Abstract
Several studies have shown that any magnetic field (MF) applied to microalgae modifies its cultivation conditions and may favor biomolecule production since it interacts with the microorganisms and affect their growth. As a result, there are changes in concentrations and compositions of biomass and biomolecules. This review aims at updating MF applications to microalga cultures that were reported by studies conducted in the last 5 years. It shows the main studies that reached positive results of carbohydrate, lipid, protein and pigment production. Effects of MFs may be positive, negative or null, depending on some factors, such as intensity, exposure time, physiological state of cells and application devices. Therefore, this review details cultivation conditions used for reaching high concentration of biomolecules, explains the action of MFs on microalgae and describes their applicability to the biorefinery concept.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae belong to a complex group of photosynthetic microorganisms that may be found in different ecosystems. They have been phytologically classified into microscopic eukaryotic microalgae and photosynthetic prokaryotic bacteria, which are also called cyanobacteria (Maltsev and Maltseva 2021).
About 30,000 species of microalgae have already been isolated and studied. In this biodiversity, some species have shown potential to be applied to biorefineries, because cultivation is simple and some may grow photoautotrophically with the use of light irradiation and CO2 as the carbon source and conversion into biomass and O2 (Elisabeth et al. 2021). In addition, several studies have reported that microalgae can accumulate carbohydrates, lipids, proteins and other secondary metabolites, such as pigments, at significant amounts (Godfray et al. 2010; Ibañez and Cifuentes 2013; Raheem et al. 2018; Maia et al. 2020; Wang et al. 2021).
In this field, conventional cultivation parameters, such as nutrients, pH, temperature and light irradiance, have been deeply studied to produce biomass and biomolecules of interest (González-Fernández and Ballesteros 2012; Ho et al. 2014; Khozin-Goldberg 2016; Hu 2019; Huo et al. 2020). However, the main challenges for industrial application are the increase in the growth rate and product synthesis by cells after scale-up, biomass pretreatment and process optimization to reduce costs. Thus, new technological approaches, such as magnetic field (MF) application, have gained prominence in microalga-based biorefinery since it is a non-toxic and low-cost technology that has emerged in bioprocesses.
Santos et al. (2017) reported that MF intervention may interact with biological systems during their growth and its effects may alter microalga metabolism, depending on intensity, exposure time and bioreactor devices. According to Silvello et al. (2022), stress caused by the magnetic effect may stimulate biomass production and modify its composition. Therefore, this new technological approach has been studied in the last years to produce biomolecules with high added value.
Despite the available number of review articles on microalga cultivation, there is little review information about MF application and its effects on microalga metabolism. These articles generally address cultivation parameters and specific applications to different industrial segments. Nevertheless, this review aims to provide an update and new insights into MF application to microalga cultures and into its applicability to biorefinery.
MF application and its effects on microalga cultures
In bioprocesses, MFs have been studied to increase production of compounds of interest as the result of stress caused by the magnetic effect on biological systems. Their effects may be classified into null, inhibitory and stimulatory, depending on the application mechanism, intensity and exposure time (Hirano et al. 1998; Zapata et al. 2002; Santos et al. 2017).
Several studies of MF application have successfully reported increment in products with the use of different types of microorganisms, such as biomass and glutathione by Saccharomyces cerevisiae (Santos et al. 2022), ethanol and peroxidase by Saccharomyces cerevisiae (Boeira et al. 2021), biomass and carotenoid by Phaffia rhodozyma (Silva et al. 2020, 2022), yellow pigment and monacolin K by Monascus purpureus (Wan et al. 2017), γ-Linolenic acid by Cunninghamella echinulate (Al-Hawash et al. 2018), red pigments by Monascus purpureus (Liao et al. 2019), pigments by Chlorella kessleri (Bauer et al. 2017), proteins by Limnospira indica (Deamici et al. 2022), lipids by Chlorella homosphaera (Costa et al. 2020) and carbohydrates by Chlorella minutissima (Menestrino et al. 2020).
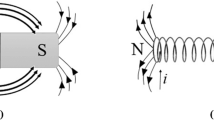
In microalga culture, MF application has become an interesting alternative to increase biomass production and, consequently, molecules of industrial interest (Santos et al. 2017; Costa et al. 2020). This emerging technological approach is considered non-toxic, low-cost and easily applicable to photobioreactors, while biomolecules may be used in several segments of chemical, pharmaceutical and food industries (Silvello et al. 2022). However, the type of photobioreactor is considered an important factor to be evaluated for product formation. Bioreactors, at different sizes, material and designs, have been widely used in microalga cultivation (Daneshvar et al. 2021) and adapted for MF application with the use of magnets and coils (Fig. 1). The design application of magnets and coils to photobioreactors cannot affect the incidence of light and must have direct magnetic interaction with cells during cultivation (Santos et al. 2017).
Depending on the type of microalga cells (prokaryotic or eukaryotic) and their physiological state, MF application may trigger different responses in cell metabolism caused by magnetic stress (Hirano et al. 1998; Luna et al. 2012). According to Sarraf et al. (2020), distinct studies have recently explained how MF application affects microalga metabolism. Its main effects are shown in Fig. 2.
MF application to microalga cultures may cause alterations in gene transcriptions of microorganisms (Hunt et al. 2009; Luna et al. 2012) and, consequently, lead to activation of some specific metabolic and enzymatic systems (Katz et al. 2005; Li et al. 2011). Activations in metabolism may occur through signaling caused by the movement of ions as the result of alteration in energy levels and orientation of electron spins (Yang et al. 2011). In addition, they may trigger alterations in the plasma membrane flux, lead to changes in osmotic pressure of cells due to salt and mineral concentrations and interact with free radical activity in the system (Hirano et al. 1998; Small et al. 2012).
In general, MF application is not the only factor that is responsible for the production of specific biomolecules, but the influence of magnetism as a stressing factor in the system, along with other parameters, such as medium composition, pH, temperature, irradiance and type of system (either open or closed), may increase yield in biomass production and, consequently, in molecules with high added value.
Carbohydrates
In microalga cultivation, different strategies have been used for increasing accumulation of carbohydrates in cells; the most common ones are nutrient starvation, irradiance exposure and temperature (Maia et al. 2020). However, MF application has been used as an alternative technological approach by activating the enzymatic system (i.e., starch synthase and sucrose synthase) involved in carbohydrate accumulation, such as the one described by Silvello et al. (2022).
Bauer et al. (2017) cultivated Chlorella kessleri in BG-11 medium for 10 d and evaluated different MF intensities and exposure times. When 30 mT was applied for 24 h d−1, carbohydrate content increased 14.4% by comparison with the control culture (without any MF application). Menestrino et al. (2020) used 30 mT and added pentoses to Chlorella minutissima cultivation. When 5% pentose was added, interaction with MF increased the carbohydrate content, which reached 60.5%. It represented increase of 16% by comparison with the culture without any MF application.
Zhu et al. (2022) evaluated MFs in Tribonema sp. cultivated in an open air system with starch wastewater in the four seasons. MF intervention did not affect carbohydrate content in any season. Tribonema sp. was able to grow in all seasons in an environment at average temperature of 16.8 ºC and MF application, although its optimal growth temperature is between 18 and 25 ºC.
Deamici et al. (2021b) evaluated production of different macromolecules in Chlorella fusca cultivation, in outdoor and indoor systems, after 15 d of cultivation. In both conditions, 25 mT was applied in raceway photobioreactors at different exposure times (1 h d−1 and 24 h d−1) and results were compared to the ones of the controls (without any MF application). No significant effect was observed (p > 0.05).
Another study carried out by Deamici et al. (2021a) evaluated the influence of 30 mT on Arthrospira platensis SAG 21.99 cultivation in a glass bottle photobioreactor for 10 d. The highest exopolysaccharide (EPS) content (34.80 g 100 gbiomass−1) was achieved when MF was applied for 24 h d−1. Characterization of EPSs produced by A. platensis under influence of MF showed that they were basically composed of neutral sugars (80.7%) and uronic acids (19.3%). Regarding Spirulina sp. LEB 18 cultivation, decrease (32.9%) in EPS content was observed when MFs were applied for 24 h d−1, by comparison with the control.
In general, microalga-based carbohydrates are considered one of the main biomolecules found in biomass. Thus, MF application may be used as a non-conventional strategy to stimulate carbohydrate production in pharmaceutical, cosmetic and food industries, such as sulfated polysaccharides as anti-inflammatory (Costa et al. 2021), EPS as anti-aging (Laroche 2022) and prebiotic food (Gouda et al. 2022), respectively. However, interest in microalga biomass with high carbohydrate content has been recently increasing to produce biofuels, mainly bioethanol (Khan et al. 2018).
Lipids
Several researchers have addressed the use of lipids obtained from microalga cultures to produce biofuels, so that fossil fuels may be replaced by less polluting energy sources (Mofijur et al. 2019; Rahpeyma and Raheb 2019; Sati et al. 2019). In addition, in microalga cultures that aim at lipids to produce biofuels, by-products and wastewater may be used as components of culture media in order to combine sustainability and cost reduction (Raheem et al. 2018).
Different strategies of microalga cultivation have been studied to increase lipid accumulation by cells (Park et al. 2019). Microalgal lipids are classified into neutral (storage) lipids, triglycerides (TAG), polar (membrane) lipids, phospholipids, glycolipids and sterols (Maltsev and Maltseva 2021). Synthesis of fatty acids may take place in three main steps: synthesis of enzyme A (CoA) in cytoplasm, synthesis of × 16–18 carbon saturated fatty acid and subsequent desaturation and carbon chain elongation and synthesis of acyl glycerol (triglycerides) (Khozin-Goldberg 2016).
The use of MF as a strategy to increase concentrations of biomolecules, such as lipids, in microalgae, has shown promising results (Table 1). However, responses to MFs are not always linear, that is, their effects on cells and formation of biomolecules may be stimulatory, inhibitory or non-existent (Santos et al. 2017). The hypothesis of MF effect on lipid accumulation is related to alteration in cell membrane flow, causing better assimilation of the substrate due to the orientation of the MF lines (Albuquerque et al. 2016) and consequently activating specific enzymatic systems for lipid accumulation, i.e., related to the synthesis of enzyme A (CoA) in the cytoplasm (Khozin-goldberg 2016).
Bauer et al. (2017) studied MF application (30 and 60 mT) at different exposure times (1 and 24 h d−1) in Chlorella kessleri cultivation in BG 11 medium. Ferrite magnets were used and, regardless of MF intensity, the best results were reached when application lasted 1 h d−1, with increases of 13.1 and 13.7% in lipid contents after the use of 30 and 60 mT, respectively. Shao et al. (2018) studied the efficiency of cadmium (Cd2+) removal with the use of 30 mT at different exposure times (3, 6 and 12 h d−1) and, consequently, lipid production by Spirulina platensis in Zarrouk medium with increase of 15.9% when application lasted 12 h d−1.
Huo et al. (2020) evaluated different temperatures (25, 30 and 33 °C) and application of 30 mT with continuous exposure to ferrite magnets in Tribonema sp. cultures in wastewater. Increases of 53.8, 30.7 and 20% in lipid productivity at 25, 30 and 33 °C, respectively, were reported when MFs were applied, by comparison with their respective controls (without any MF). Costa et al. (2020) reported high lipid content and productivity in a shorter period (1 h d−1) of MF application in Chlorella homosphaera cultivated in Bristol modified medium (BMM). Increases in lipid contents were 49.4 and 43.6% while the ones in lipid productivity were 138.4 and 108.4% when exposed to 30 and 60 mT, respectively.
Feng et al. (2020) applied 100, 200, 300, 400 and 500 mT at different times (1, 2 or 3 h d−1) to Chlorella pyrenoidosa cultures grown in wastewater to produce lipids. Two pieces of equipment were used to generate MF; one was coupled directly to the bottom of culture flasks and the other had a system of culture medium recirculation by magnets. The highest concentration of lipids was achieved by the recirculation system at 500 mT and exposure time of 1 h d−1. Chu et al. (2020) evaluated the initial concentration of nitrate (60, 100, 140, 180 and 220 mg L−1) and MF intensity (10, 20, 30 and 40 mT) continuously applied to produce lipids in Nannochloropsis oculata cultures. The best conditions for lipid production were the use of 20 mT and 140 mg L−1 nitrate, since it led to increase of 103% in lipid productivity (38 mg L d−1) by comparison with the control.
Baldev et al. (2021) applied pulsating magnetic fields (PMFs), at intensities ranging from 0.07 to 0.09 mT and frequency of 1 Hz for 4 h d−1, to Chlorella vulgaris cultures. The lipid content increased 29.8% at 0.07 mT. Zhu et al. (2022) used neodymium magnets to continuously apply 30 and 130 mT to Tribonema sp. cultivated in wastewater in all seasons (spring, summer, autumn and winter). MFs increased lipid productivity, except in winter. The best results were found in summer when 30 and 130 mT were applied, that is, increases were 33.8% (0.216 g L d−1) and 35.9% (0.216 g L d−1), respectively, by comparison with the control.
Although several studies have reported positive effects of MF on microalga lipid production, some have shown negative or null effects, as is the case of the one carried out by Li et al. (2022), who reported decrease of 15.7% in lipid content, by comparison with the control culture, when 30 mT was applied to Chlorella pyrenoidosa cultures for 24 h d−1. The authors also investigated the effects of MF on the lipid content at the same intensity and application time in Tetradesmus obliquus and no significant change was found in lipid content.
In their study, Deamici et al. (2019b) reached 15% decrease in the lipid content of C. fusca LEB 111, by comparison with the control culture, when 30 mT was applied for 24 h d−1. The authors showed no significant difference in the lipid content when 30 mT was applied for 1 h d−1 and 60 mT was applied for 1 h d−1 or 24 h d−1. Veiga et al. (2020) investigated application of 60 mT to Spirulina sp. LEB 18 for 24 h d−1 and the lipid content was not altered. Deamici et al. (2021b) studied effects of 25 mT to outdoor and indoor cultures of C. fusca LEB 111 for 1 h d−1 or 24 h d−1 and no significant difference was found in the lipid content, regardless of the cultivation system or application time.
Therefore, the use of lipids obtained from microalgae has gained prominence based on the concepts of biorefinery, where they are mainly applied to biodiesel production. Thus, MF application has shown to be an interesting approach to stimulate lipid accumulation, which is considered an alternative renewable source of high energetic capacity.
Proteins
Since the global population is estimated at 9.7 billion by 2050 – data issued by the United Nations (2019) –, there must be increase of 70% in food production worldwide (Bleakley and Hayes 2017). Thus, microalga biomass may play an important role in feeding the population because it has high amounts of protein, essential amino acids, vitamins, antioxidants and minerals (Navarro-López et al. 2020). According to Wang et al. (2021), the global microalga protein market is expected to grow as much as the overall protein market and reach USD 0.84 billion by 2023. Most microalga species exhibit high protein contents, mainly Arthrospira sp. and Chlorella sp., which are the most exploited biomass for food supplementation be-cause they have high protein contents (51–58%) (Becker 2007; Ismail et al. 2020) and are GRAS (Generally Recognized as Safe) certified by the Food and Drug Administration (Belay et al. 2008).
Microalga protein outweighs conventional sources in terms of quality and quantity, a fact that makes microalga biomass a promising source of dietary protein (Pereira et al. 2018). Furthermore, microalga proteins are susceptible to activities of proteolytic enzymes and there are compounds, such as phenolic compounds and polysaccharides, that may limit digestibility of their proteins (Ibañez and Cifuentes 2013). To increase protein contents in microalga biomass, new technologies, such as the use of MF during cultivation, have been implemented. As mentioned before, MFs may either stimulate or inhibit biomolecule production and influence metabolism of microorganisms by modifying metabolic pathways (Hunt et al. 2009; Santos et al. 2017).
Many studies of microalga cultures subject to MFs have reported changes in protein contents. Different MF application times and intensities during cultivation should be evaluated because these parameters influence protein content in the final biomass, as shown by some studies. Differences were reported when MFs were applied either continuously (24 h d−1) or for a short time (1 h d−1). Besides, most studies included in this review evaluated MF intensity between 25 to 60 mT and used the microalga Chlorella.
Bauer et al. (2017) proved that 30 mT applied for 24 h and 1 h d−1 increased protein content of Chlorella kessleri, by comparison with the control group (without any MF application). Deamici et al. (2021b) evaluated the influence of 25 mT on Chlorella fusca cultivation in outdoor and indoor conditions in raceway photobioreactors. Biomass composition was affected when MF was applied only for 1 h d−1 in indoor conditions. Regarding the protein content, it was statistically similar to outdoor assays (24 and 1 h d−1 application time), while it increased when C. fusca was cultured indoor with MF for1 h d−1 and achieved 35.7%, m m−1. Chlorella fusca was also cultured in indoor conditions in tubular vertical photobioreactors with 30 mT MF applied for 24 h d−1 by Deamici et al. (2019b). The protein content in the biomass achieved 56.2% w w−1, significantly higher (p ≤ 0.05) than the control group. In this study, biomass from other MF conditions was statistically equal to the control group. On the other hand, Deamici et al. (2019a) found that, in cultures of Arthrospira platensis, application of 30 mT for 24 h d−1 did not significantly affect (p ≤ 0.05) protein content, i. e., it was 17.5% lower than the control group and similar to MF in the 1 h d−1 condition (~ 66.7%).
Huo et al. (2020) cultivated Tribonema sp. at 30 mT for 10 days and reached maximum biomass and increase in protein productivity of 49.1 mg L−1d−1, that corresponds to 48.4% more protein in microalga biomass by comparison with the culture without any MF. In this study, MF application showed to improve biomass productivity, protein and carbohydrate contents. Besides, MFs promoted Tribonema sp. microalga growth in the late logarithmic phase of batch cultivation.
Protein content in microalga biomass subject to MF was also investigated by Li et al. (2022), who applied 30 mT to Chlorella pyrenoidosa and Tetradesmus obliquus cultures for 15 days. Regarding C. pyrenoidosa biomass, there was 44.3% increase in the protein content by comparison with the control group. In T. obliquus cultures, there was no difference (p > 0.05) in the protein content (32.9% w w−1 in the control group and 34.6% w w−1 subject to MF).
In the study conducted by Shao et al. (2018), increase in the protein content in Spirulina biomass was reported after short periods of MF application (6 h d−1), 10.1% higher than the one in the control assay (59%, CDW). In their study, when MF was applied for 12 h d−1, the protein content decreased, by comparison with the control assay.
In the study carried out by Veiga et al. (2020), influence of MF application on digestibility and protein solubility of Spirulina sp. LEB 18 was evaluated so that it could be used it in the food industry as an ingredient or food supplement. MF application to cultivation increased solubility since biomass showed the highest protein solubility (89%) at all pH under analysis. The biomass may be applied to food products and has potential to be used as an ingredient in the manufacture of protein supplements.
The use of MF in microalga culture is a relatively new technique and has been shown to be an alternative clean technique to improve microalga biomass. It has shown great results of growth and biomolecule production, and according to Li et al. (2022), it shows that MF may be a promising alternative to induce intracellular carbon partition, specially to the protein synthetic pathway.
Pigments
Main pigments found in microalgae are chlorophylls, biliproteins and carotenoids (Dasgupta 2015). The primary pigment found in all photosynthetic oxygenic organisms is chlorophyll-a, which absorbs most energy (λmax: 660–665 nm) and then serves as the primary electron donor in the electron transport chain.
To assist in the process of capturing light, microalgae have accessory pigments that absorb light at wavelengths in which chlorophyll-a has low absorptivity, such as phycobiliproteins (C-phycocyanin, allophycocyanin and phycoerythrin), carotenoids (β-carotene, violaxanthin, astaxanthin) and chlorophyll-b (Hu 2019). In this context, several studies have shown that MF interaction with microalga cells may trigger the synthesis of pigments that are shown in Table 1.
Bauer et al. (2017) evaluated MFs on Chlorella kessleri LEB 113 cultures for 10 d; 30 and 60 mT were applied for 1 and 24 h d−1. In the cases of chlorophylls and carotenoids, MF application for 1 h d−1 led to better synthesis. Intensity of 30 mT was better to produce carotenoids while 60 mT was more effective to yield chlorophylls.
Deamici et al. (2018) compared applications of 25 mT for 1 and 24 h d−1 in outdoor and indoor conditions to chlorophyll production by Spirulina. MFs for 24 h d−1 in outdoor conditions led to increase of 137.7% in chlorophyll-a content. In other conditions (indoor at MF for 1 and 24 h d−1 and outdoor at MF for1 h d−1), MFs did not change chlorophyll-a content significantly.
Application of 30 mT for 1 or 24 h d−1 to chlorophyll-a and phycocyanin production by Arthrospira platensis SAG 21.99 for 10 d was evaluated by Deamici et al. (2019a). The use of MFs for 1 h d−1 did not alter pigment concentrations. In addition, MF application for 24 h d−1 (continuous exposure) generated stressed cell and affected the molecular composition of cells, which leads to a lower pigment content by comparison with the control (without any MF action).
Ferrada et al. (2020) evaluated the effects of SMFs (500 mT) on carotenoid (violaxanthin and lutein) contents produced by Scenedesmus obliquus and Nannochloropsis gaditana with the use of different media (bold basal medium and modified f/2 prepared with seawater, respectively). In addition, they evaluated superoxide dismutase (SOD) and catalase activities. MFs decreased carotenoid content produced by S. obliquus and increased the one yielded by N. gaditana, in which high enzymatic activity and carotenoid content were observed. Violaxanthin and lutein contents in S. obliquus decreased after 96 h of MF exposure, (< 1%), while violaxanthin content in N. gaditana increased by 40.9% by comparison with the control. This behavior may be associated with oxidative stress that MFs cause to cell; to neutralize damage, the antioxidant defense machinery first activates antioxidant enzymes, such as SOD and then, production of non-enzymatic antioxidants. Furthermore, the authors showed the impact of that magnetic flux density on medium cultivations, in which dissipation of modified f/2 medium is fourfold higher than the bold basal medium and modified f/2 medium is ~ 50-fold more conductive than other media, a fact that may promote more effective electron transfer and enable better ROS formation. MF caused positive effect on carotenoids and enzymes of marine N. gaditana, and null effect on carotenoids and enzymes of freshwater S. obliquus, mainly due to ions found in seawater which interacted with MF.
Combination of mixotrophic cultivation and MF application (30 mT) was evaluated by Cordeiro et al. (2021) to verify phycocyanin production by Spirulina. With 30 mT and liquid molasses (1 g L−1), there was increase in protein (15.4%) and phycocyanin production (145%) by comparison with photoautotrophic cultivation (without any MF). In addition, chlorophyll production decreased while β-carotene synthesis was not affected. Hirano et al. (1998) and Deamici et al. (2019a) reported that increase in phycocyanin content may be connected to stimulation of photosystem II by MFs.
Menestrino et al. (2021) evaluated chlorophyll-a concentration in Spirulina sp. LEB 18 under MF application (1, 12 and 24 h d−1) in different photosynthesis cycles (dark and/or light) with the use of ferrite magnets (30 mT) and solenoids (6 mT). Increase in chlorophyll content (29.29 mg L−1) was achieved after 15 d of cultivation when 30 mT was applied for 12 h d−1 in a light cycle by comparison with the control condition (14.75 mg L−1).
Influence of MFs on carotenoid and enzyme (superoxide dismutase and catalase) contents produced by Scenedesmus obliquus and Nannochloropsis gaditana was evaluated by Serrano et al. (2021). Two exposure modes (24 h d−1 and 1 h d−1) and two magnet configurations (north and south) of 1.164 T were studied. After 96 h, in the case of N. gaditana, violaxanthin increased by 10 and 40% under continuous (24 h d−1) and pulsed (1 h d−1) MFs, respectively. Enzymatic activities were measured after 48 h of MF exposure: superoxide dismutase (SOD) from S. obliquus increased up to 65% (north) and 42% (south) in the continuous mode, while in the pulse mode, enzymatic activity decreased by 26.3% (north) and 50.5% (south). SOD from N. gaditana increased in continuous and pulse modes in both configurations (north and south). Regarding catalase (CAT), activity of S. obliquus increased by 37% when combined continuous mode and south configuration and N. gaditana increased by 19% and 9% for north and south configuration, respectively, under continuous mode. Thereby, MFs may induce a non-enzymatic defense response, such as carotenoids, while MFs may impact the antioxidant response and induce a non-enzymatic defense response as carotenoids in microalgae.
Deamici et al. (2021b) evaluated the application of 25 mT to chlorophyll content for 1 or 24 h d−1 in Chlorella fusca LEB 111 cultivations in indoor and outdoor conditions for 15 d. Outdoor cultures exposed to MFs for 1 h d−1 had the highest chlorophyll content (~ 20 µg mL−1) after 4 d of cultivation.
These studies show that effects of MFs on microalga pigments may be positive, negative or null. The effect of MF on pigment production is not clear, but it knows that MFs interact with ions (Mg, Cl, Ca, Fe and Cu) that are essential for photosystems I and II. In addition, if biomass concentration increased after MF application, pigment concentration may also have increased. Therefore, it is important to examine pigment concentration in terms of “mg or µg of pigment per g of biomass”.
Conclusion and perspectives
This review has given new insights into biomolecule production by microalgae subject to MFs. It is known that the magnetic effect is not the only factor responsible for the stimulatory effect on the biological system, but it depends on minimal operating conditions, such as light irradiation, temperature, pH and agitation/aeration. However, synergistic effects caused by magnetic intervention positively influence production of biomolecules of industrial interest.
The main challenges in this process are scale-up, biomass productivity and costs. Despite technological advances achieved in recent years, the cost of the process that collects the compounds of interest has been considered high. Therefore, the use of the circular bioeconomy concept has gained prominence in bioprocesses since it aims to fill the gap between laboratory and industrial-scale processes. Furthermore, the use of MFs as an emerging technology has shown to be an innovative and low-cost alternative to be applied to microalga biorefineries.
Concerning future perspectives, some issues need to be highlighted: (i) the technological approach that uses MFs needs to be adapted on an industrial scale; (ii) downstream strategies need to be better investigated in extraction and recovery of microalga-based molecules; (iii) evaluation of the influence of MF in co-cultures aiming at production of compounds of interest; (iv) genetic engineering as a tool to improve microalga biomass productivity added to MF application to obtain specific biomolecules; and (v) evaluation of costs in the upstream and downstream stages of the process to verify the economic feasibility of its application to biorefineries.
Data availability
Not applicable.
Code availability
Not applicable.
References
Albuquerque W, Costa R, Porto A, Fernandes TS (2016) Evidences of the static magnetic field influence on cellular systems. Prog Biophys Mol Biol. https://doi.org/10.1016/j.pbiomolbio.2016.03.003
Al-Hawash AB, Li S, Zhang X et al (2018) Productivity of γ-Linoleic acid by oleaginous fungus Cunninghamella echinulata using a pulsed high magnetic field. Food Biosci 21:1–7. https://doi.org/10.1016/j.fbio.2017.10.007
Baldev E, MubarakAli D, Sivasubramanian V et al (2021) Unveiling the induced lipid production in Chlorella vulgaris under pulsed magnetic field treatment. Chemosphere 279:130673. https://doi.org/10.1016/j.chemosphere.2021.130673
Bauer LM, Costa JAV, Rosa APC, Santos LO (2017) Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour Technol 244:1425–1432. https://doi.org/10.1016/j.biortech.2017.06.036
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210. https://doi.org/10.1016/j.biotechadv.2006.11.002
Belay A, Berestov V, Bertolin TE et al (2008) Factors affecting oocyte quality: who is driving the follicle? Reprod Domest Anim 5:809–822
Bleakley S, Hayes M (2017) Algal proteins: extraction, application, and challenges concerning production. Foods 6:1–34. https://doi.org/10.3390/foods6050033
Boeira CZ, Silvello MAC, Remedi RD et al (2021) Mitigation of nivalenol using alcoholic fermentation and magnetic field application. Food Chem 340:127935. https://doi.org/10.1016/j.foodchem.2020.127935
Chu FJ, Wan TJ, Pai TY et al (2020) Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J Environ Manage 253:109680. https://doi.org/10.1016/j.jenvman.2019.109680
Cordeiro AP, Nogueira AOM, Salgado HZ et al (2021) Simultaneous application of mixotrophic culture and magnetic fields as a strategy to improve Spirulina sp. LEB 18 phycocyanin synthesis. Curr Microbiol 78:4014–4022. https://doi.org/10.1007/s00284-021-02666-8
Costa SS, Peres BP, Machado BR et al (2020) Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.123880
Costa JAV, Lucas BF, Alvarenga AGP et al (2021) Microalgae polysaccharides: an overview of production, characterization, and potential applications. Polysaccharides 2:759–772. https://doi.org/10.3390/polysaccharides2040046
Daneshvar E, Sik Ok Y, Tavakoli S et al (2021) Insights into upstream processing of microalgae: a review. Bioresour Technol. https://doi.org/10.1016/j.biortech.2021.124870
Dasgupta CN (2015) Algae as a source of phycocyanin and other industrially important pigments. algal biorefinery: an integrated approach. Springer International Publishing, Cham, pp 253–276
Deamici KM, Santos LO, Costa JAV (2018) Magnetic field action on outdoor and indoor cultures of Spirulina: evaluation of growth, medium consumption and protein profile. Bioresour Technol 249:168–174. https://doi.org/10.1016/j.biortech.2017.09.185
Deamici KM, Cuellar-Bermudez SP, Muylaert K et al (2019a) Quantum yield alterations due to the static magnetic fields action on Arthrospira platensis SAG 21.99: evaluation of photosystem activity. Bioresour Technol 292:121945. https://doi.org/10.1016/j.biortech.2019a.121945
Deamici KM, Santos LO, Costa JAV (2019b) Use of static magnetic fields to increase CO2 biofixation by the microalga Chlorella fusca. Bioresour Technol 276:103–109. https://doi.org/10.1016/j.biortech.2018.12.080
Deamici KM, Morais MG, Santos LO et al (2021a) Static magnetic fields effects on polysaccharides production by different microalgae strains. Appl Sci. https://doi.org/10.3390/app11115299
Deamici KM, Santos LO, Costa JAV (2021b) Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst Eng 44:1453–1460. https://doi.org/10.1007/s00449-021-02526-6
Deamici KM, Morais MG, Santos LO et al (2022) Magnetic field action on Limnospira indica PCC8005 cultures: enhancement of biomass yield and protein content. Appl Sci 12:1533. https://doi.org/10.3390/app12031533
Elisabeth B, Rayen F, Behnam T (2021) Microalgae culture quality indicators: a review. Crit Rev Biotechnol 41:457–473. https://doi.org/10.1080/07388551.2020.1854672
Feng X, Chen Y, Lv J et al (2020) Enhanced lipid production by Chlorella pyrenoidosa through magnetic field pretreatment of wastewater and treatment of microalgae-wastewater culture solution: magnetic field treatment modes and conditions. Bioresour Technol 306:123102. https://doi.org/10.1016/j.biortech.2020.123102
Ferrada P, Rodríguez S, Serrano G et al (2020) An analytical-experimental approach to quantifying the effects of static magnetic fields for cell culture applications. Appl Sci. https://doi.org/10.3390/app10020531
Godfray HCJ, Beddington JR, Crute IR et al (2010) Food security: the challenge of feeding 9 billion people. Science (80- ) 327:812–818. https://doi.org/10.1109/CIS.2016.52
González-Fernández C, Ballesteros M (2012) Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol Adv 30:1655–1661. https://doi.org/10.1016/j.biotechadv.2012.07.003
Gouda M, Tadda MA, Zhao Y et al (2022) Microalgae bioactive carbohydrates as a novel sustainable and eco-friendly source of prebiotics: emerging health functionality and recent technologies for extraction and detection. Front Nutr 9:1–20. https://doi.org/10.3389/fnut.2022.806692
Hirano M, Ohta A, Abe K (1998) Magnetic field effects on photosynthesis and growth of the cyanobacterium Spirulina platensis. J Ferment Bioeng 86:313–316. https://doi.org/10.1016/S0922-338X(98)80136-0
Ho SH, Ye X, Hasunuma T et al (2014) Perspectives on engineering strategies for improving biofuel production from microalgae: a critical review. Biotechnol Adv 32:1448–1459. https://doi.org/10.1016/j.biotechadv.2014.09.002
Hu I-C (2019) Production of potential coproducts from microalgae. Biofuels from algae. Second Edi. Elsevier, Amsterdam, pp 345–358
Hunt RW, Zavalin A, Bhatnagar A et al (2009) Electromagnetic biostimulation of living cultures for biotechnology, biofuel and bioenergy applications. Int J Mol Sci 10:4515–4558. https://doi.org/10.3390/ijms10104515
Huo S, Chen X, Zhu F et al (2020) Magnetic field intervention on growth of the filamentous microalgae Tribonema sp. in starch wastewater for algal biomass production and nutrients removal: influence of ambient temperature and operational strategy. Bioresour Technol 303:122884. https://doi.org/10.1016/j.biortech.2020.122884
Ibañez E, Cifuentes A (2013) Benefits of using algae as natural sources of functional ingredients. J Sci Food Agric 93:703–709. https://doi.org/10.1002/jsfa.6023
Ismail I, Hwang YH, Joo ST (2020) Meat analog as future food: a review. J Anim Sci Technol 62:111–120. https://doi.org/10.5187/jast.2020.62.2.111
Katz E, Lioubashevski O, Willner I (2005) Magnetic field effects on bioelectrocatalytic reactions of surface-confined enzyme systems: enhanced performance of biofuel cells. J Am Chem Soc 127:3979–3988. https://doi.org/10.1021/ja044157t
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17:1–21. https://doi.org/10.1186/s12934-018-0879-x
Khozin-goldberg I (2016) The physiology of microalgae. Springer International Publishing, Cham
Laroche C (2022) Exopolysaccharides from microalgae and cyanobacteria: diversity of strains, production strategies, and applications. Mar Drugs 20:336. https://doi.org/10.3390/md20050336
Li WW, Sheng GP, Liu XW et al (2011) Impact of a static magnetic field on the electricity production of Shewanella-inoculated microbial fuel cells. Biosens Bioelectron 26:3987–3992. https://doi.org/10.1016/j.bios.2010.11.027
Li C, Hu Z, Gao Y et al (2022) Bioeffects of static magnetic fields on the growth and metabolites of C. pyrenoidosa and T. obliquus. J Biotechnol 351:1–8. https://doi.org/10.1016/j.jbiotec.2022.04.004
Liao Q, Liu Y, Zhang J et al (2019) A low-frequency magnetic field regulates Monascus pigments synthesis via reactive oxygen species in M. purpureus. Process Biochem 86:16–24. https://doi.org/10.1016/j.procbio.2019.08.009
Luna LG, Àlvarez I, Rivero R (2012) Cultivo de Chlorella vulgaris sobre residual de soja con la aplicación de un campo magnético. Rev Colomb Biotecnol 13:27–38
Maia JL, Cardoso JS, Mastrantonio DJS et al (2020) Microalgae starch: a promising raw material for the bioethanol production. Int J Biol Macromol 165:2739–2749. https://doi.org/10.1016/j.ijbiomac.2020.10.159
Maltsev Y, Maltseva K (2021) Fatty acids of microalgae: diversity and applications. Springer, Netherlands
Menestrino BC, Pintos THC, Sala L et al (2020) Application of static magnetic fields on the mixotrophic culture of Chlorella minutissima for carbohydrate production. Appl Biochem Biotechnol 192:822–830. https://doi.org/10.1007/s12010-020-03364-0
Menestrino BC, Sala L, Costa JAV et al (2021) Magnetic fields exhibit a positive impact on lipid and biomass yield during phototrophic cultivation of Spirulina sp. Bioprocess Biosyst Eng 44:2087–2097. https://doi.org/10.1007/s00449-021-02585-9
Mofijur M, Rasul MG, Hassan NMS, Nabi MN (2019) Recent development in the production of third generation biodiesel from microalgae. Energy Procedia 156:53–58. https://doi.org/10.1016/j.egypro.2018.11.088
Navarro-López E, Ruíz-Nieto A, Ferreira A et al (2020) Biostimulant potential of Scenedesmus obliquus grown in brewery wastewater. Molecules 25:1–16. https://doi.org/10.3390/molecules25030664
Pereira AM, Lisboa CR, Costa JAV (2018) High protein ingredients of microalgal origin: obtainment and functional properties. Innov Food Sci Emerg Technol 47:187–194. https://doi.org/10.1016/j.ifset.2018.02.015
Raheem A, Prinsen P, Vuppaladadiyam AK et al (2018) A review on sustainable microalgae based biofuel and bioenergy production: recent developments. J Clean Prod 181:42–59. https://doi.org/10.1016/j.jclepro.2018.01.125
Rahpeyma SS, Raheb J (2019) Microalgae biodiesel as a valuable alternative to fossil fuels. Bioenergy Res 12:958–965. https://doi.org/10.1007/s12155-019-10033-6
Santos LO, Deamici KM, Menestrino BC et al (2017) Magnetic treatment of microalgae for enhanced product formation. World J Microbiol Biotechnol 33:1–6. https://doi.org/10.1007/s11274-017-2332-4
Santos LO, Silva PGP, Lemos Junior WJF et al (2022) Glutathione production by Saccharomyces cerevisiae: current state and perspectives. Appl Microbiol Biotechnol 106:1879–1894. https://doi.org/10.1007/s00253-022-11826-0
Sarraf M, Kataria S, Taimourya H et al (2020) Magnetic field (MF) applications in plants: an overview. Plants 9:1–17. https://doi.org/10.3390/plants9091139
Sati H, Mitra M, Mishra S, Baredar P (2019) Microalgal lipid extraction strategies for biodiesel production: a review. Algal Res 38:101413. https://doi.org/10.1016/j.algal.2019.101413
Serrano G, Miranda-Ostojic C, Ferrada P et al (2021) Response to static magnetic field-induced stress in Scenedesmus obliquus and Nannochloropsis gaditana. Mar Drugs. https://doi.org/10.3390/md19090527
Shao W, Ebaid R, Abomohra AEF, Shahen M (2018) Enhancement of Spirulina biomass production and cadmium biosorption using combined static magnetic field. Bioresour Technol 265:163–169. https://doi.org/10.1016/j.biortech.2018.06.009
Silva PGP, Prescendo Júnior D, Sala L et al (2020) Magnetic field as a trigger of carotenoid production by Phaffia rhodozyma. Process Biochem 98:131–138. https://doi.org/10.1016/j.procbio.2020.08.001
Silva PGP, Prescendo Júnior D, Burkert JFM, Santos LO (2022) Carotenoid extraction from Phaffia rhodozyma biomass: downstream strategies and economic evaluation of energy. Brazilian J Chem Eng. https://doi.org/10.1007/s43153-022-00225-7
Silvello MAC, Gonçalves IS, Azambuja SPH et al (2022) Microalgae-based carbohydrates: a green innovative source of bioenergy. Bioresour Technol 344:126304. https://doi.org/10.1016/j.biortech.2021.126304
Small DP, Hüner NPA, Wan W (2012) Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 33:298–308. https://doi.org/10.1002/bem.20706
Veiga MC, Fontoura MM, Oliveira MG et al (2020) Magnetic fields: biomass potential of Spirulina sp for food supplement. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-020-02318-4
Wan Y, Zhang J, Han H et al (2017) Citrinin-producing capacity of Monascus purpureus in response to low −frequency magnetic fields. Process Biochem 53:25–29. https://doi.org/10.1016/j.procbio.2016.11.009
Wang Y, Tibbetts SM, McGinn PJ (2021) Microalgae as sources of high-quality protein for human food and protein supplements. Foods 10:1–18. https://doi.org/10.3390/foods10123002
Yang G, Wang J, Mei Y, Luan Z (2011) Effect of magnetic field on protein and oxygen-production of Chlorella vulgaris. Math Phys Fish Sci 9:116–126
Zapata JEM, Moreno GO, Márquez EJF (2002) Efectos de los campos magnéticos sobre el crecimiento de Saccharomyces cerevisiae. Interciencia 27:544–550
Zhu F, Chen X, Cui Y et al (2022) Weak magnetic field intervention on outdoor production of oil-rich filamentous microalgae: influence of seasonal changes. Bioresour Technol 348:126707. https://doi.org/10.1016/j.biortech.2022.126707
Funding
This study was partially funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES—Finance Code 001) and the Foundation Research Support in the State of Rio Grande do Sul (FAPERGS – Grant number 21/2551–0002268-0). The authors are also thankful for research fellow provided by the National Counsel for Technological and Scientific Development—Brazil (Proc. 309921/2019–8).
Author information
Authors and Affiliations
Contributions
LOS: project administration, supervision, writing, review and editing—original draft; PGPS: conceptualization, writing, review and editing—original draft; BRM: visualization and writing—original draft; LS: visualization and writing—original draft; KMD: visualization and writing—original draft. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict interest
The authors declare no competing interests.
Ethical approval
Neither human beings nor animals were used for carrying out the study reported by this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, L.O., Silva, P.G.P., Machado, B.R. et al. Update on the application of magnetic fields to microalgal cultures. World J Microbiol Biotechnol 38, 211 (2022). https://doi.org/10.1007/s11274-022-03398-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03398-y