Abstract
Microorganisms associated with plants have a great biotechnological potential, but investigations of these microorganisms associated with native plants in peculiar environments has been incipient. The objective of this study was to analyze the plant growth-promoting bacteria potential of cultivable bacteria associated with rare plants from the ferruginous rocky fields of the Brazilian Iron Quadrangle. The roots and rhizospheres of nine endemic plants species and samples of a root found in a lateritiric duricrust (canga) cave were collected, the culturable bacteria isolated and prospected for distinct biotechnological and ecological potentials. Out of the 148 isolates obtained, 8 (5.4%) showed potential to promote plant growth, whereas 4 (2.7%) isolates acted as biocontrol agents against Xanthomonas citri pathotype A (Xac306), reducing the cancrotic lesions by more than 60% when co-inoculated with this phytopathogen in Citrus sinensis plants. Moreover, other 4 (2.7%) isolates were classified as potential bioremediation agents, being able to withstand high concentrations of arsenite (5 mM As3+) and arsenate (800 mM As5+), by removing up to 35% and 15% of this metalloid in solution, respectively. These same four isolates had a positive influence on the growth of both the roots and the aerial parts when inoculated with tomato seeds in the soil contaminated with arsenic. This is the first time that an investigation highlights the potentialities of bacteria associated with rare plants of ferruginous rocky fields as a reservoir of microbiota of biotechnological and ecological interest, highlighting the importance of conservation of this area that is undergoing intense anthropic activity.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron geosystems represent one of the oldest landscapes on the planet; they were formed by rocky packages known as banded iron formations (BIF) between 3.0 and 2.65 Ga ago (Gibson et al. 2015; Salgado and Carmo 2015). In Brazil, iron duricrusts (known as canga) were formed in situ over BIF weathering, with records of origin that start at ca. 50 Ma (Monteiro et al. 2014). The canga duricrusts can reach about 30 m thick, and the mineralogical constitution can reach 90% iron oxide and hydroxide. The soil developments in canga—when present—are acidic and shallow, with a remarkably high level of manganese and iron, and very low nutrient status, notably P (Jacobi et al. 2015). Carmo and Jacobi (2016) analyzed edaphic fertility in canga outcrops and found extremely low concentrations of P (< 3.9 mg kg−1), which are lower than some of the world’s most nutrient-poor soils in ancient landscapes.
Besides these adverse edaphic conditions, plants from iron duricrusts exhibit biological characteristics that support their adaptation to a greater variety of environmental stressors, such as the ability to deal with high radiation, strong winds, seasonal rainfall distribution, and exposed rock substratum with temperatures that reach 67 °C (Jacobi et al. 2015; Silveira et al. 2016). These conditions act as powerful selective pressure factors, and certainly favor the evolution and consequent adaptation of plants (Gibson et al. 2010). Restricted areas of the canga soils concentrate a high diversity of vascular plants, as in the case of Iron Quadrangle (IQ), southeastern Brazil, defined as a priority area of extremely high importance for the conservation and sustainable use of Brazilian biodiversity (MMA 2007). In this region, more than 1100 species were inventoried in only 14 canga outcrops sampled, with a total area of less than 550 ha (Jacobi and Carmo 2012). This increased diversity is also associated with the structural heterogeneity of the canga, represented by fractures, depressions, pores, and canals that develop toward the rocky interior (Carmo et al. 2016). This heterogeneity favors root development even in the absence of soil, forming exceptional structures inside subsurface caves, such as rhizomes and suspended tufts. However, natural areas in metalliferous regions are suffering intensive losses in biodiversity due to the unprecedented global demand for ores, specifically iron (Jacobi and Carmo 2012; Jacobi et al. 2011).
From an ecological perspective, the dynamics among the communities living in these high-stress environments confer a functionally diverse relationship between living beings and their interactions and abiotic factors. For example, by influencing the interactions between plants and microorganisms, plants can provide an unique niche for the maintenance of several microbial communities, and in contrast, the microorganisms assist the survival of plants by acting as plant growth promoters (Barrow et al. 2008). Through this process of interaction and mutual benefit, it is possible that diversified and elaborated biochemical mechanisms are employed by bacteria to play a role in the growth of plants. These so-called plant growth-promoting bacteria (PGPBs) act through direct and indirect mechanisms. The ability of the associated microbial community to supplement the plant with nutrients or hormones is an example of direct mechanism. Among the PGPBs acting by indirect mechanisms, the ability to fix atmospheric nitrogen (N2), solubilize phosphate, produce siderophores and phytohormones [such as indole acetic acid (IAA)], allows the classification of these microorganisms as potential biofertilizers (Bulgarelli et al. 2013). Among the PGPBs acting by indirect mechanisms, those that can act as biocontrollers or soil bioremediators stand out. Bioremediators reduce the bioavailability of toxic compounds to plants by metabolizing, biotransforming, or chelating such compounds (Ma et al. 2016). On the other hand, biocontrollers prevent phytopathogens (bacteria, fungi, nematodes, or viruses) from causing damage to the host plant through the production of acylases or lactonases, antibiotics, toxins, and other molecules, such as hydrocyanic acid (HCN) (Combes-Meynet et al. 2011). Furthermore, such biocontrol mechanisms can also be provided by systemic induced responses. In this case, the protection occurs through molecular signals induced by the microorganisms that activate the basal system of plant defense, culminating in the integral protection of the plant against the action of pathogens (Vlot et al. 2008).
Based on interactions between plants and microorganisms in this unique scenario, and on the ecological importance of the IQ for Brazilian biodiversity, we describe bioprospecting data on the potential of cultivable bacteria obtained from nine endemic plant species of the IQ—most of which are restricted to ferruginous rocky fields—to act as biofertilizers, biocontrollers, and bioremediators of metalliferous soils. In addition, this work emphasizes the opportunity for further research to understand the relationship between plant endemism and the actions of the microbiota involved in this process.
Materials and methods
Part of the methodologies summarized below are described in detail in Supplementary Materials and Methods in order to facilitate experimental reproducibility.
Area of study and sample collection
In the IQ (Fig. 1), the predominant climate, according to the classification of Köppen, is a subtropical altitude characterized by strong seasonality, which presents a dry winter and rainy summer. In winter, the average does not usually reach 18 °C, and in summer the average does not reach 22 °C. The soils developed on the canga reflect the heterogeneity of the metalliferous areas, being considered endemic soils. In this way, the canga make up a metalliferous system, insularly covering a geological matrix constituted by BIFs. Samples of roots and rhizospheres associated with nine plants endemic of the IQ were investigated: Sinningia rupicola, Arthrocereus glaziovii, Jacquemontia linarioides, Vriesea minarum, Gomesa gracilis, Croton serratoideus, Paspalum brachytrichum, Chromolaena multiflosculosa, and Calibrachoa elegans. Additionally, root samples found inside a local cave were incorporated into the analyses (Supplemental Table 1). The authorization for the collection of samples was granted by the Instituto Chico Mendes de Conservação da Biodiversidade, issued by the number 54015-3, valid until 03/01/2019.
Selection, isolation, and preservation of cultivable bacteria
For the isolation of root-associated bacteria we use the protocols described by Caneschi et al. (2018). In order to facilitate simultaneous investigation of the isolates potential, some of the methodologies summarized below were performed from the replication of multiple bacteria using a 96-point manual microplate replicator. This mechanical apparatus is composed of 96 metal tips that perfectly fit a 96-well plate, thus allowing up to 96 isolates per plate to be deposited on the surface of solid culture media simultaneously.
Indoleacetic acid (IAA) production assay
To quantify IAA production by the isolates the colorimetric method adapted from Bric et al. (1991) using the LB culture medium enriched with 5 mM tryptophan was performed.
Nitrogen fixation assay
To verify a possible nitrogen fixation by the isolates, the protocols described by Estrada-De Los Santos et al. (2001) with the appropriate adaptations.
Phosphate solubilization assay
The phosphate solubilization ability was evaluated based on the method of Nautiyal (1999) with the appropriate adaptations.
Siderophore production assay
Detection of siderophore production was based on the method of Schwyn and Neilands (1987), with the appropriate adaptations.
Production of hydrocyanic acid (HCN) assay
To verify the HCN production the method was adapted from Bakker and Schippers (1987).
Amylase and protease production assays
For the amylase and protease activities was used the methods described by Strauss et al. (2001), with the appropriate adaptations.
In vitro antagonistic assay
To investigate the possible antimicrobial activity of the isolates, inhibition assays against the phytopathogen Xanthomonas citri subsp. citri pathotype A strain 306 (Xac 306) were performed. For the direct inhibition analysis, the isolates were grown in liquid LB at 28 °C for 2 days. After this period, approximately 2 µL aliquots of isolate suspension (between 0.6 and 0.8 OD600 nm) were transferred to 90 × 15 mm Petri dishes (using 96-point manual microplate replicators) containing the target phytopathogen, which was was homogeneously spread over solid LB medium. The bacterial isolates were spread over the phytopathogen cover, and a formation of a halo around the isolate colony demonstrated the inhibition of phytopathogen growth. Isolates the inhibited the phytopathogen were subjected to an indirect inhibition assay. This assay was performed by growing the target phytopathogen and inhibitor isolate in the adjacent chambers of a specialized plastic petri dish, containing a center partition, maintained at 28 °C for 2 days. If inhibition was caused by volatile compounds, the center partition of the dish would not prevent inhibition.
In vivo antagonistic assay
In vivo assays of antagonism against X. citri subsp. citri pathotype A (Xac 306) were carried out under controlled growth conditions in the FCAV Technology Department of São Paulo State University, SP, Brazil. Sweet orange plants (C. sinensis) were grown in a greenhouse under controlled temperature and humidity conditions, according to Caicedo et al. (2016).
Resistance and arsenic removal assays
To study the resistance of the isolates to different concentrations of arsenic was used the methods described by Felestrino et al. (2017a).
SEM/DXRS analysis of the arsenic resistant isolates
The scanning electron microscopy (SEM) and the image analysis using energy dispersion X-ray spectroscopy (SEM/DXRS) was performed according to the protocols previously described by Felestrino et al. (2017a) and De et al. (2008).
In vivo plant growth-promoting assay on tomato growing on polluted soil
To evaluate the potential of the arsenic-resistant isolates to remove this metal in vivo, tomato plants were grown in artificially contaminated soil, and the phenotype change was analyzed in the presence of the bacteria of interest, according to the protocols described by Felestrino et al. (2017a).
Statistical analysis
To determinate if the in vivo removal of the metalloid by the isolates was statistically significant (p values < 0.05 and < 0.01), the Kruskal–Wallis non-parametric ANOVA test with Dunn’s post-hoc test was used. Statistical analyses were performed using GraphPad Prism 5.
The lesions of five infiltrated leaves (in triplicate) were quantified, and infected areas were calculated using Image J v1.48 (Schneider et al. 2012). This program measures the area, mean, standard deviation, minimum and maximum selection of the entire image, lengths, and angles, all using real-world measurement units, such as millimeters. The mean and standard deviation of the lesions, in the presence and absence of the biocontrol isolates, was calculated using GraphPad Prism (version 6.01; GraphPad Software, Inc.).
Identification of isolates by 16S region sequencing and phylogenetic analysis
DNA of the selected isolates was extracted using a QIAGEN DNeasy Blood and Tissue Kit. Conserved regions of the 16S rRNA were amplified using the 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) universal primers (Hongoh et al. 2003). The integrity of the samples was checked on 0.8% agarose gel, and the purified 16S rRNA products were quantified using a Thermo Fisher Scientific™ NanoDrop Lite spectrophotometer. The sequencing reaction was performed and the phylogenetic analysis were performed according to Felestrino et al. (2017b). All sequences obtained in this study were deposited at GenBank; the accession numbers are given in Tables 1, 2 and 3.
Results
From the nine endemic plant samples, a total of 148 cultivable bacteria were isolated. There was a great variation in the number of isolates obtained from the samples investigated under the growth conditions established in this study (see methodologies). The highest representativeness in the number of isolates was associated with Arthrocereus glaziovii (35 isolates), Sinningia rupicola (22 isolates), and roots into the cave (23 isolates), whereas the lowest representations were associated with Vriesea minarum (3 isolates), Gomesa gracilis (5 isolates), and Paspalum brachytricum (6 isolates) (Fig. 2).
Characterization of the biochemical potential of the isolates obtained from the investigated samples. The numbers (1–10) in the upper left corner of the images represent the respective samples collected. n represents the total number of bacterial isolates. The X-axis of the graph represents the number of isolates that produced positive results for the production of indol-acetic acid (IAA), amylases (Amy), siderophores (Sid), hydrocyanic acid (HCN) and proteases (Prot), or were able to inhibit Xanthomonas citri (Xac306), assimilate nitrogen (N2), solubilize phosphate (PO4), or to confer resistance to arsenite (As3+) and arsenate (As5+)
Supplementary Table 1 compiles all results from these assays, which are summarized graphically in Fig. 2 for each investigated sample. To facilitate the description of PGPBs potential, these isolates were classified according to their ability to act as biocontrollers, bioreactors, or soil biofertilizers, as described in detail below. The isolates that obtained the best results and demonstrated promising use in different contexts were identified by sequencing of the 16S rRNA of the conserved region (Tables 1, 2, 3).
Out of the 16 identified isolates, 10 belonged to the Firmicutes phylum, comprising mostly potential biofertilizers (eight of the total), three are from the phylum Actinomicetales (all biocontrollers), and three belong to the Proteobacteria phylum, presenting diverse functions (Fig. 3).
Phylogenetic analysis of isolates that had at least one of the tested activities of plant growth-promotion. Tree clades were identified as proteobacteria (blue), actinobacteria (pink), and firmicutes (salmon). The isolates identified in this study are identified in the respective colors of their clade. The black circles identify the nodes whose bootstrap values are equal to 100
Biocontrollers
The obtained isolates were evaluated for their ability to act as biocontrollers in plants. Out of the 148 isolates, 15 (10.1%) were able to synthesize siderophores, 34 (23.0%) produced HCN, and 56 (37.8%) produced protease (Supplementary Fig. 1). Investigations for the antagonistic potential against a phytopathogen revealed that four isolates (2.7%) were able to inhibit the growth of Xac 306 in vitro, a quarantine pest and causative agent of citrus canker (Table 1; Fig. 4a). From this result, these four isolates were also subjected to an indirect inhibition, but none of the isolates were able to inhibit Xac 306 growth, indicating that the inhibitory substances were non-volatile (Fig. 4b). Highlighting the two strains of Serratia (R166Sr and R108C) identified the positive responses to the four trials of antagonistic activity (Table 1).
Biocontrol potential of the investigated isolates. a Direct inhibition assay of Xanthomonas citri subsp. Citri (Xac306), pelos isolados 108, 166, 115 and 268 (em duplicate). NC negative control of inhibition (Escherichia coli), PC positive controls of inhibition (Alcaligenes faecalis strain Mc250 and Serratia sp.). b Indirect inhibition test mediated by volatile compounds. c Xac306 and Xac306 + putative biocontroller isolate virulence phenotype on the adaxial (above) and abaxial (below) sides of Citrus sinensis leaves after 14 days of infection, highlighting the percentage average and the standard deviation of inhibition
To verify whether this inhibitory potential was also observed in vivo, these isolates were co-infected with Xac 306 in sweet orange plants (Citrus × sinensis). The virulence phenotype was investigated for 14 days and after this period, the injured areas were quantified. The results showed that the cancrosis lesions decreased significantly in relation to the infection control in the four isolates, varying around 30–62% reduction in the virulence phenotype, in comparison to the control condition, where the leaf was infected with the causal agent of the cancrosis on both sides of the leaf (Fig. 4c).
Bioremediators
In order to evaluate the potential of the isolates for resistance to arsenic, all 148 isolates were grown in arsenic medium at concentrations ranging from 0.5 to 10 mM As3+ and 1–1000 mM As5+. Four of these isolates (2.7%) were able to withstand high concentrations of both arsenic species, from 5 mM As3+ and up to 800 mM As5+ (Table 2). These isolates were grown in the presence of both species of arsenic, and using X-ray fluorescence spectroscopy with total reflection, it was possible to verify that all the isolates demonstrated a significant capacity of arsenic removal after 5 days, ranging from 21 to 36% removal of As3+, and 5–15% of As5+ (Table 2).
To verify the possible structural modifications in the cells of these isolates owing to the arsenic removal process, SEM was performed, followed by energy dispersive X-ray spectroscopy (EDS) analysis. No significant modifications in the cellular structures were observed in any of the five isolates, which could imply deep internalization of these ions. However, it was possible in all of them to detect the typical outcome of bacterial cells subjected to a stress condition, manifested as morphological and turgescence alterations (Fig. 5). In addition, in the presence of As3+ and As5+, it was found that a small amount of the metalloids were adsorbed on the isolate membranes.
Scanning electron microscopy of the arsenic resistant isolates. Resistance up to 5 mM As3+ (arsenite) for all isolates, 50 mM As5+ (arsenate) for isolates 274 and 208, 500 mM As5+ for isolate 209, and 800 mM As5+ for isolate 291 (see Table 2)
It was also evaluated whether bacterial removal could influence the development of plants in soils contaminated with arsenic. For this, Santa Clara tomato seeds were grown in soil contaminated with arsenic in the presence and absence of the four resistant isolates. Before starting the test, a growth curve of tomato plants at different concentrations of As3+ and As5+ was determined in order to verify the minimum inhibitory concentration after 21 days. For both metalloid species investigated, the 2.5 mM concentration was established for removal trials (Fig. 6a, c). As expected, for both As3+ and As5+, the development of control plants grown on unpolluted soils (C) was significantly different compared to control plants grown in the presence of metalloids (CAs). The length of aerial parts and roots of these plants showed differences in growth, with values of p = 0.0100 and p = 0.0069 for arsenite, and p = 0.0174 and p = 0.0073 for arsenate, respectively (Fig. 6b, d). In the presence of the four isolates investigated (208, 209, 274, and 291), although they reflected a visual improvement in the quality of the plant growth, both for the root and aerial parts, variations of statistical significance between plants grown in the presence of As3+ or As5+ were not identified (Fig. 6b, d, e).
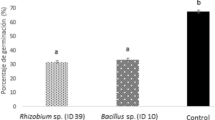
The bioremediation potential of the isolates. Growth curves of tomato plants in the presence of increasing concentrations of arsenite (a) and arsenate (c). The rectangle highlights the concentration chosen for the assays in the presence of the isolates. The growth rate of tomato plants in the presence of arsenite (b) and arsenate (d), and the recovery of plant growth by inoculation of the potential soil bioremediation isolates. C, Control without arsenic; CAs, non-bacterized plants growing in the As contaminated soil. The bar graph denotes the mean of plants elongation with/without the presence of arsenic, containing/not containing the potential bio-controller isolates (208 and 209, Curtobacterium; 274, Leucobacter; and 291, Stenotrophomonas). *p values ≤ 0.05 and **p values ≤ 0.01. e Visual comparison of the growth rate of plants in the presence of arsenic and rhizoremediation in the presence of Leucobacter (274)
Biofertilizers
All isolates were evaluated for their ability to act as biofertilizers, being able to act directly or indirectly on plant growth (Supplementary Fig. 2). Of the 148 isolates obtained, nine (6.08%) were able to solubilize phosphate, 34 (23.0%) produced amylase, five (3.38%) showed a positive result for the biochemical assay of N2 fixation, and the capacity to produce IAA was showed by 36 isolates (24.32%), three of which had a production of more than 9 µg mL−1 (Table 3).
Discussion
The diversity of plants present in the ferruginous rocky fields reinforces the idea that this environment is not homogeneous, but formed by different clusters of microhabitats (Silveira et al. 2016). This structural complexity allows ecological interactions to favor the establishment of a species in a hostile environment, such as canga. The characteristics of the plants thriving in this type of soil are due to adaptive processes that aim for the conservation of resources (Negreiros et al. 2014). In this context, we can infer that association with a specific microbiota can be part of this adaptation. This specificity has already been described previously by (Sanchez-Canizares et al. 2011), who demonstrated the speciation of nodulating bacteria in Lupinus mariae-josephi, an endemic plant in eastern Spain. These researchers proved that bacteria from the Bradyrhizobium genus showed different phenotypic and symbiotic characteristics compared to Bradyrhizobium found in the non-endemic plant Lupinus sp. at a different site. In this way, they concluded that the speciation of bacteria can result from the colonization of a single habitat.
Factors that could assist these bacteria in the establishment of endemic plants can be diverse, thereby placing them as important PGPBs. The exploratory results of this study corroborate this perspective. The production of IAA by 24.32% of the isolates investigated might be associated with the mechanism of plant rooting in a rocky soil, although this has not been empirically observed in tomato plants (data not shown). The plants of these regions possess a large volume of secondary roots that grow parallel to the rocks to compensate for the difficulty of penetrating into the canga, and this might have a direct relationship with the contribution of these bacteria to this physiological process. Likewise, some bacteria isolated in this study, which were able to solubilize phosphate, probably played a crucial role in the establishment of endemic plants in the soils lacking this micronutrient (Schaefer et al. 2015; Wang et al. 2010). The canga soils also present very low levels of total N, which reflects their general chemical scarcity (Schaefer et al. 2015). At this point, nitrogen fixing bacteria are important, and, although they were found in a smaller number in this study (2.5%), they might be essential for the development and maintenance of these plants, allowing recycling of this fundamental component for their development and establishment.
Besides the ability to solubilize phosphate or fix N2, some bacteria have the ability to secrete hydrolases, such as amylase, that biochemically assist in a number of factors, including increasing the bioavailability of some nutrients. Amylase, for example, is an important enzyme required for energy metabolism in plants, and plays a significant role in starch breakdown, particularly in the conversion of starch to maltose (Thoma et al. 1971). Studies have indicated that the activities of amylase can be influenced by different factors, such as vegetation types, environment, and soil types (Pancholy and Rice 1973). Root exudates, for example, may influence the synthetic amylase activity of microorganisms (Pancholy and Rice 1973; Ross 1975). These factors might be related to the production of amylase by about a quarter of the isolates (23.0%), and might justify their importance in the nutrient cycling process in this extremely unique environment (Schaefer et al. 2016).
PGPBs can also benefit plant growth indirectly through biocontrolling bacteria. This potential antagonism might occur through a wide range of mechanisms, such as the production of secondary compounds, siderophores, antibiotics, volatile metabolites, and enzymes, as well as the induction of systemic responses (Saraf et al. 2014). An important mechanism for biocontrol is the production of enzymes that degrade the membrane components of phytopathogenic organisms, such as proteases (Aeron et al. 2011). Of the total isolates obtained in this study, 37.8% were able to produce some type of protease, including the four isolates that inhibited the growth of Xac 306, demonstrating the potential and importance of these organisms in a possible plant protection process. Similarly, the production of HCN, a volatile metabolite produced by some PGPBs, is another potential strategy that can cause the inhibition of phytopathogens, as described in Pseudomonas sp. LBUM300, which showed the potential control of cankers induced by Clavibacter michiganensis in tomato plants (Lanteigne et al. 2012). In the present study, 23.0% of the total isolates produced HCN, including the four isolates that inhibited Xac 306, all belonging to the Firmicutes phylum (Serratia sp. strains R166Sr and R108C, Lysinibacillus sp. strain R268Cm, and Bacillus sp. strain R115C).
Although canga soils are rich in iron, this metal is not frequently found in its bioavailable form (Schaefer et al. 2015). The bioavailability of this metal for bacterial metabolism depends on the ability of the microorganism to produce and secrete iron chelators in the medium, molecules known as siderophores (Vacheron et al. 2013). Once chelated to the metal, by means of specific transporters, these siderophores are again internalized. From the total isolates obtained in this study, 10.1% were able to synthesize siderophores. In the context of this study, this is a molecule that complexes with iron to make it available for the plant, thereby directly supplying the need for this element by the plant, and restricting its availability for use by the surrounding phytopathogens (Ahmed and Holmstrom 2014).
Finally, to promote growth and protection from phytopathogens, many PGPBs assist in the adaptation and maintenance of plants in environments with high concentrations of metals (Kumari et al. 2016). Arsenic is a highly toxic metalloid; it causes severe metabolic changes, culminating in significant cellular damage (Finnegan and Chen 2012). The geological formations of the canga soils from the IQ, where the studied plants were collected, naturally present high concentrations of arsenic (Costa et al. 2015). The resistance of some of the isolates to high concentrations of this metal might justify their adaptation to these contaminated environments and, consequently, to the plants associated with these microorganisms. This has been proven by the capacity to remove the metalloid in vitro and directly from the soil, thereby improving the growth of tomato plants. Although scanning microscopy did not show significant changes in cell morphology that would have indicated the bioaccumulation of the metal, EDS analysis showed the presence of adsorbed arsenic in the membrane, indicating another important mechanism of removal. Although the plants in the IQ have already been classified as metallophytes, being adapted to accumulate high concentrations of metals to adapt to the ferruginous soils, and thus also avoiding herbivorous processes (Ribeiro et al. 2017), this is the first time that bacteria associated with these plants are described as potential soil mediators.
Therefore, in addition to the role of these isolates in canga ecosystems and possibly in the establishment and maintenance of endemic plants in hostile conditions, these isolates can be characterized as potential PGPBs for future applications in contaminated soils, either by weathering or by anthropogenic action. This perspective, despite some successful initiatives (Akhtar et al. 2013; Khan et al. 2013; Mulligan et al. 2001), is still far from being implemented as a promising and sustainable biological process.
Conclusion
The dynamics among communities living in unique environments can form ecological relationships with diverse functions. This is justified by the interesting find of microorganisms associated with endemic plants of the IQ in ferruginous subtrates soils. This is the first time that the bacterial isolates identified and analyzed from root and rhizosphere samples associated with these plants have been demonstrated to act as potential PGPBs, showing themselves as efficient biofertilizers, biocontrollers, and bioremediators of contaminated environments. Finally, the importance of this study was also focused, on a secondary basis, on the preservation of the IQ; this is an environment with intense anthropogenic actions, which are imminent risk factors for decreases in diversity and endemism.
References
Aeron A, Kumar S, Pandey P, Maheshwari DK (2011) Emerging role of plant growth promoting rhizobacteria in agrobiology. In: Maheshwari DK (ed) Bacteria in agrobiology: crop ecosystems. Springer, New York, pp 1–36. https://doi.org/10.1007/978-3-642-18357-7
Ahmed E, Holmstrom SJ (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208. https://doi.org/10.1111/1751-7915.12117
Akhtar MS, Chali B, Azam T (2013) Bioremediation of arsenic and lead by plants and microbes from contaminated soil. Res Plant Sci 1:68–73. https://doi.org/10.12691/plant-1-3-4
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biol Biochem 19:451–457. https://doi.org/10.1016/0038-0717(87)90037-X
Barrow JR, Lucero ME, Reyes-Vera I, Havstad KM (2008) Do symbiotic microbes have a role in plant evolution, performance and response to stress? Commun Integr Biol 1:69–73
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Ann Rev Plant Biol 64:807–838. https://doi.org/10.1146/annurev-arplant-050312-120106
Caicedo JC, Villamizar S, Ferro MIT, Kupper KC, Ferro JA (2016) Bacteria from the citrus phylloplane can disrupt cell–cell signalling in Xanthomonas citri and reduce citrus canker disease severity. Plant Pathol 65:782–791. https://doi.org/10.1111/ppa.12466
Caneschi WL et al (2018) Brazilian ironstone plant communities as reservoirs of culturable bacteria with diverse biotechnological potential. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01638
Carmo FF, Jacobi CM (2016) Diversity and plant trait-soil relationships among rock outcrops in the Brazilian Atlantic rainforest. Plant soil 403:7–20. https://doi.org/10.1007/s11104-015-2735-7
Carmo FF, Campos IC, Jacobi CM (2016) Effects of fine-scale surface heterogeneity on rock outcrop plant community structure. J Veg Sci 27:50–59. https://doi.org/10.1111/jvs.12342
Combes-Meynet E, Pothier JF, Moenne-Loccoz Y, Prigent-Combaret C (2011) The Pseudomonas secondary metabolite 2,4-diacetylphloroglucinol is a signal inducing rhizoplane expression of Azospirillum genes involved in plant-growth promotion. Mol Plant Microbe Interact. 24:271–284. https://doi.org/10.1094/MPMI-07-10-0148
Costa RVF, Leite MGP, Mendonça FPC Jr, Nalini HA (2015) Geochemical mapping of arsenic in surface waters and stream sediments of the Quadrilátero Ferrífero, Brazil. Rem 68:43–51. https://doi.org/10.1590/0370-44672015680077
De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar Biotechnol 10:471–477. https://doi.org/10.1007/s10126-008-9083-z
Estrada-De Los Santos P, Bustillos-Cristales R, Caballero-Mellado J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental geographic distribution. Appl Environ Microbiol 67:2790–2798. https://doi.org/10.1128/AEM.67.6.2790-2798.2001
Felestrino ÉB et al (2017a) Alcaligenes faecalis associated with Mimosa calodendron rizhosphere assist plant survival in arsenic rich soils. J Soil Sci Plant Nutr 17:1102–1115. https://doi.org/10.4067/S0718-95162017000400019
Felestrino EB, Santiago IF, Freitas LD, Rosa LH, Ribeiro SP, Moreira LM (2017b) Plant growth promoting bacteria associated with Langsdorffia hypogaea-rhizosphere-host biological interface: a neglected model of bacterial prospection. Front Microbiol 8:172. https://doi.org/10.3389/fmicb.2017.00172
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:182. https://doi.org/10.3389/fphys.2012.00182
Gibson N, Yates CJ, Dillon R (2010) Plant communities of the ironstone ranges of South Western Australia: hotspots for plant diversity and mineral deposits. Biodivers Conserv 19:3951–3962. https://doi.org/10.1007/s10531-010-9939-1
Gibson N, Coates D, van Leeuwen S, Yates C (2015) Hot, dry and ancient: banded iron formations of western Australia. In: Carmo FF, Kamino LHY (eds) Geossistemas Ferruginosos do Brasil: áreas prioritárias para conservação da diversidade geológica e biológica, patrimônio cultural e serviços ambientais. 3i Editora Ltda, Belo Horizonte, pp 23–46
Hongoh Y, Yuzawa H, Ohkuma M, Kudo T (2003) Evaluation of primers and PCR conditions for the analysis of 16S rRNA genes from a natural environment. FEMS Microbiol Lett 221:299–304
Jacobi CM, Carmo FF (2012) Diversidade Florística nas cangas do Quadrilátero Ferrífero. 3i Editora Ltda, Belo Horizonte
Jacobi CM, do Carmo FF, de Campos IC (2011) Soaring extinction threats to endemic plants in Brazilian metal-rich regions. Ambio 40:540–543
Jacobi CM, Carmo FF, Campos IC (2015) Iron geosystems: priority areas for conservation in Brazil. In: Tibbett M (ed) Mining in ecologically sensitive landscapes. CRC Press, Boca Raton
Khan S, Afzal M, Iqbal S, Khan QM (2013) Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 90:1317–1332. https://doi.org/10.1016/j.chemosphere.2012.09.045
Kumari D, Qian XY, Pan X, Achal V, Li Q, Gadd GM (2016) Microbially-induced carbonate precipitation for immobilization of toxic metals. Adv Appl Microbiol 94:79–108. https://doi.org/10.1016/bs.aambs.2015.12.002
Lanteigne C, Gadkar VJ, Wallon T, Novinscak A, Filion M (2012) Production of DAPG and HCN by Pseudomonas sp. LBUM300 contributes to the biological control of bacterial canker of tomato. Phytopathology 102:967–973. https://doi.org/10.1094/PHYTO-11-11-0312
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manage 174:14–25. https://doi.org/10.1016/j.jenvman.2016.02.047
MMA (2007) Áreas Prioritárias para Conservação, Uso Sustentável e Repartição de Benefícios da Biodiversidade Brasileira: Atualização—Portaria MMA no. 9, de 23 de janeiro de 2007. Série Biodiversidade, 31. Ministério do Meio Ambiente, Secretaria de Biodiversidade e Florestas, Brasília
Monteiro HS, Vasconcelos PM, Farley KA, Spier CA, Mello CL (2014) (U–Th)/He geochronology of goethite and the origin and evolution of cangas. Geochim Cosmochim Acta 131:267–289. https://doi.org/10.1016/j.gca.2014.01.036
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207. https://doi.org/10.1016/S0013-7952(00)00101-0
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Negreiros D, Stradic SL, Fernandes GW, Rennó HC (2014) CSR analysis of plant functional types in highly diverse tropical grasslands of harsh environments. Plant Ecol 215:379–388. https://doi.org/10.1007/s11258-014-0302-6
Pancholy SK, Rice EL (1973) Soil enzymes in relation to old field succession: amylase, cellulase, invertase, dehydrogenase, and urease soil. Sci Soc Am J 37:47–50. https://doi.org/10.2136/sssaj1973.03615995003700010018x
Ribeiro SP et al (2017) Plant defense against leaf herbivory based on metal accumulation: examples from a tropical high altitude ecosystem. Plant Species Biol 32:147–155. https://doi.org/10.1111/1442-1984.12136
Ross DJ (1975) Studies on a climosequence of soils in tussock grasslands. 5. Invertase and amylase activities of topsoils and their relationships with other properties. J Sci 18:511–518
Salgado AAR, Carmo FF (2015) Quadrilátero Ferrífero: a beautiful and neglected landscape between the gold and iron ore reservoirs. In: Vieira BC, Salgado AAR, Santas L (eds) Landscapes and landforms of Brazil. Springer, London, pp 319–330
Sanchez-Canizares C et al (2011) Endosymbiotic bacteria nodulating a new endemic lupine Lupinus mariae-josephi from alkaline soils in Eastern Spain represent a new lineage within the Bradyrhizobium genus. Syst Appl Microbiol 34:207–215. https://doi.org/10.1016/j.syapm.2010.11.020
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169:18–29. https://doi.org/10.1016/j.micres.2013.08.009
Schaefer CE et al (2015) Solos desenvolvidos sobre canga ferruginosa no Brasil: uma revisão crítica e papel ecológico de termiteiros. In: Carmo FFD, Kamino LHY (eds) Geossistemas Ferruginosos do Brasil. 3i Editora Ltda, Belo Horizonte, pp 77–102
Schaefer CEGR et al (2016) The physical environment of Rupestrian Grasslands (Campos Rupestres) in Brazil: geological, geomorphological and pedological characteristics, and interplays. In: Fernandes GW (ed) Ecology and conservation of mountaintop grasslands in Brazil. Springer, Cham, pp 15–53. https://doi.org/10.1007/978-3-319-29808-5_2
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Analyt Biochem 160:47–56
Silveira FAO et al (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403:129–152. https://doi.org/10.1007/s11104-015-2637-8
Strauss ML, Jolly NP, Lambrechts MG, van Rensburg P (2001) Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J Appl Microbiol 91:182–190
Thoma JA, Spradlin JE, Dygert S (1971) Plant and animal amylases. In: Boyer PD (ed) The enzymes, vol 5, 3rd edn. Academic Press, New York, pp 115–189
Vacheron J et al (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356. https://doi.org/10.3389/fpls.2013.00356
Vlot AC, Klessig DF, Park SW (2008) Systemic acquired resistance: the elusive signal(s). Curr Opin Plant Biol 11:436–442. https://doi.org/10.1016/j.pbi.2008.05.003
Wang Y, Wang E, Wang D, Huang S, Ma Y, Smith CJ, Wang L (2010) Crop productivity and nutrient use efficiency as affected by long-term fertilisation in North China Plain. Nutr Cycl Agroecosyst 86:105–119. https://doi.org/10.1007/s10705-009-9276-5
Acknowledgements
Thanks to all members of the Laboratory of Biochemistry and Molecular Biology (LBBM, Federal University of Ouro Preto, UFOP) for their support. Thanks to the NanoLab Laboratory of the Department of Metalurgy of Mines, Federal University of Ouro Preto, for SEM and EDS analysis.
Funding
This work was supported by the following agencies: the National Council of Technological and Scientific Development (CNPq Process 481226/2013-3), Foundation of Protection to Research of the State of Minas Gerais—FAPEMIG (process APQ-02387-14), and Coordination for the Improvement of Higher Education Personnel (CAPES) (the BIGA Project, CFP 51/2013, process 3385/2013).
Author information
Authors and Affiliations
Contributions
EBF, LMM, FFC, and LHYK designed the work and selected the plant samples investigated. EBF, WLC, LMM, FFC, LHYK, and CGCL collected the plant samples. EBF, ITV, WLC, IFC, RABA, CGCL, NPF, and ABS performed all biochemical assays. EBF, JAF, JCCC, and CGCL performed the experiments in Citrus plants. EBF, LMM, ITV, FFC, CCMG, and LHYK interpreted findings. EBF and LMM wrote the paper. ITV, WLC, IFC, RABA, CGCL, NPF, ABS, JAF, JCCC, FFC, CCMG and LHYK contributed additional interpretations and general manuscript comments. EBF, LMM, RABA, and ABS revised the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Felestrino, É.B., Vieira, I.T., Caneschi, W.L. et al. Biotechnological potential of plant growth-promoting bacteria from the roots and rhizospheres of endemic plants in ironstone vegetation in southeastern Brazil. World J Microbiol Biotechnol 34, 156 (2018). https://doi.org/10.1007/s11274-018-2538-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2538-0