biomass compared with inorganic Se (SeO32−). Additionally, groups of poultry receiving SeNPs were characterized by a higher concentration of Se in eggs, as well as an 8% reduction in total lipid content in yolk, 6% less total cholesterol, and a 10% increase in the proportion of HDL (high-density lipoprotein) cholesterol [83]. Consumption of eggs fortified with SeNPs could facilitate the daily supply of the recommended amount of Se for human consumption.
The effect of Se supplementation through SeNPs was also evaluated in the diet of Tor putitora fish, in conjunction with vitamin C. This combination presented a synergistic effect, increasing the weight of the treated fish by 125.4% with respect to the control. In addition, the red blood cell count and hemoglobin level were increased in the group of fish fed a diet supplemented with 0.68 mg/kg SeNPs [97].
In cows, promising results were achieved with 0.30 mg/kg SeNP supplementation in the diet, highlighting a 31% increase in the Se level milk, 4.7% more protein, and 23% more activity of the GPX enzyme compared with the intake of an inorganic form of Se (SeO32−). Transcriptional levels of glutathione peroxidase, thioredoxin reductase, and the selenoproteins W, T, K, and F were upregulated in the mammary glands of dairy cows [80].
Potential Adverse Effects of SeNPs in Food Technology
SeNPs have an extremely narrow threshold between beneficial and toxic doses. Particularly in the field of food technology, no extensive research has been conducted to determine the harmful effects of SeNPs when used in food packaging and preservation or when used as feed supplements for human food animals. One of the reasons could lie in the fact that SeNPs are not in direct contact with food for human consumption, such as in the case of their use in snack packaging [79, 87], SeNPs are in the middle layers of packaging or in cleaning products (paper towels) [78, 85], where apparently there would be no toxic effect on the consumer. When SeNPs were used for their antimicrobial activity, no adverse effects were reported, as SeNPs were used to inhibit the growth and biofilm production of some foodborne pathogens [77]. This is one of the few cases where a range of concentrations (5–100 µg/mL) was evaluated and 20 µg/mL was reported to be the optimal dose, while 100 µg/mL did not cause toxic effects in an animal model (Artemia larvae). The SeNPs used in this study are of biogenic origin. Only two studies used biologically synthesized NPs, and the rest were obtained by a chemical approach (81.8% of a total of 11 research articles).
With respect to the use of SeNPs as a dietary supplement in animal feed, some changes in nutritional quality were identified, including a decrease in the protein and total lipid contents [83, 96] as well as cellular alterations related to oxidative stress, mainly the production of MDA, which is the product of lipid peroxidation caused by ROS. Uncontrolled lipid peroxidation can cause damage to macromolecules (DNA and proteins) and subsequently cause cell death [65].
High concentrations of Se may cause selenosis. In animals, selenosis usually manifests itself in two clinical forms: chronic and acute. The first form can cause weight loss, anemia, hair loss, joint stiffness, myocardial atrophy, and cirrhosis. In cattle, this type of selenosis decreases fertility because it favors the growth of ovarian cysts and prolongs the state of sexual inactivity in females. In the second case, toxicity symptoms included vision problems, abdominal pain, paralysis, and death. One of the frequent problems with the use of Se is its ease of transfer to the placenta and secretion through the milk, and thus, even in lactating animals, it is possible to observe symptoms of selenosis [17].
However, from the research included in this review, no tangible report was found where toxic effects can be observed for the application of SeNPs in the diet of farm animals or their use in aquaculture. However, in red seabream (Pagrus major) production, with the highest dose evaluated (2 mg SeNPs per kg diet), a possible adverse effect on fish growth and feed efficiency was suggested, only compared to 1 mg SeNPs, without this effect being negative with respect to the control (SeNP-free feed) [96].
However, a potential adverse effect is observed as the concentration of SeNPs increases [81,82,83, 96], and this fact is related to the chemical synthesis of SeNPs, as mentioned previously. The use of SeNPs in food technology is in increasing development; however, extensive research is required on the toxic effects of SeNPs, as they may directly or indirectly affect consumers. In addition, the mechanisms that convert SeNPs into assimilable forms of Se for human food animals fed diets supplemented with SeNPs as well as their use in the coating of postharvest fruits or as nanomaterials for the production of perishable food packaging need to be investigated.
Conclusion and Perspectives
Due to their biological properties, SeNPs are being studied in different research areas, leading to a large number of studies focused on providing solutions to diverse problems that converge on the health of living organisms. The application of SeNPs is considered to be highly versatile and is being successfully used in agriculture and food technology. In these two major areas, the activity of SeNPs as antimicrobial and antioxidant agents is highlighted. Particularly, in agricultural areas, SeNPs act by stimulating plant growth, increasing the Se content in edible foods through biofortification, and improving the quality of crops with a higher nutraceutical value. In the area of food technology, SeNPs represent an important innovation in food packaging by improving shelf life and in the production of animal source foods with higher Se content.
The characteristics of SeNPs, such as the size, synthesis, concentration, and application, are factors that directly influence their biological activity and should be considered during their assessment. Conversely, significant advances have been made in the functionalization of SeNPs as a way to increase their efficacy by combining them with other compounds. Therefore, it is also a scope that can be widely exploited due to the great diversity of compounds that are present in nature, which have wide biological activities and can be a viable alternative in the areas reviewed herein.
Further research and in vivo testing are needed to ensure that SeNPs do not represent risks to the health of humans, animals, and the environment due to a lack of evidence on their toxic effects in some applications.
Data Availability
Not applicable.
Abbreviations
- SeNPs:
-
Selenium nanoparticles
- CAT:
-
Catalase
- GSH:
-
Glutathione
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- JA:
-
Jasmonic acid
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- APX:
-
Ascorbate peroxidase
- OH• :
-
Hydroxyl radical
- H2O2 :
-
Hydrogen peroxide
- O2 • − :
-
Superoxide radical
- O2H• :
-
Peroxyl radical
- NO• :
-
Nitric oxide
- MDA:
-
Malondialdehyde
- PAL:
-
Phenylalanine ammonium lyase
- PLL:
-
Poly-L-lysine
- PAA:
-
Polyacrylic acid
- PVP:
-
Polyvinylpyrrolidone
- LC50 :
-
Median lethal concentration
- GSH-Px:
-
Glutathione peroxidase
- 4CL:
-
4-Coumarate-CoA ligase
- LOX:
-
Lipoxygenase
- ABA:
-
Abscisic acid
- SA:
-
Salicylic acid
- Glu:
-
Glutamate
- Pro:
-
Proline
- P5C5:
-
Δ1-Pyrroline-5-carboxylate synthetase
- P5CDH:
-
P5C dehydrogenase
- ProDH:
-
Proline dehydrogenase
- GSA:
-
Glutamyl-5-semi-aldehyde
- HCT1:
-
Hydroxycinnamoyl-CoA quinate transferase
- HQT1:
-
Hydroxycinnamoyl-CoA Quinate/shikimate hydroxycinnamoyl transferase
- bZIP:
-
Basic leucine zipper domain
- RNS:
-
Reactive nitrogen species
- Mo:
-
Molybdenum
- Fe:
-
Iron
- ATP:
-
Adenosine triphosphate
- C20:4n6:
-
Arachidonic acid
- C18:2n6/C18:3n3:
-
Linoleic acid/arachidic acid
- SFA/USFA:
-
Saturated fatty acids/unsaturated fatty acids
- MRSA:
-
Multidrug-resistant strain S. aureus
- GPx 1,2,4, :
-
Glutathione peroxidase 1,2,4
References
Kieliszek M (2019) Selenium–fascinating microelement, properties and sources in food. Molecules 24:1298. https://doi.org/10.3390/molecules24071298
Bodnar M, Konieczka P, Namiesnik J (2012) The properties, functions, and use of selenium compounds in living organisms. J Environ Sci Health C 30:225–252. https://doi.org/10.1080/10590501.2012.705164
Domokos-Szabolcsy E, Márton L, Sztrik A, Babka B, Prokisch J, Fari M (2012) Accumulation of red elemental selenium nanoparticles and their biological effects in Nicotinia tabacum. Plant Growth Regul 68:525–531. https://doi.org/10.1007/s10725-012-9735-x
Jiang L, Ni J, Liu Q (2012) Evolution of selenoproteins in the metazoan. BMC Genomics 13:446. https://doi.org/10.1186/1471-2164-13-446
Araie H, Shiraiwa Y (2016) Selenium in Algae. In: Borowitzka M, Beardall J, Raven J (eds) The physiology of microalgae. Developments in Applied Phycology, Springer, Cham, pp 281–288. https://doi.org/10.1007/978-3-319-24945-2_12
Vinkovic Vrcek I (2018) Selenium nanoparticles: biomedical applications. In: Michalke B (ed) Selenium. Molecular and Integrative Toxicology. Springer, Cham, pp 393–412. https://doi.org/10.1007/978-3-319-95390-8_21
Mangiapane E, Pessione A, Pessione E (2014) Selenium and selenoproteins: an overview on different biological systems. Curr Protein Pept Sci 15:598–607. https://doi.org/10.2174/1389203715666140608151134
Jampílek J, Králová K (2017) Nanomaterials for delivery of nutrients and growth-promoting compounds to plants. In: Prasad R, Kumar M, Kumar V (eds) Nanotechnology, Springer, Singapore, pp 177–226. https://doi.org/10.1007/978-981-10-4573-8_9
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241. https://doi.org/10.1016/S0140-6736(00)02490-9
Schiavon M, Nardi S, Vecchia F, Ertani A (2020) Selenium biofortification in the 21st century: status and challenges for healthy human nutrition. Plant Soil 453:245–270. https://doi.org/10.1007/s11104-020-04635-9
Kipp AP, Strohm D, Brigelius-Flohé R, Schomburg L, Bechthold A, Leschik-Bonnet E, Heseker H (2015) Revised reference values for selenium intake. J Trace Elem Med Biol 32:195–199. https://doi.org/10.1016/j.jtemb.2015.07.005
Kieliszek M, Błażejak S (2016) Current knowledge on the importance of selenium in food for living organisms: a review. Molecules 21:609. https://doi.org/10.3390/molecules21050609
Liu H, Yu F, Shao W, Ding D, Yu Z, Chen F, Geng D, Tan X, Lammi MJ, Guo X (2018) Associations between selenium content in hair and Kashin-Beck disease/Keshan disease in children in Northwestern China: a prospective cohort study. Biol Trace Elem Res 184:16–23. https://doi.org/10.1007/s12011-017-1169-x
Sonkusre P (2020) Specificity of biogenic selenium nanoparticles for prostate cancer therapy with reduced risk of toxicity: an in vitro and in vivo study. Front Oncol 9:1541. https://doi.org/10.3389/fonc.2019.01541
Sonkusre P, Nanduri R, Gupta P, Cameotra SS (2014) Improved extraction of intracellular biogenic selenium nanoparticles and their specificity for cancer chemoprevention. J Nanomed Nanotechnol 5:1000194. https://doi.org/10.4172/2157-7439.1000194
Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C (2019) Therapeutic applications of selenium nanoparticles. Biomed Pharmacother 111:802–812. https://doi.org/10.1016/j.biopha.2018.12.146
Żarczyńska K, Sobiech P, Radwińska J, Rękawek W (2013) Effects of selenium on animal health. J Elem 18:329–340. https://doi.org/10.5601/jelem.2013.18.2.12
Kargozar S, Mozafari M (2018) Nanotechnology and Nanomedicine: Start small, think big. Mater Today Proc 5:15492–15500. https://doi.org/10.1016/j.matpr.2018.04.155
Hernández-Díaz JA, Garza-García JJO, Zamudio-Ojeda A, León-Morales JM, López-Velázquez JC, García-Morales S (2021) Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J Sci Food Agric 101:1270–1287. https://doi.org/10.1002/jsfa.10767
Rafique M, Sadaf I, Rafique MS, Tahir MB (2016) A review on green synthesis of silver nanoparticles and their applications. Artif Cell Nanomed Biotechnol 45:1272–1291. https://doi.org/10.1080/21691401.2016.1241792
Menon S, Shanmugam V (2020) Chemopreventive mechanism of action by oxidative stress and toxicity induced surface decorated selenium nanoparticles. J Trace Elem Med Biol 62:126549. https://doi.org/10.1016/j.jtemb.2020.126549
Kondaparthi P, Flora S, Naqvi S (2019) Selenium nanoparticles: An insight on its Pro-oxidant and antioxidant properties. Front Nanosci Nanotechnol 6(1). https://doi.org/10.15761/fnn.1000189
Shang Y, Hasan MK, Ahammed GJ, Li M, Yin H, Zhou J (2019) Applications of nanotechnology in plant growth and crop protection: a review. Molecules 24:2558. https://doi.org/10.3390/molecules24142558
Prasad R, Bhattacharyya A, Nguyen QD (2017) Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol 8:1014. https://doi.org/10.3389/fmicb.2017.01014
Quiterio-Gutiérrez T, Cadenas-Pliego G, Hernández-Fuentes AD, Sandoval-Rangel A, Benavides-Mendoza A, Cabrera-de la Fuente M, Juárez-Maldonado A (2019) The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int J Mol Sci 20:1950. https://doi.org/10.3390/ijms20081950
Sarwar N, Akhtar M, Kamran MA, Imran M, Riaz MA, Kamran K, Hussain S (2020) Selenium biofortification in food crops: key mechanisms and future perspectives. J Food Compos Anal 93:103615. https://doi.org/10.1016/j.jfca.2020.103615
Elbatala I, Sidkey N, Ismaila A, Arafa RA, Fathy R (2016) Impact of silver and selenium nanoparticles synthesized by gamma irradiation and their physiological response on early blight disease of potato. J Chem Pharm Res 8:934–951
Li D, Zhou C, Zhang J, An Q, Wu Y, Li J, Pan C (2020) Nano-selenium foliar applications enhance the nutrient quality of pepper by activating the capsaicinoid synthetic pathway. J Agric Food Chem 68:9888–9895. https://doi.org/10.1021/acs.jafc.0c03044
Udalova ZV, Folmanis GE, Khasanov FK, Zinovieva SV (2018) Selenium nanoparticles–an inducer of tomato resistance to the root-knot nematode Meloidogyne incognita (Kofoid et White, 1919) Chitwood 1949. Dokl Biochem Biophys 482:264–267. https://doi.org/10.1134/s1607672918050095
Abedi S, Iranbakhsh A, Oraghi Ardebili Z, Ebadi M (2021) Nitric oxide and selenium nanoparticles confer changes in growth, metabolism, antioxidant machinery, gene expression, and flowering in chicory (Cichorium intybus L.): potential benefits and risk assessment. Environ Sci Pollut Res 28:3136–3148. https://doi.org/10.1007/s11356-020-10706-2
Schiavon M, Lima LW, Jiang Y, Hawkesford MJ (2017) Effects of selenium on plant metabolism and implications for crops and consumers. In: Pilon-Smits E, Winkel L, Lin ZQ (eds) Selenium in plants. Plant Ecophysiology. Springer, Cham, pp 257–275. https://doi.org/10.1007/978-3-319-56249-0_15
Ahmed HS, Ahmed MF, Shoala T, Salah M (2018) Impact of single or fractionated radiation and selenium nano-particles on acid lime (Citrus aurantifolia L.) seed germination ability and seedlings growth. Adv Agric Environ Sci 1:91–100. https://doi.org/10.30881/aaeoa.00016
Tocai M, Laslo V, Vicas SI (2018) Antioxidant capacity and total phenols content changes on cress (Lepidium Sativum) sprouts after exogenous supply with nano selenium. Nat Resour Sustain Dev 8:131–137. https://doi.org/10.31924/nrsd.v8i2.014
Gunti L, Dass RS, Kalagatur NK (2019) Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: antioxidant, antimicrobial, and biocompatibility. Front Microbiol 10:931. https://doi.org/10.3389/fmicb.2019.00931
Alagesan V, Venugopal S (2019) Green synthesis of selenium nanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. Bionanosci 9:105–116. https://doi.org/10.1007/s12668-018-0566-8
Fardsadegh B, Jafarizadeh-Malmiri H (2019) Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process Synth 8:399–407. https://doi.org/10.1515/gps-2019-0007
Nandini B, Hariprasad P, Prakash HS, Shetty HS, Geetha N (2017) Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerospora graminicola in pearl millet. Sci Rep 7:2612. https://doi.org/10.1038/s41598-017-02737-6
Hu D, Yu S, Yu D, Liu N, Tang Y, Fan Y, Wu A (2019) Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control 106:106748. https://doi.org/10.1016/j.foodcont.2019.106748
Vrandečić K, Ćosić J, Ilić J, Ravnjak B, Selmani A, Galić E, Vinković T (2020) Antifungal activities of silver and selenium nanoparticles stabilized with different surface coating agents. Pest Manag Sci 76:2021–2029. https://doi.org/10.1002/ps.5735
Huang X, Chen X, Chen Q, Yu Q, Sun D, Liu J (2016) Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater 30:397–407. https://doi.org/10.1016/j.actbio.2015.10.041
Benelli G (2015) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res 115:23–34. https://doi.org/10.1007/s00436-015-4800-9
Sowndarya P, Ramkumar G, Shivakumar MS (2017) Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif Cell Nanomed Biotechnol 45:1490–1495. https://doi.org/10.1080/21691401.2016.1252383
Salem SS, Fouda MMG, Fouda A, Awad MA, Al-Olayan EM, Allam AA, Shaheen TI (2021) Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. J Clust Sci 32:351–361. https://doi.org/10.1007/s10876-020-01794-8
Ramya S, Shanmugasundaram T, Balagurunathan R (2019) Actinobacterial enzyme mediated synthesis of selenium nanoparticles for antibacterial, mosquito larvicidal and anthelminthic applications. Particul Sci Technol 38:63–72. https://doi.org/10.1080/02726351.2018.1508098
Krishnan M, Ranganathan K, Maadhu P, Thangavelu P, Kundan S, Arjunan N (2020) Leaf extract of Dillenia indica as a source of selenium nanoparticles with larvicidal and antimicrobial potential toward vector mosquitoes and pathogenic microbes. Coatings 10:626. https://doi.org/10.3390/coatings10070626
Gudkov SV, Shafeev GA, Glinushkin AP, Shkirin AV, Barmina EV, Rakov II, Kalinitchenko VP (2020) Production and use of selenium nanoparticles as fertilizers. ACS Omega 5:17767–17774. https://doi.org/10.1021/acsomega.0c02448
Nazerieh H, Oraghi Ardebili Z, Iranbakhsh A (2018) Potential benefits and toxicity of nanoselenium and nitric oxide in peppermint. Acta Agric Slov 111:357–368. https://doi.org/10.14720/aas.2018.111.2.11
Ragavan P, Ananth A, Rajan MR (2017) Impact of selenium nanoparticles on growth, biochemical characteristics and yield of cluster bean Cyamopsis tetragonoloba. Int J Environ Agric Biotechnol 2:2917–2926. https://doi.org/10.22161/ijeab/2.6.19
Sotoodehnia-Korani S, Iranbakhsh A, Ebadi M, Majd A, Ardebili ZO (2020) Selenium nanoparticles induced variations in growth, morphology, anatomy, biochemistry, gene expression, and epigenetic DNA methylation in Capsicum annuum; an in vitro study. Environ Pollut 265:114727. https://doi.org/10.1016/j.envpol.2020.114727
Hernández-Hernández H, Quiterio-Gutiérrez T, Cadenas-Pliego G, Ortega-Ortiz H, Hernández-Fuentes AD, Cabrera de la Fuente M, Juárez-Maldonado A (2019) Impact of selenium and copper nanoparticles on yield, antioxidant system, and fruit quality of tomato plants. Plants 8:355. https://doi.org/10.3390/plants8100355
Juárez-Maldonado A, Ortega-Ortíz H, Morales-Díaz A, González-Morales S, Morelos-Moreno Á, Cabrera-De la Fuente M, Sandoval-Rangel A, Cadenas-Pliego G, Benavides-Mendoza A (2019) Nanoparticles and nanomaterials as plant biostimulants. Int J Mol Sci 20:162. https://doi.org/10.3390/ijms20010162
Hussein HAA, Darwesh OM, Mekki BB, El-Hallouty SM (2019) Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol Rep 24:e00377. https://doi.org/10.1016/j.btre.2019.e00377
Golubkina NA, Folmanis GE, Tananaev IG, Krivenkov LV, Kosheleva OV, Soldatenko AV (2017) Comparative evaluation of spinach biofortification with selenium nanoparticles and ionic forms of the element. Nanotechnol Russ 12:569–576. https://doi.org/10.1134/S1995078017050032
Zahedi SM, Abdelrahman M, Hosseini MS, Hoveizeh NF, Tran LSP (2019) Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ Pollut 253:246–258. https://doi.org/10.1016/j.envpol.2019.04.078
El Lateef Gharib FA, Zeid IM, Ghazi SM, Ahmed EZ (2019) The response of cowpea (Vigna unguiculata L) plants to foliar application of sodium selenate and selenium nanoparticles (SeNPs). J Nanomater Mol Nanotechnol 8:4
Babajani A, Iranbakhsh A, Oraghi Ardebili Z, Eslami B (2019) Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ Sci Pollut Res 26:24430–24444. https://doi.org/10.1007/s11356-019-05676-z
Li Y, Zhu N, Liang X, Zheng L, Zhang C, Li YF, Zhang Z, Gao Y, Zhao J (2019) A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotoxicol Environ Saf 189:109955. https://doi.org/10.1016/j.ecoenv.2019.109955
Golubkina NA, Folmanis GE, Tananaev IG (2012) Comparative evaluation of selenium accumulation by allium species after foliar application of selenium nanoparticles, sodium selenite and sodium selenite. Dokl Biol Sci 444:176–179. https://doi.org/10.1134/S0012496612030076
Wang K, Wang Y, Li K, Wan Y, Wang Q, Zhuang Z, Guo Y, Li H (2020) Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J Nanobiotechnology 18:103. https://doi.org/10.1186/s12951-020-00659-6
Hu T, Li H, Li J, Zhao G, Wu W, Liu L, Wang Q, Guo Y (2018) Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front Plant Sci 9:597. https://doi.org/10.3389/fpls.2018.00597
He M, He CQ, Ding NZ (2018) Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front Plant Sci 9:1771. https://doi.org/10.3389/fpls.2018.01771
Habibi G, Aleyasin Y (2020) Green synthesis of Se nanoparticles and its effect on salt tolerance of barley plants. Int J Nano Dimens 11:145–157
Soleymanzadeh R, Iranbakhsh A, Habibi G, Ardebili ZO (2020) Selenium nanoparticle protected strawberry against salt stress through modifications in salicylic acid, ion homeostasis, antioxidant machinery, and photosynthesis performance. Acta Biol Crac Ser Bot 62:33–42. https://doi.org/10.24425/abcsb.2019.127751
Amina Z, Samar O (2019) Nano Selenium: Reduction of severe hazards of atrazine and promotion of changes in growth and gene expression patterns on Vicia faba seedlings. Afr J Biotechnol 18:502–510. https://doi.org/10.5897/ajb2019.16773
Djanaguiraman M, Belliraj N, Bossmann SH, Prasad PVV (2018) High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 3:2479–2491. https://doi.org/10.1021/acsomega.7b01934
Dudeja P, Gupta RK (2017) Nutraceuticals. In: Gupta RK, Minhas D, Minhas S (eds) Food Safety in the 21st Century. Academic Press, Massachusetts, pp 491–496. https://doi.org/10.1016/B978-0-12-801773-9.00040-6
Zeid IM, Gharib FAEL, Ghazi SM, Ahmed EZ (2019) Promotive effect of ascorbic acid, gallic acid, selenium and nano-selenium on seed germination, seedling growth and some hydrolytic enzymes activity of cowpea (Vigna unguiculata) seedling. J Plant Physiol Pathol 7:1. https://doi.org/10.4172/2329-955X.1000193
El-Kinany RG, Brengi SH, Nassar AK, El-Batal A (2019) Enhancement of plant growth, chemical composition and secondary metabolites of essential oil of salt-stressed coriander (Coriandrum Sativum L.) plants using selenium, nano-selenium, and glycine betaine. Sci J Flower Ornam Plant 6:151–173. https://doi.org/10.21608/sjfop.2019.84973
Zahedi SM, Hosseini MS, Daneshvar Hakimi Meybodi N, Teixeira da Silva JA (2019) Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. S Afr J Bot 124:350–358. https://doi.org/10.1016/j.sajb.2019.05.019
EFSA Scientific Committee (2011) Scientific Opinion on Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J 9:2140. https://doi.org/10.2903/j.efsa.2011.2140
Zanella M, Ciappellano SG, Venturini M, Tedesco E, Manodori L, Benett F (2015) Nutraceuticals and nanotechnology. Agro Food Industry Hi Tech 26:26–31
Humphrey Adebayo A, Faith Yakubu O, Bakare-Akpata O (2020) Uptake, metabolism and toxicity of selenium in tropical plants. In: Rahman MM, Asiri AM, Khan A, Inamuddi (eds) Importance of selenium in the environment and human health. IntechOpen, London. https://doi.org/10.5772/intechopen.90295
Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK, Rather IA (2017) Application of nanotechnology in food science: perception and overview. Front Microbiol 8:1501. https://doi.org/10.3389/fmicb.2017.01501
Ezhilarasi PN, Karthik P, Chhanwal N, Anandharamakrishnan C (2013) Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol 6:628–647. https://doi.org/10.1007/s11947-012-0944-0
Yu H, Park JY, Kwon CW, Hong SC, Park KM, Chang PS (2018) An overview of nanotechnology in food science: preparative methods, practical applications, and safety. J Chem 2018:5427978. https://doi.org/10.1155/2018/5427978
Biji KB, Ravishankar CN, Mohan CO, Srinivasa Gopal TK (2015) Smart packaging systems for food applications: a review. J Food Sci Technol 52:6125–6135. https://doi.org/10.1007/s13197-015-1766-7
Khiralla GM, El-Deeb BA (2015) Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT Food Sci Technol 63:1001–1007. https://doi.org/10.1016/j.lwt.2015.03.086
Webster TJ, Wang Q (2013) Short communication: inhibiting biofilm formation on paper towels through the use of selenium nanoparticles coatings. Int J Nanomedicine 8:407–411. https://doi.org/10.2147/IJN.S38777
Vera P, Canellas E, Nerín C (2018) New antioxidant multilayer packaging with nanoselenium to enhance the shelf-life of market food products. Nanomaterials 8:837. https://doi.org/10.3390/nano8100837
Han L, Pan K, Fu T, Phillips CJC, Gao T (2021) Nano-selenium supplementation increases selenoprotein (Sel) gene expression profiles and milk selenium concentration in lactating dairy cows. Biol Trace Elem Res 199:113–119. https://doi.org/10.1007/s12011-020-02139-2
Cai SJ, Wu CW, Gong LM, Song T, Wu H, Zhang LY (2012) Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult Sci 91:2532–2539. https://doi.org/10.3382/ps.2012-02160
Shi L, Xuna W, Yuea W, Zhanga C, Rena Y, Shi L, Wanga Q, Yanga R, Lei F (2011) Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin Res 96:49–52. https://doi.org/10.1016/j.smallrumres.2010.11.005
Radwan NL, Eldin TAS, Zaiat AAE, Mostafa MASA (2015) Effect of dietary nano-selenium supplementation on selenium content and oxidative stability in table eggs and productive performance of laying hens. Int J Poult Sci 14:161–176. https://doi.org/10.3923/ijps.2015.161.176
Vilela C, Kurek M, Hayouka Z, Röcker B, Yildirim S, Antunes MDC, Freire CSR (2018) A concise guide to active agents for active food packaging. Trends Food Sci Tech 80:212–222. https://doi.org/10.1016/j.tifs.2018.08.006
Wang Q, Webster TJ (2013) Bacteria fighting paper towels: the influence of selenium nanoparticles. 39th Annual Northeast Bioengineering Conference. IEEE Xplore. https://doi.org/10.1109/NEBEC.2013.55
Jamróz E, Kopel P, Juszczak L, Kawecka A, Bytesnikova Z, Milosavljević V, Adam V (2018) Development and characterisation of furcellaran-gelatin films containing SeNPs and AgNPs that have antimicrobial activity. Food Hydrocoll 83:9–16. https://doi.org/10.1016/j.foodhyd.2018.04.028
Jamróz E, Kopel P, Juszczak L, Kawecka A, Bytesnikova Z, Milosavljevic V, Makarewicz M (2019) Development of furcellaran-gelatin films with Se-AgNPs as an active packaging system for extension of mini kiwi shelf life. Food Packag Shelf Life 21:100339. https://doi.org/10.1016/j.fpsl.2019.100339
Sharma C, Dhiman R, Rokana N, Panwar H (2017) Nanotechnology: an untapped resource for food packaging. Front Microbiol 8:1735. https://doi.org/10.3389/fmicb.2017.01735
Vera P, Echegoyen Y, Canellas E, Nerín C, Palomo M, Madrid Y, Cámara C (2016) Nano selenium as antioxidant agent in a multilayer food packaging material. Anal Bioanal Chem 408:6659–6670. https://doi.org/10.1007/s00216-016-9780-9
Guillard V, Gaucel S, Fornaciari C, Angellier-Coussy H, Buche P, Gontard N (2018) The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front Nutr 5:121. https://doi.org/10.3389/fnut.2018.00121
Lauriano Souza VG, Fernando AL (2016) Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag Shelf Life 8:63–70. https://doi.org/10.1016/j.fpsl.2016.04.001
Konkol D, Wojnarowski K (2018) The Use of nanominerals in animal nutrition as a way to improve the composition and quality of animal products. J Chem 2018:5927058. https://doi.org/10.1155/2018/5927058
Gopi M, Pearlin B, Kumar RD, Shanmathy M, Prabakar G (2017) Role of nanoparticles in animal and poultry nutrition: modes of action and applications in formulating feed additives and food processing. Int J Pharmacol 13:724–731. https://doi.org/10.3923/ijp.2017.724.731
Pelyhe C, Mézes M (2013) Myths and facts about the effects of nano-selenium in farm animals— mini-review. Eur Chem Bull 2:1049–1052
Gangadoo S, Dinev I, Willson NL, Moore RJ, Chapman J, Stanley D (2020) Nanoparticles of selenium as high bioavailable and non-toxic supplement alternatives for broiler chickens. Environ Sci Pollut Res 27:16159–16166. https://doi.org/10.1007/s11356-020-07962-7
Dawood MAO, Koshio S, Zaineldin AI, Van Doan H, Ahmed HA, Elsabagh M, Abdel-Daim MM (2019) An evaluation of dietary selenium nanoparticles for red sea bream (Pagrus major) aquaculture: growth, tissue bioaccumulation, and antioxidative responses. Environ Sci Pollut Res 26:30876–30884. https://doi.org/10.1007/s11356-019-06223-6
Khan KU, Zuberi A, Nazir S, Ullah I, Jamil Z, Sarwar H (2017) Synergistic effects of dietary nano selenium and vitamin C on growth, feeding, and physiological parameters of mahseer fish (Tor putitora). Aquac Reports 5:70–75. https://doi.org/10.1016/j.aqrep.2017.01.002
Acknowledgements
The authors would like to thank the Consejo Nacional de Ciencia y Tecnología (CONACYT, Mexico) for the M. Sc. scholarship of Jorge JO Garza-García (717217) and José A Hernández-Díaz (717214). SGM and JMLM are part of PLANTECC National Laboratory.
Author information
Authors and Affiliations
Contributions
JJOGG investigated, designed figure sets, and wrote—original draft. JAHD investigated, designed figure sets, and wrote—original draft. AZO investigated and analyzed the literature. JMLM investigated, verified, and discussed the information. AGG investigated and organized the information. DRSC investigated and organized the information. JCLV investigated and verified the information. SGM conceptualized the idea, supervised, and wrote—revised and edited. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jorge J. O. Garza-García and José A. Hernández-Díaz contributed equally to this work
Rights and permissions
About this article
Cite this article
Garza-García, J.J.O., Hernández-Díaz, J.A., Zamudio-Ojeda, A. et al. The Role of Selenium Nanoparticles in Agriculture and Food Technology. Biol Trace Elem Res 200, 2528–2548 (2022). https://doi.org/10.1007/s12011-021-02847-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02847-3