Abstract
Several studies have demonstrated the benefits of magnetic treatment on the productivity of secondary metabolites, growth, and the state of microalgae cultures. This technology offers advantages such as low operating costs, absence of toxic effects, absence of secondary pollution, a wide range of applications, and a long useful life. Despite these advantages, its use in industrial microalgae culture systems has been limited due to the lack of consensus on the mechanisms that explain the observed effects on microalgae. In this paper, the effect of magnetic field treatments on microalgae and cyanobacterial cultures and the possible mechanisms are critically reviewed. However, it is still necessary to conduct more studies directly relating the experimental effects observed with one or several proposed mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are a group of microscopic photosynthetic organisms (Osanai et al. 2017). These include eukaryotic and prokaryotic microorganisms, which can be found in all terrestrial and aquatic habitats (Siqueira et al. 2018). In microalgae, typically, plant metabolic properties are combined with characteristics of microbial cells, such as the capacity for rapid growth in liquid culture, simplicity of nutritional requirements, metabolic plasticity, tolerance to extreme conditions, ability to synthesize and secrete some metabolites, and the potential to develop genetically engineered strains in the interest of biotechnology industry (Khavari et al. 2021).
These characteristics have made these microorganisms a promising strategy for wastewater treatment. They accumulate and metabolize nutrients in liquid wastewater (Molazadeh et al. 2019). In addition, the biomass obtained is a source of biomolecules of industrial interest, containing lipids, proteins, and carbohydrates, highly valued as a source of raw materials for different industries such as the pharmaceutical sectors (Yan et al. 2016), aquaculture (Shah et al. 2018), food (Gateau et al. 2017), cosmetics (Ariede et al. 2017) or bioenergy (Su et al. 2017). Despite all the applications microalgae cultivation provides, large-scale production still needs to be improved. This is because cultivation systems are not yet efficient enough (Garrido-Cardenas et al. 2018). Therefore, new cultivation methods and strategies are being explored and developed to reduce large-scale production costs.
These production methods often focus on the establishment of microalgae inoculum isolated from natural sources with specific and highly efficient growth characteristics (Jiang et al. 2018), effective conditions of culture management, and strategies to avoid or detect predation to maximize biomass productivity and the concentration of some biomolecules of interest (Markou et al. 2014; Joe et al. 2018), the development of methods for the efficient separation of biomass from the culture medium and for metabolite extraction (Vandamme et al. 2013; Sathasivam et al. 2019). A relatively novel alternative method for productivity improvement of microalgae cultures with wastewater treatment is applying magnetic field treatment. This technology has the advantages of low capital and operating cost, has no proven toxic effects, and does not produce secondary contamination (Zhang et al. 2017).
Research carried out in this area has repeatedly reported that with the application of magnetic field treatment, the productivity of the algal biomass and intracellular biomolecule concentration improved (Deamici et al. 2016; Bauer et al. 2017; Huo et al. 2020). Moreover, it has also been observed that CO2 fixation of microalgae increased (Deamici et al. 2019b). It also improved the rate of nutrient uptake, heavy metals absorption from wastewater (Shao et al. 2018), and the rate of photosynthetic oxygen production (Tu et al. 2015).
However, implementing this technology presents limitations that impede its development since the theoretical foundations that support it still need to be understood more. Hence, this review provides an overview of the current understanding of the action mechanisms reported.
Definition and classification of magnetic fields
All life forms on earth are under the action of a natural and stable geomagnetic field of around 0.5 G. Although this intensity is considered relatively large concerning most manufactured sources, this does not prevent the evolution of living beings subjected to this geomagnetic field. This phenomenon is probably one of the reasons why various researchers worldwide have been interested in studying the biological effects and possible applications of magnetic fields on biological systems (Kataria et al. 2017; Mohammadi et al. 2018; Radhakrishnan 2019; da Silva et al. 2020; Sarraf et al. 2020; Chen et al. 2021; Lew et al. 2021).
An electromagnetic field (EMF) is produced by accelerating electric charges. It can be viewed as the combination of an electric field and a magnetic field (Zhang et al. 2017). These EMFs are divided along the electromagnetic spectrum based on frequency. They can be classified as non-ionizing (low-level radiation generally perceived as harmless to humans) or ionizing (high-level radiation potentially damaging cells and DNA).
Classification according to its variation over time
Magnetic fields (MFs) can be classified according to their variation in time: static or dynamic. When the intensity of the magnetic field does not vary in time, it is referred to as a static magnetic field (SMF). On the other hand, when the intensity of the magnetic field changes over time, they are referred to as a dynamic magnetic field (DMF) (Zhang et al. 2017). One of the most studied DMFs is pulsed magnetic fields (PMF), generated by a strong electromagnet passing an electric current through its windings, generating a brief but strong magnetic field pulse (Shneerson et al. 2014). The effect of PMF on biological systems has been reported in several studies, for example, bacteria, cancer cell tissues, as well as plant cells and microalgae cells (Schmiedchen et al. 2018; Sengupta and Balla 2018; Bodewein et al. 2019; Lin et al. 2019; Akaberi et al. 2020). Studies carried out with these MFs have shown various cellular responses depending on the intensity of the applied EMF and the frequency. In studies carried out by Baldev et al. (2021) with cultures of Chlorella vulgaris and a PMF in a magnetic flux density range of 600–900 mG, it was observed that there was no linear relationship between the magnetic flux density and the average dry cell weight of the cultures. However, research with these PMFs has taken a back seat in recent decades. Specifically, in microalgae cultivation, there are fewer and fewer research reports on PMFs. This is because the variability of parameters such as frequency and intensity make it difficult to understand the mechanisms of action of these magnetic fields on biological systems.
In this context SMFs have re-emerged as a viable option for studies of the biological mechanisms affected by the action of this physical agent. In contrast to DMFs, SMFs have fewer variables in their configuration. The devices most used for these studies are built from permanent magnets, allowing more accessible experimental facilities to be developed (Chu et al. 2020; Deamici et al. 2021). In addition, the results obtained with this type of magnetic field are more accessible to scale due to its simple configuration.
Classification according to the intensity of SMFs
In biological systems studies, the intensity of the applied SMF can be classified as weak (< 1 mT), moderate (1 mT to 1 T), high (1 T to 20 T), and very high (> 20 T). The magnetic field density is a vector B, expressed in Tesla (T) according to the International System of Units (SI). However, to be compatible with biological investigations, it can also be expressed in Gauss (G), where:
Various authors have widely studied the intensity of SMF on organisms (Chu et al. 2020; Chen et al. 2021). As with other characteristics, the intensity of the SMF does not generate the same effects from one organism to another. Furthermore, there is not always a linear behavior about the increase or decrease of the intensities of the SMF studied (Chen et al. 2021; Hassanpour and Pourhabibian 2022; Khorshidi et al. 2022). In the research carried out by the Khorshidi research group (Khorshidi et al. 2022), different intensities of SMF (0, 2, 4 and 8 mT) were applied to the suspension culture of Haematococcus lacustris cells. This study showed that applying 8 mT SMF at the beginning of the logarithmic phase of growth increased the maximum cell concentration. In comparison, the maximum accumulation of astaxanthin was obtained by applying 4 mT SMF.
On the other hand, in studies developed with the bacterium Clostridium pasteurianum and the application of different intensities of SMF, the fermentation of glucose to obtain hydrogen was evaluated (Chen et al. 2021). The hydrogen production study demonstrates that SMF is a physical agent capable of improving the total glucose consumption and increasing the maximum rate of hydrogen production of the cultures, independent of the intensity of SMF applied compared to the control cultures. Furthermore, it was observed that there was no linear sequence of the effects of SMF observed concerning the intensity of the SMF applied.
Spatial distribution and exposure time of SMFs
The spatial distribution of magnetic forces is also characteristic of SMFs, which can influence biological systems. The observed effects of the spatial distribution of SMFs on biological systems depend on the interaction of SMFs with the magnetic moments of organisms. Binhi and Prato (2018) have explained these effects through the Level Mixing Mechanism (LMM). This LMM expresses that, in some conditions, the precession of the magnetic moments that reside on rotating molecules can be slowed relative to the immediate biophysical structures. This means that variations in the quantum levels of magnetic moments can be observed. Therefore, a relationship is established between the biological effects in response to the inversion of the SMF vector. SMFs can be classified according to their spatial distribution into homogeneous (when the field strength is spatially constant) and non-homogeneous (when the field strength varies spatially).
Another critical characteristic that changes the effects of MF on biological samples is the exposure time to MFs. In this sense, various changes found in biological systems depending on the time of exposure to MFs are still being investigated in depth. These changes are not always linear and can change profoundly from one biological system to another. This has been corroborated in different studies with different organisms. In the study developed with Spirulina platensis (current name Arthrospira platensis) with different exposure times to SMF (0 min, 3 h, 6 h and 12 h), it was observed that this physical agent improves the biomass productivity of the microorganisms in two of the exposure schemes evaluated (Shao et al. 2018). Applying the magnetic treatment for three and six hours was the best scheme compared with the control cultures. Nevertheless, applying SMF for 12 h inhibited biomass productivity concerning control cultures. In addition, with 1 h exposure to SMF, stimulator effects on cell growth have been observed in different microalgae such as H. lacustris (Khorshidi et al. 2022), and Chlorella fusca (current name Desmodesmus abundans) (Deamici et al. 2021). The results above constitute evidence of the variability of the effects of SMF depending on the type of organism to which it will be applied and the time of exposure to the MF.
Overview of reported general characteristics of microalgae and cyanobacteria cultures treated with MF
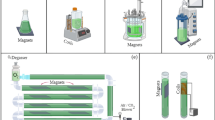
Table 1 summarizes some critical characteristic culture cultivation and treatment conditions and the main effects found in MF-treated microalgae and cyanobacteria culture studies. Research carried out in recent years has mainly used microalgae belonging to the genera Chlorella and Spirulina (Arthrospira) (Bauer et al. 2017; Shao et al. 2018; Deamici et al. 2019b; da Costa et al. 2020; Deamici et al. 2021). These microalgae and cyanobacteria can be considered model microorganisms due to the great industrial interest and adaptability they present in biotechnology. However, research with the SMF has been extended to studying other species such as Nannochloropsis, Tetraselmis, and Tribonema. There is a wide range of MF intensities that have been studied. Not much variety is observed in terms of the material with which the devices for magnetic treatment are made; the most commonly used are ferrite magnets (Bauer et al. 2017; Shao et al. 2018; Deamici et al. 2019b; Chu et al. 2020; Huo et al. 2020; da Costa et al. 2020; Deamici et al. 2021).
The exposure time of the MFs on the microalgae and cyanobacteria cultures has been one parameter of great importance in defining the best protocol for the magnetic treatment application. Most of the analyzed studies showed a wide range of exposure times to MF, ranging from 1 min to 24 h of exposure (Brailo et al. 2018; Shao et al. 2018; Deamici et al. 2021). Although in almost all the cases studied for microalgae and cyanobacteria, positive effects were observed in terms of the increase in cell concentration, improvement of growth parameters, and increase in the concentration of metabolites of interest. The exposure (Bauer et al. 2017; Deamici et al. 2019b, 2021).
The MF application protocols on cultures have been varied. In most of the analyzed research, the MF was applied during the whole cultivation period (Shao et al. 2018; Deamici et al. 2019b; Huo et al. 2020; da Costa et al. 2020). However, applying MF every day, but only during the light photoperiod of the microorganisms, increased the cell concentration and CO2 biofixation of the microorganisms (Bauer et al. 2017; Deamici et al. 2019b). These results open a window for a better understanding of the mechanism through which MF acts on photosynthetic microorganisms. It has been proven that this physical agent acts positively on photosystem II of microorganisms, increasing the quantum yield and positively modifying the parameters related to the photochemistry flux ratios and specific energy fluxes of the reaction centres (Deamici et al. 2019a).
Mechanisms that explain the behaviour of MFs on biological systems
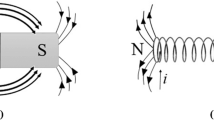
The effect of the MFs on biological systems has been extensively studied in recent years. Positive and negative effects of this phenomenon have been observed. Despite the extensive literature, there still needs to be a complete and detailed understanding of the biological mechanisms and effects exerted by MF (Santos et al. 2017; Zhang et al. 2017; Sarraf et al. 2020). First, it is essential to note that all the proposed mechanisms are closely related to the magnetic properties of the atoms and molecules that make up biological systems. As is known, atoms and molecules can be classified according to their magnetic properties (Zhang et al. 2017): paramagnetic (when exposed to an external MF, the substances are weakly magnetized in the same sense of the external field), diamagnetic (when exposed to an external MF the substances are weakly magnetized but in the opposite direction of the external field) and ferromagnetic (these are the substances that when exposed to an external MF are strongly magnetized in the same sense of the external field).
So far the results have followed one or more of the reported mechanisms that explain the effects of SMF found in biological systems. These mechanisms focus on three fundamental phenomena associated with MFs (Santos et al. 2017): (1) magnetic induction, (2) the magneto-mechanical effect, and (3) the formation of radical pairs. The mechanisms above are based on observations, reliable experimental data, and basic concepts of biophysical and biochemistry. However, the reproducibility of the results obtained is not always achieved, so currently the need to reach a consensus on this subject is one of the objectives of specialists in this area.
The first mechanism, magnetic induction, is theoretically based on the "Hall effect" phenomenon. The Hall effect produces an electric field E transverse across a conductor and perpendicular to an external magnetic field B made perpendicular to the conduction direction (Parke 2020). The electric field E = vB is generated by the Lorentz force acting to shift charges to one side as they move with speed v through the magnetic field. The Hall effect has also been used to assess electron transfer between electrogenic bacteria and microbial fuel cell appliances (Cao et al. 2020). This study verified through the measurement of the Hall effect, with the intensity of the magnetic field B and the electric current of 0.63 T and 50 μA, respectively, that Geobacter sulfurreducens has the ability to transfer electrons to a surface without the use of electrical conductive pili. Currently, this knowledge about magnetic induction is the theoretical basis for developing equipment for continuous real-time monitoring of cerebral blood flow control for the treatment and prevention of cerebrovascular diseases (Zeng et al. 2022).
The magneto-mechanical effect is another mechanism and is an intrinsic feature of ferromagnetism (Bulte et al. 2002). Specific molecules or ferromagnetic material, under the action of an external MF, will undergo mechanical movements to align their magnetic moments with the directions of the applied field (Nikitin et al. 2022). The development of this mechanism is based on the presence of magnetite in various organisms such as bacteria, sharks, pigeons, and phytoplankton (Lefèvre and Bazylinski 2013; Nimpf et al. 2019; Amor et al. 2020). Magnetite is an iron oxide (Fe3O4), which under the action of an external MF, acquires ferromagnetic properties by orienting its domains in the direction of the applied MF, becoming a magnet. The presence of this magnetoreceptor in various organisms has allowed them to orient themselves with the earth's magnetic field and obtain additional biological responses. In the case of magnetotactic bacteria, the magnetite crystals are organized into magnetosomes. Magnetite crystals in bacteria are considered to be surrounded by a membrane, and this structure is connected to the cell wall through cytoskeleton filaments, providing a biochemical mechanism for force translation. Currently, this mechanism is studied due to its potential use in biomedical applications related to cell labelling, drug delivery, and biosensing, among others (Anik et al. 2021; Baki et al. 2021).
The last mechanism is related to the formation of radical pairs. It is frequently used to explain the variation of cell biological reactions and the influence of the MF on the energy levels and spin orientation of electrons (Santos et al. 2017). Various authors have extensively studied this mechanism (Chidsey et al. 1985; Gilch et al. 1998; Hore and Mouritsen 2016; Zadeh-Haghighi and Simon 2022). However, it is still considered that several physical parameters are not considered related to magnetic moments (Binhi 2016). The reorientation of the spins and the generation of radical pairs under the action of an MF is closely related to each atom's electronic configuration and, consequently, its intrinsic magnetic properties. This mechanism involves magnetically sensitive intermediate molecules due to the presence of radical pairs (Zadeh-Haghighi and Simon 2022). So far, only two magnetoreceptor candidate molecules have been found for radical pair formation, i.e., cryptochrome and chlorophylls in photosynthetic organisms (Hore and Mouritsen 2016).
Sensitive magnetic reactions generally involve radicals. A radical pair is a short-lived reaction intermediate comprising two tandemly formed radicals whose unpaired electron spines can be antiparallel (a singlet state, S) or parallel (a triplet state, T) (Rodgers and Hore 2009). Under an external MF, a spin correlation is produced between two unpaired electrons, coming from a donor and an acceptor molecule (Wong et al. 2021). The external magnetic field drives the conversion from state S to state T, or vice versa, which gives rise to different reaction products. Photoexcitation is another necessary characteristic for the development of radical pair reactions, which gives rise to forming radicals (Kattnig et al. 2016). For example, in the case of cryptochrome, upon exposure to blue light, transfers an electron to the flavin adenine dinucleotide (FAD), which results in both the protein and the flavonoid having unpaired electrons, that is, the radical pair necessary for a radical pair reaction.
In the context of microalgae and cyanobacteria, considering the theoretical foundations of the mechanisms described above, it can be said that in the same cell culture, the effect of the three mechanisms could be observed simultaneously. This assumption is based on the fact that in the culture of microalgae and cyanobacteria, magnetite or other ferromagnetic molecules could be present intracellularly, giving rise to intracellular molecule rotations, which could open or close channels in cell membranes. However, to date, no scientific report has been found to corroborate the presence of some of these ferromagnetic molecules in these microorganisms. These magnetoreceptors have only been found in phytoplankton, which generally have a high concentration of magnetotactic bacteria (Yuan et al. 2020).
On the other hand, in photoautotrophic cultures light plays a fundamental role by providing the photons that cells need to initiate photosynthesis. However, it also constitutes one of the essential factors to activate the formation mechanism of radical pairs together with the presence of chlorophyll molecules under the action of MF. Even though the radical pair mechanism contains elements that directly relate it to microalgae, such as the presence of chlorophylls, the results obtained so far related to this mechanism are not solid enough since it is not possible to establish the direct relationship between the photochemical effects found with the formation of the radical pair.
Finally, these cultures are carried out in liquid media disposed of by ions that can be influenced, like intracellular ions, by the presence of an external MF, due to the Hall effect. However, no solid experimental evidence demonstrates the influence of SMF on the ionic transfer from the culture medium to the cells or vice versa.
Effect of the magnetic field on the microalgae and cyanobacteria cells
The results obtained for microalgae and cyanobacteria suggest that the effect of MF on these microorganisms could involve the three mechanisms outlined above. However, there is not enough practical evidence to demonstrate this approach. Most of the effects found are associated with genetic, metabolic and physiological changes in microorganisms (Han et al. 2016; Shao et al. 2018), which can then be measured through the increase in the cell concentration of the cultures (Deamici et al. 2021), variations in growth parameters, changes in the biochemical composition of the biomass (da Costa et al. 2020) and improvement in the photosynthetic capacity of the microorganisms (Small et al. 2012; Deamici et al. 2019a). Furthermore, these results can be grouped into three main topics which will be addressed further: (1) metabolic and/or genetic changes, (2) structural changes in cells, and (3) electron transition changes linked to reaction centres.
Metabolic and / or genetic changes
Multiple studies have demonstrated that the application of SMF affects metabolic and/or genetic changes that positively stimulate cell concentrations in culture as well as their biochemical composition. For example, Han et al. (2016) explained that the changes in the counts of Chlorella pyrenoidosa could be attributed to the influence of SMF on the biological functions of the organism by changes in hormones, the activity of some enzymes, the transport of membrane ions or the transcription of DNA. Shao et al. (2018) related the increase in the growth rate of S. platensis (A. platensis) during the exponential phase, with the effect of the SMF on possible improvement of metabolic pathways related to growth, which counteract the toxic effect of Cd2+ ions present in the culture medium. da Costa et al. (2020) observed that with the application of an SMF of 30 mT in cultures of C. minutissima (M. homosphaera), the production of proteins and carbohydrates in cultures were stimulated. The authors relate the increase in protein and carbohydrate content to interactions between MF and cells, altering the intracellular macromolecule production. In addition, Deamici et al. (2016) evaluated the effect of different intensities of SMF (30 and 60 mT) and EMF (5 mT) on the growth and the composition of the biomass of Spirulina sp. In this research, the SMF of 30 and 60 mT stimulated cell growth and the content of proteins and carbohydrates, which was attributed to possible changes at the enzymatic level. However, MF intensities do not always relate in a linear way to cell concentrations and their composition. As such, Luo et al. (2020) observed variability in their results when applying different intensities of SMF to cultures of Chlorella vulgaris. A wide variability in the content of extracellular polysaccharides and total proteins was seen at day 16 of the culture and an increase in cell concentration was not observed at all the intensities of the SMF applied with respect to the control cultures. The protein content and enzymatic activity of the treated cultures increased, which could be an indication of the modifications at the metabolic level that MF can exert.
Although findings in strictly controlled conditions show similar positive results, care should be taken when applying MF outdoors, where environmental characteristics cannot be controlled. In a study carried out by the Deamici research group (Deamici et al. 2021) in in- and outdoor settings, it was observed that an application of 25 mT SMF during 1 h or 24 h to C. fusca (D. abundans), resulted in increases in the concentration of biomass and modifications in the biochemical composition of the biomass, concerning the control cultures. These researchers attributed the observed effects to metabolic modifications, such as variations in growth kinetics, which result from the applied SMF and do not occur linearly due to interference with environmental characteristics. Therefore, variations were observed between crops exposed outdoors and indoors. In addition, da Costa et al. (2021) explained that the effect of SMF on microalgae was also dependent on the moment in the light: dark cycle when it is applied. When the SMF is applied for 12 h in the light cycle cells alter their metabolic pathways and electron transport chain systems could be affected to keep metabolism functioning under the action of this physical agent. However, when the SMF is applied during 12 h in a dark cycle this does not happen.
Furthermore, the results changed when the SMF was applied for only 1 h since it stimulated cell growth during light and dark cycles. From these results, the SMF is a physical agent that stimulates the reactions associated with the photosynthetic apparatus of microalgae. Moreover, the degree of stimulus is directly related to the time the microorganisms are exposed to this treatment.
Structural changes in cells
Other possible explanations that have been addressed about the effects of MF on microalgae cultures are related to modifications of the membrane channels, which facilitate the transport of substances from the culture medium to the cells or from the cells to the culture medium. Deamici et al. (2019b) attributed the increased growth rate of cultures exposed to SMF to a possible enhancement of membrane permeability. Better absorption of nutrients by microorganisms could improve the efficiency of the metabolic reactions that microalgae develop during their growth. More recently, Chu et al. (2020) evaluated the influence of SMFs of different intensities (20, 30, and 40 mT) and nitrate concentration on the growth and lipid productivity of Nannochloropsis oculata. It was observed that the treatment carried out with the lowest field induction (20 mT), was the one that stimulated the growth of N. oculata. This was associated with improving the permeability of the membrane of the algae, which could facilitate the transport of the substrate of the culture medium toward the cytoplasm of the cell. It could also be linked to the physical–chemical changes in the culture medium when an external MF is applied. This issue must be addressed so far and could constitute a key aspect in understanding the effects of MFs on microalgae cultures.
Electron transition changes linked to reaction centres of microorganisms
Various authors have associated the results achieved in their research with electronic interactions and energetic changes generated in cells when the MF is applied (Small et al. 2012; Deamici et al. 2019a; Sukhov et al. 2021). First, it is necessary to explain that microalgae and cyanobacteria can absorb light energy through the chlorophyll molecules in their structure (Heimann and Huerlimann 2015; Osanai et al. 2017). This absorbed energy begins a set of redox reactions, driven by the light energy in the reaction centers of photosystems I and II (Mamedov et al. 2015) as the first stage of oxygenic photosynthesis. The effect of SMF on photosystem II has already been studied by various authors (Small et al. 2012; Deamici et al. 2019a; Sukhov et al. 2021). The results have allowed us to conclude that the SMF positively affects PS II, increasing its quantum yield (Deamici et al. 2019a). Although these results are closely linked to the mechanism that explains the formation of pairs of radicals, the experimental evidence that supports this relationship still needs to be improved. For example, Tu et al. (2015) attributed the growth of algae and the production of oxygen to the stimulation of MFs in the energy flow and to the cyclic system of electron transfer in the photosynthesis process, which leads to the release of oxygen and the generation of electrons from high potential, so oxidation–reduction reaction process is facilitated. Likewise, Huo et al. (2020) relate the increases in cell concentration when the 30 mT SMF was applied to improving the bio-electrocatalytic reactions of a set of enzymes and other modifications at electronic levels. The studies carried out by Deamici et al. (2016) proposes a possible explanation related to the fact that SMF affects microalgae growth by changing the concentrations of free radicals or the reaction rates that involving these radicals.
Also in the research carried out by Huo et al. (2020) an increase in the concentration of biomass was observed, when the filamentous microalga Tribonema sp. was grown at low temperatures (25 °C) with the application of the SMF. These researchers attribute this behavior to the fact that MF can affect the bio-electrocatalytic transformations of various sets of enzymes in algae cells by enhancing electron transfer at the interface of the electrode solution. In the study carried out by Deamici et al. (2019a) with the cyanobacterium A. platensis, it was observed that during the action of a SMF of 30 mT the photochemical processes in the electron transport chain improved, compared to the control groups. However, the absorption flux per reaction centre was not affected.
Another critical point is that application of MF might trigger the formation of pairs of radicals in the media the cells are cultured in, which might affect cell growth. Chu et al. (2020) considered that a decrease in the specific growth rate could be related to an increase in free radicals in the media due to applying an SMF with intensities of 30 mT and 40 mT. As mentioned before, it is clear that the application of MF does not have a linear behavior in terms of positive observed results (Zhang et al. 2017; Deamici et al. 2021). To protect cells from the stress they are exposed to, such as radicals in the media, MF might mediate metabolic reactions in the cells, ultimately limiting their growth and altering their biochemical composition. So, investment in defense mechanisms is at the expense of cellular growth.
With the results of the phototaxis and hypoosmotic shock, experiments reported for the dinoflagellate Gymnodinium catenatum, Vale (2017) presented a hypothesis for two separate damaging mechanisms that condition the natural blooms of G. catenatum. One mechanism depends on solar radiation, and the other relates to geomagnetic activity. In the particular case of geomagnetic activity, the researchers explained that EMFs could alter the activity of ion channels, specifically voltage-gated calcium channels. Variations in EMFs caused by tectonic activity may influence the physiology of G. catenatum (Vale 2017).
To better understand the processes triggered during the light phase of photosynthesis when SMF is applied, it is necessary to explain some basic processes related to light capture by photosynthetic organisms. From the moment, we will focus only on the photochemical process that occurs in the RCs of the PS II, which is where the effect of the SMF has been evaluated.
During the light reactions of photosynthesis the light captured by the light-harvesting complex (LHC) is transferred to the reaction centers (RC) of photosystem II (PS II) (Junge 2019). Special chlorophyll P680 is found in the RCs of PS II (Vinyard et al. 2013). These special chlorophylls will be excited by the absorption of a photon of light energy, generating the oxidized form of chlorophyll (P680+) (Mamedov et al. 2015). From this moment on, the excited chlorophylls of the RCs will reduce to a pheophytin molecule (Phe), forming the radical pairs P680•+ and Phe•− in the PS II (Barber 2003; Mamedov et al. 2015). The radical Phe.− reduces a plastoquinone molecule bound to the protein quinone A (QA), beginning an electron transfer process between photosystem II and I (PS II and PS I) where quinone B (QB) also intervenes through the electron transport chain (Mamedov et al. 2015; Shen 2015). These oxidation–reduction reactions in a chain release the energy necessary for synthesizing adenosine triphosphate (ATP) (Leister 2022). Then the ATP generated together with nicotinamide adenine dinucleotide phosphate (NADPH) will be used by the cells as a source of chemical energy during the dark phase of photosynthesis.
Results have verified that variations in the primary photochemistry of PS II occur when SMF is applied to cells. These variations are related to the increase in the respiration rate and the photosynthetic evolution of oxygen (Small et al. 2012). This photosynthetic oxygen increase process constitutes evidence demonstrating that the effects of SMF have a direct relationship with light absorption by microorganisms; they are also associated with the photochemical processes that occur in PS II, which is where the photolysis of water takes place. The photolysis of water is produced by breaking the chemical bonds that constitute the water molecule due to the incidence of light energy. This photochemical reaction gives rise to hydrogen and electrons as reaction products, which will form part of the subsequent reactions, in addition to the gaseous dioxygen that is expelled into the medium (McEvoy and Brudvig 2006).
On the other hand, the primary photochemistry of PS II has also been studied by Deamici et al. (2019a). They observed that when the SMF is applied for 1 h and 24 h per day, the maximum quantum yield for primary photochemistry (φpo) increases concerning control cultures. This improvement in PS II photosynthetic efficiency is closely related to the increase in the probability that a trapped exciton moves an electron into the electron chain beyond QA (ψo) and quantum yield for electron transport (φeo). However, there is another parameter that these authors evaluated that is closely related to the formation of radical pairs, and that was not analyzed in depth. This parameter is the absorption flux per reaction center (ABS/RC) and is considered an estimate of the antenna size of the photosynthetic apparatus. The ABS/RC value is inversely proportional to the number of active RCs of the PS II. The results showed that with the application of the SMF, the ABS/RC decreased for all the cultures exposed to the magnetic treatment. This finding indicates that in PS II there is an increase in active RCs. The concentration of the oxidized form of chlorophyll (P 680+) increases, which will then reduce the pheophytin molecule, increasing the concentration of the radical pairs P680•+ and Phe•− in the PS II. With this interpretation of these results, it could be said that the mechanism of action that best explains the photochemical processes of SMF in microalgae is the formation of radical pairs of the RC of PS II.
What happens to the microalgae and cyanobacteria culture medium under the action of MF?
Water is one of the essential substances for the development of any living organism. Therefore, any external or internal disturbance exerted on this substance could affect the development of living organisms. The effects of MFs on the physical–chemical characteristics of water have already been confirmed by several authors (Wang et al. 2018; Yuan et al. 2020). However, it has been verified that the effects of magnetically treated water on biological systems are generally associated with the presence of dissolved salts in the liquid medium.
For example, Abdulraheem and Jameel (2021) evaluated the effect of water treated with different MF intensities (1000 and 3000 G) and different salinities with electrical conductivities of (0.7, 4, and 8 dS m−1) on the growth of the sunflower plant. They observed that the use of saline water with magnetic treatment could increase germination, root depth, stem height, and humidity of both the aboveground parts and the roots of the sunflower plant at different levels depending on the part of the plant, the value of the electrical conductivity of the irrigation water and the intensity of the MF. Similar results were obtained by Samarah et al. (2021) in the evaluation studies of the application of magnetically treated saline solutions on tomato seeds. Magnetic treatment of water or seeds improved seed germination percentage, germination rate, and seedling growth in two laboratory experiments, especially under salinity stress of 5 and 10 dS m−1. Based on these results, we could say that MFs applied to saline solutions induce the formation of an electric field due to the “Hall effect”, discussed in the mechanisms section. Nevertheless, more studies are still needed to corroborate this statement and its relationship with the triggered biological processes.
The previous results are vital for microalgae and cyanobacteria cultivation since any variation can positively or negatively influence these microorganisms in the culture medium. That is why this knowledge constitutes the basis for the development of technologies for wastewater treatment. The research carried out by Poshtarenko (2021) has verified that MF technology in wastewater treatment reduced the turbidity of the water and eliminated its impurities, mainly in the presence of iron ions. This author addressed the polarizing effect of the magnetic field on ions and water molecules as a possible hypothesis for the effect of MF on wastewater.
Although still with a limited number of studies, some authors have already begun to report changes in the microalgae culture medium when this physical agent is applied. Han et al. (2016) investigated the cultivation of Chlorella pyrenoidosa in wastewater and the application of an SMF of 0.18–0.70 T. They reported that magnetic treatment of wastewater resulted in a 9% significant reduction in water turbidity. This effect is not only scientifically crucial from a chemical point of view. Also, from a physiological point of view, a decrease in the turbidity of the wastewater is of great importance since there will be a larger availability of light for the microorganisms to carry out photosynthesis. Moreover, it was observed that the pre-treatment of municipal wastewater before microalgae cultivation had a more beneficial effect on the accumulation of biomass and lipids (Feng et al. 2020). However, there were no significant differences when the magnetic treatment was applied in an installation with wastewater and microalgae. These experimental results prove that MF application on culture media from the chemical point of view affects its physical and chemical characteristics.
General conclusion and future perspectives
This review discusses the main biological effects induced by magnetic fields (MFs) in microalgae and cyanobacteria. Currently, only three mechanisms are reported to attempt to explain the effects of MFs on biological systems, including (1) magnetic induction, (2) magneto-mechanical effect, and (3) the formation of radical pairs. Despite the existence of these mechanisms, the technology currently available has not allowed the scientific community to directly evaluate them. Most effects observed in microalgae and cyanobacteria are based on biochemical responses and cell growth, which have been manifested through metabolic and/or genetic changes, electron transition changes linked to reaction centers of the microorganisms, and structural changes in cells. Evaluating the effects of static magnetic fields (SMF) at an electronic level is a complex process that requires adequate equipment for experimental studies and correct and profound interpretation to establish relationships between the experimental results obtained with the proposed mechanism of action. The most robust mechanism discovered so far is related to the formation of radical pairs associated with electron transition changes linked to the reaction centers of the photosynthetic apparatus of microorganisms.
On the other hand, many questions about the effects of magnetic fields (MF) on microalgae and cyanobacteria and all the factors associated with their cultivation remain unanswered, such as the effects of MF on culture media. These questions can be resolved by standardizing the results obtained by different authors and reporting essential parameters such as magnetic intensity, type of magnetic field, type of spatial distribution of the magnetic field, and chemical composition of devices used for magnetic treatment. Furthermore, future studies should report the specific strain used in their research, as even within the same species, variations may depend on the physiological state of the microorganisms and their isolation source, among others. Other crucial factors to consider are the growth phase at which the MF is applied to the microorganism and the duration of the exposure to the MF.
Many emerging concepts focus on new approaches for the application of MF on microalgae, ranging from the production of magnetically modified cells (Savvidou et al. 2019), to the development of techniques for flocculation of these microorganisms from magnetic nanoparticles (Álvarez-Manzaneda and de Vicente 2017; Yin et al. 2020) and also as pre-treatment for the wet extraction of the biomass, observing excellent results during the extraction (Guo et al. 2019). Magnetic treatment technology has the added value of solving significant environmental problems, such as wastewater treatment integrated with microalgae cultivation. In addition, this technology has the advantages of low operating cost, no proven toxic effects, does not produce secondary contamination, provides a wide range of applications, and has a long service life (Zhang et al. 2017). Therefore, commercially exploiting the full potential of products derived from microalgae and applying MFs can improve the economic feasibility of microalgae and cyanobacterial biorefineries.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abdulraheem LH, Jameel W (2021) Effects of magnetic treatment of different qualities of irrigation water on plant growth. IOP Conf Ser: Earth Environ Sci 1:012030
Akaberi S, Krust D, MŘller G, Frey W, Gusbeth C (2020) Impact of incubation conditions on protein and C-phycocyanin recovery from Arthrospira platensis post-pulsed electric field treatment. Bioresour Technol 306:123099
Álvarez-Manzaneda I, de Vicente I (2017) Assessment of toxic effects of magnetic particles used for lake restoration on Chlorella sp. and on Brachionus calyciflorus. Chemosphere 187:347–356
Amor M, Ceballos A, Wan J, Simon CP, Aron AT, Chang CJ, Hellman F, Komeili A (2020) Magnetotactic bacteria accumulate a large pool of iron distinct from their magnetite crystals. Appl Environ Microbiol 86:e01278–20
Anik MI, Hossain MK, Hossain I, Mahfuz A, Rahman MT, Ahmed I (2021) Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Select 2:1146–1186
Ariede MB, Candido TM, Jacome ALM, Velasco MVR, de Carvalho JCM, Baby AR (2017) Cosmetic attributes of algae-A review. Algal Res 25:483–487
Baki A, Wiekhorst F, Bleul R (2021) Advances in magnetic nanoparticles engineering for biomedical applications—A review. Bioengineering 8:134
Baldev E, MubarakAli D, Sivasubramanian V, Pugazhendhi A, Thajuddin N (2021) Unveiling the induced lipid production in Chlorella vulgaris under pulsed magnetic field treatment. Chemosphere 279:130673
Barber J (2003) Photosystem II: the engine of life. Quart Rev Biophys 36:71–89
Bauer LM, Costa JAV, da Rosa APC, Santos LO (2017) Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour Technol 244:1425–1432
Binhi V (2016) Primary physical mechanism of the biological effects of weak magnetic fields. Biophysics 61:170–176
Binhi VN, Prato FS (2018) Rotations of macromolecules affect nonspecific biological responses to magnetic fields. Scie Rep 8:13495
Bodewein L, Schmiedchen K, Dechent D, Stunder D, Graefrath D, Winter L, Kraus T, Driessen S (2019) Systematic review on the biological effects of electric, magnetic and electromagnetic fields in the intermediate frequency range (300 Hz to 1 MHz). Environ Res 171:247–259
Brailo M, Pećarević M, Grilec D, Mišković M, Lale D, Jurjević M, Čalić M, Mikuš J, Bratoš Cetinić A (2018) The influence of electromagnetic field on viability of marine microalgae Tetraselmis suecica and bacteria Escherichia coli and Enterococcus faecalis. Naše More 65:71–77
Cao DX, Yan H, Brus VV, Wong MS, Bazan GC, Nguyen T-Q (2020) Visualization of charge transfer from bacteria to a self-doped conjugated polymer electrode surface using conductive atomic force microscopy. ACS Appl Mat Interf 12:40778–40785
Chen L, Zhang K, Wang M, Zhang Z, Feng Y (2021) Enhancement of magnetic field on fermentative hydrogen production by Clostridium pasteurianum. Bioresour Technol 341:125764
Chidsey CE, Takiff L, Goldstein RA, Boxer SG (1985) Effect of magnetic fields on the triplet state lifetime in photosynthetic reaction centers: Evidence for thermal repopulation of the initial radical pair. Proc Nat Acad Sci 82:6850–6854
Chu F-J, Wan T-J, Pai T-Y, Lin H-W, Liu S-H, Huang C-F (2020) Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J Environ Manage 253:109680
da Costa MB, Pintos THC, Sala L, Costa JAV, Santos LO (2020) Application of static magnetic fields on the mixotrophic culture of Chlorella minutissima for carbohydrate production. Appl Biochem Biotech 192:822–830
da Costa MB, Sala L, Costa JAV, Buffon JG, Santos LO (2021) Magnetic fields exhibit a positive impact on lipid and biomass yield during phototrophic cultivation of Spirulina sp. Bioproc Biosyst Eng 44:2087–2097
da Silva PGP, Júnior DP, Sala L, de Medeiros Burkert JF, Santos LO (2020) Magnetic field as a trigger of carotenoid production by Phaffia rhodozyma. Process Biochem 98:131–138
Deamici KM, Costa JAV, Santos LO (2016) Magnetic fields as triggers of microalga growth: evaluation of its effect on Spirulina sp. Bioresour Technol 220:62–67
Deamici KM, Cuellar-Bermudez SP, Muylaert K, Santos LO, Costa JAV (2019a) Quantum yield alterations due to the static magnetic fields action on Arthrospira platensis SAG 21.99: Evaluation of photosystem activity. Bioresour Technol 292:121945
Deamici KM, Santos LO, Costa JAV (2019b) Use of static magnetic fields to increase CO2 biofixation by the microalga Chlorella fusca. Bioresour Technol 276:103–109
Deamici KM, Santos LO, Costa JAV (2021) Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioproc Biosyst Eng 44:1453–1460
Feng X, Chen Y, Lv J, Han S, Tu R, Zhou X, Jin W, Ren N (2020) Enhanced lipid production by Chlorella pyrenoidosa through magnetic field pretreatment of wastewater and treatment of microalgae-wastewater culture solution: Magnetic field treatment modes and conditions. Bioresour Technol 306:123102
Garrido-Cardenas JA, Manzano-Agugliaro F, Acien-Fernandez FG, Molina-Grima E (2018) Microalgae research worldwide. Algal Res 35:50–60
Gateau H, Solymosi K, Marchand J, Schoefs B (2017) Carotenoids of microalgae used in food industry and medicine. Mini Rev Med Chem 17:1140–1172
Gilch P, Pollinger-Dammer F, Musewald C, Michel-Beyerle ME, Steiner U (1998) Magnetic field effect on picosecond electron transfer. Science 281:982–984
Guo B, Yang B, Silve A, Akaberi S, Scherer D, Papachristou I, Frey W, Hornung U, Dahmen N (2019) Hydrothermal liquefaction of residual microalgae biomass after pulsed electric field-assisted valuables extraction. Algal Res 43:101650
Han S, Jin W, Chen Y, Tu R, Abomohra AE-F (2016) Enhancement of lipid production of Chlorella pyrenoidosa cultivated in municipal wastewater by magnetic treatment. App Biochem Biotech 180:1043–1055
Hassanpour H, Pourhabibian R (2022) Impact of sodium pyrophosphate and static magnetic field on Haematococcus pluvialis: enhancement of astaxanthin accumulation, PAL, and antioxidant enzyme activities. Physiol Mol Biol Plants 28:1207–1216
Heimann K, Huerlimann R (2015) Microalgal classification: major classes and genera of commercial microalgal species. In: Kim S-K (ed) Handbook of marine microalgae. Elsevier, Amsterdam, pp 25–41
Hore PJ, Mouritsen H (2016) The radical-pair mechanism of magnetoreception. Annu Rev Biophys 45:299–344
Huo S, Chen X, Zhu F, Zhang W, Chen D, Jin N, Cobb K, Cheng Y, Wang L, Ruan R (2020) Magnetic field intervention on growth of the filamentous microalgae Tribonema sp. in starch wastewater for algal biomass production and nutrients removal: influence of ambient temperature and operational strategy. Bioresour Technol 303:122884
Jiang Y, Wang H, Zhao C, Huang F, Deng L, Wang W (2018) Establishment of stable microalgal-bacterial consortium in liquid digestate for nutrient removal and biomass accumulation. Bioresour Technol 268:300–307
Joe M-H, Kim D-H, Choi DS, Bai S (2018) Optimization of phototrophic growth and lipid production of a newly isolated microalga, Desmodesmus sp. KAERI-NJ5. Microbiol Botechnol Lett 46:377–389
Junge W (2019) Oxygenic photosynthesis: history, status and perspective. Quart Rev Biophys 52:e1
Kataria S, Baghel L, Guruprasad K (2017) Pre-treatment of seeds with static magnetic field improves germination and early growth characteristics under salt stress in maize and soybean. Biocatal Ag Biotech 10:83–90
Kattnig DR, Evans EW, Déjean V, Dodson CA, Wallace MI, Mackenzie SR, Timmel CR, Hore P (2016) Chemical amplification of magnetic field effects relevant to avian magnetoreception. Nat Chem 8:384–391
Khavari F, Saidijam M, Taheri M, Nouri F (2021) Microalgae: therapeutic potentials and applications. Mol Biol Rep 48:4757–4765
Khorshidi N, Hassanpour H, Ziyadi H (2022) Static magnetic field improved growth and astaxanthin production in Haematococcus lacustris via the regulation of carbohydrate accumulation, H2O2 level, and antioxidant defense system. J Appl Phycol 34:2283–2295
Lefèvre CT, Bazylinski DA (2013) Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol Mol Biol Rev 77:497–526
Leister D (2022) Enhancing the light reactions of photosynthesis: Strategies, controversies and perspectives. Mol Plant 16:4–22
Lew W-Z, Feng S-W, Lee S-Y, Huang H-M (2021) The review of bioeffects of static magnetic fields on the oral tissue-derived cells and its application in regenerative medicine. Cells 10:2662
Lin L, Wang X, Cui H (2019) Synergistic efficacy of pulsed magnetic fields and Litseacubeba essential oil treatment against Escherichia coli O157:H7 in vegetable juices. Food Control 106:106686
Luo X, Zhang H, Li Q, Zhang J (2020) Effects of static magnetic field on Chlorella vulgaris: growth and extracellular polysaccharide (EPS) production. J Appl Phycol 32:2819–2828
Mamedov M, Nadtochenko V, Semenov A (2015) Primary electron transfer processes in photosynthetic reaction centers from oxygenic organisms. Photosynth Res 125:51–63
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: The supply of nutrients. Water rRes 65:186–202
Mohammadi F, Ghanati F, Sharifi M, Chashmi NA (2018) On the mechanism of the cell cycle control of suspension-cultured tobacco cells after exposure to static magnetic field. Plant Sci 277:139–144
Molazadeh M, Ahmadzadeh H, Pourianfar HR, Lyon S, Rampelotto PH (2019) The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front Bioeng Biotech 7:42
Nikitin AA, Ivanova AV, Semkina AS, Lazareva PA, Abakumov MA (2022) Magneto-mechanical approach in biomedicine: Benefits, challenges, and future perspectives. Int J Mol Sci 23:11134
Nimpf S, Nordmann GC, Kagerbauer D, Malkemper EP, Landler L, Papadaki-Anastasopoulou A, Ushakova L, Wenninger-Weinzierl A, Novatchkova M, Vincent P (2019) A putative mechanism for magnetoreception by electromagnetic induction in the pigeon inner ear. Curr Biol 29:4052–4059
Osanai T, Park Y-I, Nakamura Y (2017) Biotechnology of microalgae, based on molecular biology and biochemistry of eukaryotic algae and cyanobacteria. Front Microbiol 8:118
Parke WC (2020) Electric and magnetic fields in life. In: Biophysics. Springer, Cham. https://doi.org/10.1007/978-3-030-44146-3_6
Poshtarenko A (2021) Application of alternating magnetic field in wastewater treatment at yeast enterprises. Ukr J Ecol 11:156–160
Radhakrishnan R (2019) Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol Mol Biol Plants 25:1107–1119
Rodgers CT, Hore PJ (2009) Chemical magnetoreception in birds: the radical pair mechanism. Nat Acad Sci 106:353–360
Samarah NH, Hani B, Makhadmeh IM (2021) Effect of magnetic treatment of water or seeds on germination and productivity of tomato plants under salinity stress. Horticulturae 7:220
Santos LO, Deamici KM, Menestrino BC, Garda-Buffon J, Costa JAV (2017) Magnetic treatment of microalgae for enhanced product formation. World J Microbiol Biotechnol 33:169
Sarraf M, Kataria S, Taimourya H, Santos LO, Menegatti RD, Jain M, Ihtisham M, Liu S (2020) Magnetic field (MF) applications in plants: An overview. Plants 9:1139
Sathasivam R, Radhakrishnan R, Hashem A, Abd_Allah EF (2019) Microalgae metabolites: A rich source for food and medicine. Saudi J Biol Sci 26:709-722
Savvidou MG, Banis G, Ferraro A, Molino A, Karatza D, Chianese S, Musmarra D, Kolisis F, Hristoforou E (2019) Production of magnetic modified microalgae using iron oxide nanoparticles and electroporation technique. Chem Eng Trans 73:13–18
Schmiedchen K, Petri A-K, Driessen S, Bailey WH (2018) Systematic review of biological effects of exposure to static electric fields. Part II: Invertebrates and plants. Env Res 160:60–76
Sengupta S, Balla VK (2018) A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J Adv Res 14:97–111
Shah MR, Lutzu GA, Alam A, Sarker P, Chowdhury MK, Parsaeimehr A, Liang Y, Daroch M (2018) Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol 30:197–213
Shao W, Ebaid R, Abomohra AE-F, Shahen M (2018) Enhancement of Spirulina biomass production and cadmium biosorption using combined static magnetic field. Bioresour Technol 265:163–169
Shen J-R (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48
Shneerson GA, Dolotenko MI, Krivosheev SI (2014) Strong and superstrong pulsed magnetic fields generation. De Gruyter, Berlin
Siqueira SF, Queiroz MI, Zepka LQ, Jacob-Lopes E (2018) Introductory chapter: Microalgae biotechnology. A brief introduction. In: Jacob-Lopes E, Zepka LQ, Queiroz MI (eds) Microalgal Biotechnology. IntechOpen, London, pp 1–11
Small DP, Hüner NP, Wan W (2012) Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 33:298–308
Su Y, Song K, Zhang P, Su Y, Cheng J, Chen X (2017) Progress of microalgae biofuel’s commercialization. Renew Sust Energy Rev 74:402–411
Sukhov V, Sukhova E, Sinitsyna Y, Gromova E, Mshenskaya N, Ryabkova A, Ilin N, Vodeneev V, Mareev E, Price C (2021) Influence of magnetic field with Schumann resonance frequencies on photosynthetic light reactions in wheat and pea. Cells 10:149
Tu R, Jin W, Xi T, Yang Q, Han S-F, Abomohra AE-F (2015) Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivated in municipal wastewater. Water Res 86:132–138
Vale P (2017) Influence of static magnetic fields in phototaxis and osmotic stress in Gymnodinium catenatum (Dinophyceae). Gen Physiol Biophys 36:235–245
Vandamme D, Foubert I, Muylaert K (2013) Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotech 31:233–239
Vinyard DJ, Ananyev GM, Charles Dismukes G (2013) Photosystem II: the reaction center of oxygenic photosynthesis. Annu Rev Biochem 82:577–606
Wang Y, Wei H, Li Z (2018) Effect of magnetic field on the physical properties of water. Results Phys 8:262–267
Wong SY, Wei Y, Mouritsen H, Solov’yov IA, Hore P (2021) Cryptochrome magnetoreception: four tryptophans could be better than three. J Roy Soc Interface 18:20210601
Yan N, Fan C, Chen Y, Hu Z (2016) The potential for microalgae as bioreactors to produce pharmaceuticals. Int J Mol Sci 17:962
Yin Z, Zhu L, Li S, Hu T, Chu R, Mo F, Hu D, Liu C, Li B (2020) A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions. Bioresour Technol 301:122804
Yuan W, Zhou H, Yang Z, Hein JR, Yang Q (2020) Magnetite magnetofossils record biogeochemical remanent magnetization in hydrogenetic ferromanganese crusts. Geology 48:298–302
Zadeh-Haghighi H, Simon C (2022) Magnetic field effects in biology from the perspective of the radical pair mechanism. J R Soc Interface 19:20220325
Zeng L, Li G, Zhang M, Zhu R, Chen J, Li M, Yin S, Bai Z, Zhuang W, Sun J (2022) A noninvasive and comprehensive method for continuous assessment of cerebral blood flow pulsation based on magnetic induction phase shift. PeerJ 10:e13002
Zhang X, Yarema K, Xu A (2017) Biological effects of static magnetic fields. Springer, Singapore
Funding
This research was supported by VLIR-UOS Belgium project CU2019IUC030A105-77143 and UHasselt BOF-BILA fellowship R-9903.
Author information
Authors and Affiliations
Contributions
Y.S. Font: Conceptualization, Investigation, validation, visualization, writing-original Draft Y.O. Díaz: Conceptualization, writing – Review and editing A. Cuypers: Conceptualization, Resources, Writing – Review and Editing, funding acquisition, E.I Alemán: writing – review and editing, validation, funding acquisition, supervision; D. Vandamme: conceptualization, resources, writing – Review and editing – supervision – funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Font, Y.S., Díaz, Y.O., Cuypers, A. et al. The effect of magnetic field treatment on the cultivation of microalgae: An overview of involved mechanisms. J Appl Phycol 35, 1525–1536 (2023). https://doi.org/10.1007/s10811-023-02994-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02994-1