Abstract
Chromium (Cr) is a highly toxic metal for microorganisms as well as plants and animal cells. Due to its widespread industrial use, Cr has become a serious pollutant in diverse environmental settings. The hexavalent form of the metal, Cr(VI), is considered a more toxic species than the relatively innocuous and less mobile Cr(III) form. The study of the interactions between microorganisms and Cr has been helpful to unravel the mechanisms allowing organisms to survive in the presence of high concentrations of Cr(VI) and to detoxify and remove the oxyanion. Various mechanisms of interactions with Cr have been identified in diverse species of bacteria and fungi, including biosorption, bioaccumulation, reduction of Cr(VI) to Cr(III), and chromate efflux. Some of these systems have been proposed as potential biotechnological tools for the bioremediation of Cr pollution using bioreactors or by in situ treatments. In this review, the interactions of microorganisms with Cr are summarised, emphasising the importance of new research avenues using advanced methodologies, including proteomic, transcriptomic, and metabolomic analyses, as well as the use of techniques based on X-ray absorption spectroscopy and electron paramagnetic resonance spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium compounds are environmental contaminants present in ground water, soil and industrial effluents due to their extensive use in various industries (Madhavi et al. 2013). The trivalent Cr(III) and hexavalent Cr(VI) are the most stable and abundant forms in the environment (Cervantes et al. 2001); the common Cr(VI) species present in aqueous solution are hydrochromate (HCrO4 −), chromate (CrO4 2−) and dichromate (Cr2O7 2−), depending on the solution pH, the Cr(VI) concentration and on the redox potential. On the other hand, Cr(III) derivatives are much less mobile and exist in the environment mostly forming stable complexes with both organic and inorganic ligands; in aqueous solution at neutral pH, Cr(III) tend to associate with hydroxide OH− ions in a pH-dependent manner and precipitates as hydroxide [Cr(OH)3] or hydrated oxide (Cr2O·H2O) (Barrera-Díaz et al. 2012; Ramírez Díaz et al. 2008). The biological effects of Cr strongly depend on its oxidation state and cellular localization. Cr(VI) is considered the most toxic form of Cr for microorganisms and mammalian cells. In the intracellular medium Cr toxicity is mainly related to the process of reduction of Cr(VI) by the action of flavoenzymes or metabolites (i.e. ascorbic acid, glutathione) to produce the active intermediates Cr(V) and/or Cr(IV) and the reactive oxygen species (ROS) peroxide, hydrogen peroxide and hydroxyl radicals, with Cr(III) as the final product. ROS production causes oxidative damage to DNA, proteins and lipids and Cr(III) affects DNA replication, causes mutagenesis, and alters enzyme structure and activity by reacting with their carboxyl and thiol groups (Viti et al. 2014; Ramirez-Diaz et al. 2008). Bacteria of different genera (for example, Arthrobacter, Cupriavidus, Escherichia, Pseudomonas, Shewanella) and few cases of fungi (the yeast Saccharomyces cerevisiae) have been used in transcriptomic and/or proteomic studies on their responses to Cr(VI). Overall, the results obtained indicated changes in functions related to central metabolism and energy production and conversion, as well as effects on different transporters and DNA metabolism and repair (Viti et al. 2014).
Microbial interactions with chromium
Microbial mechanisms of the interaction with Cr are of both fundamental and biotechnological interest, because microorganisms have a variety of properties that can affect changes in Cr speciation, toxicity, and mobility. Therefore, these microbial mechanisms of Cr detoxification may serve as the basis for the development of new technologies to remove Cr from contaminated settings.
Some previous articles have reviewed the information published regarding the mechanisms of chromate resistance in microorganisms and/or those related to the enzymatic and non-enzymatic processes of Cr(VI) reduction (Cheung and Gu 2007; Ramírez-Diaz et al. 2008; Poljsak et al. 2010; Viti et al. 2014; Thatoi et al. 2014; Joutey et al. 2015). In this overview, our aim was to summarise the recent literature addressing the different microbial mechanisms of interactions with Cr, in an attempt to connect the basic biological processes to biotechnological applications.
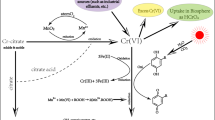
Microbial mechanisms of the interactions with Cr involve active or passive processes, which are schematically shown in Fig. 1. These processes include transport, accumulation, biosorption, and the enzymatic and non-enzymatic reduction of the oxyanion to Cr(III).
Chromium transport
Due to the structural similarity of Cr(VI) with SO4 2− anion, it can easily be transported across biological membranes via active sulphate transporters in bacteria and fungi (Cervantes et al. 2001) (Fig. 1). Another chromate transport system is represented by the ChrA protein, which belongs to the chromate ion transporter (CHR) superfamily that includes hundreds of homologues from all three domains of life. ChrA protein is composed of two families of sequences: the short-chain monodomain family and the long-chain bidomain family (Díaz-Pérez et al. 2007; Viti et al. 2014). The efflux of chromate is a resistance mechanism in bacteria conferred by the ChrA protein, which functions as a chemiosmotic pump that effluxes chromate from the cytoplasm using the proton motive force (Ramírez Díaz et al. 2008; Viti et al. 2014). ChrA homologues in fungi appear to function in an opposite manner to those in bacteria. In the filamentous fungus Neurospora crassa, the CHR-1 protein is a bidomain member of the CHR superfamily, which acts as a transporter of chromate favouring the accumulation of the oxyanion in cells, resulting in chromate sensitivity (Flores-Alvarez et al. 2012).
Besides the classic chromate transport systems such as sulphate (chromate) permeases, another chromate transport system was described in the yeast Saccharomyces cerevisiae, which is limited by actin-mediated endocytosis. This system was proposed to involve the ubiquitin-dependent endocytic inactivation of plasma membrane Cr transporter(s), independent of the sulphate (and chromate) permeases Sul1p and Sul2p (Holland and Avery 2009). Figure 1 summarises the action of these various chromate transport systems, highlighting the differences between bacteria and fungi.
By contrast, in microbial cells Cr(III) crosses cell membranes with low efficiency because it tends to form insoluble compounds. However, it has also been observed that microbial reduction of Cr(VI) in the extracellular medium may transiently generate soluble Cr(III) in the form of Cr3+ ion and/or hydroxyl complexes which can have deleterious effects on cells (Bencheikh-latmani et al. 2007). In any case, the transporter responsible for incorporating Cr(III) into microbial cells has not been identified to date, although this ion can be formed inside cells by either enzymatic or non-enzymatic Cr(VI) reduction (Fig. 1).
Bioaccumulation of chromium
Microorganisms are capable of incorporating metals into their cells through two processes, one called “passive uptake” or “biosorption” and the other known as “active uptake”. The former process is considered as the metabolism-independent accumulation of metals by living or inactive non-living biomass or biological materials, whereas active uptake, which occurs only in living cells, requires metabolism and energy for the transport of metals across the cell membrane into the cells. The combination of the active and passive modes of metal uptake is called “bioaccumulation” (Mohan and Pittman 2006; Wang and Chen 2009).
The cell wall of microorganisms is the first component that comes into contact with metal ions, and is therefore largely responsible for the biosorption of metals (Wang and Chen 2009). The cell wall chemical functional groups participating in Cr binding have been identified using different analytical techniques, including Fourier transform infrared spectroscopy (FTIR), scanning/transmission electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX/TEM-EDX), X-ray diffraction (XRD) analysis, X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS) (Mukherjee et al. 2013) (Table 1). For instance, the use of FTIR revealed the functional groups involved in Cr(VI) binding in the cell walls of the bacterium Streptomyces werraensis LD22, including amine, hydroxyl, and carboxyl. The same technique indicated that the functional groups involved in Cr(VI) binding in the cell walls of the fungus Aspergillus niger were hydroxyl, carboxyl, amino, and carbonyl (Table 1).
The adsorption coupled reduction of Cr(VI) is a process by which Cr(VI) is reduced to Cr(III) in the aqueous phase or in the biomass via contact with electron-donor groups of the biomass under an acidic pH (Park et al. 2006; Fig. 1); this process has been demonstrated in fungi (Park et al. 2007) and bacteria (Polti et al. 2011) using analytical techniques such as XPS, XAS, and SEM-EDX/TEM-EDX. However, several studies have also demonstrated that some bacteria species such as cyanobacteria (Ozturk and Aslim 2008), Azotobacter (Joshi and Juwarkar 2009), Arthrobacter (Shuhong et al. 2014), and Bacillus (Dogan et al. 2015), as well as fungal species such as Trichoderma (Chang et al. 2016) and Schwanniomyces (Mohite et al. 2015) produced exopolymeric substances (EPS) with the capacity to remove Cr(VI) by an adsorption coupled reduction process.
Recently, it has been reported that chromium oxide nanoparticle synthesis follows Cr(VI) reduction in the Bacillus cereus strain XMCr-6 (Dong et al. 2013) and the biosorption process in Bacillus subtilis cells (Annamalai et al. 2014) exposed to Cr(VI); in both studies, the size, morphology, and location of the biosynthesised nanoparticles were determined by SEM, TEM, XRD, or FTIR. In the yeast Schwanniomyces, the presence of EPS generates nanoparticles that show a coupled adsorption-reduction system (Mohite et al. 2015). Table 2 summarises the results obtained in the last 9 years on Cr removal by biosorption with bacteria and fungi.
Enzymatic reduction
In bacteria, the enzymatic Cr(VI) reduction system can be attributed to the catalysis performed either by soluble cytosolic proteins or insoluble cell membrane enzymes (Ramirez-Diaz et al. 2008; Thatoi et al. 2014; Viti et al. 2014) (Fig. 1). Numerous bacterial genera, including Pseudomonas, Bacillus, and Arthrobacter, have been widely reported to reduce Cr(VI) using an enzymatic process, either aerobically or anaerobically or under both conditions (Thatoi et al. 2014; Viti et al. 2014). In Pseudomonas species, the genes related to Cr(VI) reduction are located in plasmids, whereas in several bacilli and Enterobacteriaceae species, these genes are located on the chromosomal DNA (Thatoi et al. 2014).
Some bacterial chromate reductases such as ChrR, YieF, NemA, and LpDH have been identified, which are located either in soluble fractions (cytoplasm) or bound to the membrane of the bacterial cell; the conditions under which these enzymes are functional can be aerobic or anaerobic, or sometimes both.
Chromate reductase activity has been studied in the fungi Penicillium sp. (Arévalo-Rangel et al. 2013) and A. niger (Gu et al. 2015). In both cases, it was found that the chromate reductase was mainly located in the soluble fraction of cells. In Aspergillus flavus, chromate reductase activity is accompanied by a biosorption process, indicating that there is a complex remediation mechanism of Cr(VI), which includes an interconnection of different interactions with the metal (Singh and Bishnoi 2015). Cr(VI) reduction can also be achieved indirectly by enzymes such as glucose oxidase from A. niger, which produces molecules such as gluconolactone and hydrogen peroxide that can reduce Cr(VI) to Cr(III) (Romo-Rodríguez et al. 2015) (Fig. 1).
In addition, some recent studies have focused on determining the ability of Cr(VI) reduction by chromate reductase enzymes present in the supernatant of microbial cultures. Rath et al. (2014) reported high extracellular chromate reductase activity under an optimised set of conditions from a Bacillus amyloliquefaciens strain isolated from chromite mine soil. An isolate of Arthrobacter sp. from this same environment showed extracellular chromate reductase activity to reduce the Cr(VI) present in the chromite mine effluent (Dey and Paul 2016). In B. cereus RMLAU1, which catalyses the co-remediation of pentachlorophenol and chromate, chromate reductase activity was observed in the culture supernatant, cytosolic fraction, as well as in cell debris under aerobic conditions; the maximum enzyme activity was found in the cytosolic fraction (48 %), followed by that in the culture supernatant (39.7 %) and cell debris (12.3 %) (Tripathi et al. 2013).
Non-enzymatic reduction
Reduction of Cr(VI) may take place by chemical reactions associated with compounds present in intra/extracellular compounds produced during microbial metabolism, including amino acids, nucleotides, sugars, vitamins, organic acids, or glutathione (Cervantes et al. 2001; Dhal et al. 2013) (Fig. 1). For instance, ascorbate is capable of reducing Cr(VI), and the riboflavin derivatives FAD and FMN are essential coenzymes for chromate-reducing flavoenzymes (Cervantes et al. 2001). In addition, it is well known that Fe(II) and H2S, the anaerobic metabolic end products of iron- and sulphate-reducing bacteria (SRB), can reduce hexavalent Cr, both individually and in combination (Somasundaram et al. 2009).
In bacteria, direct Cr(VI) reduction has been demonstrated by means of the production of the siderophore pyridine-2,6-bis (thiocarboxylic acid) (pdtc) by Pseudomonas stutzeri KC, which reduces 86 % of hexavalent Cr. In addition, the pdtc hydrolysis products pyridine-2-carboxylic-6-thiocarboxylic acid and dipicolinic acid also reduce Cr(VI) (Zawadzka et al. 2007).
Excreted fungal metabolites such as the organic acids citrate (Barrera-Diaz et al. 2012) and oxalate (Barrera-Diaz et al. 2012; Wrobel et al. 2015) have also been reported to participate in the reduction of Cr(VI) through the photocatalytic effect of Fe(III) or by Mn without light (Fig. 1). The importance of this process in a biological context was illustrated by the finding that the extracellular reduction of Cr(VI) to Cr(III) in the presence of Mn2+ is negatively affected in the oxalic acid-non producer mutants of Aspergillus tubingensis Ed8 (Fig. 2) (unpublished data).
Cr(VI) reduction in A. tubingensis Ed8 and the null mutants Δoah IT4-9 and IT5-13. The Cr(VI) reduction capacity of wild-type A. tubingensis Ed8 (black squares) and its derived null mutants in the oah gene, IT4-9 (dark grey squares) and IT5-13 (light grey squares), at 24 h of culture was determined by the diphenyl-carbazide method. The culture medium was supplemented with 30 mg L−1 Cr(VI) and 5 mM MnCl2
Biotechnological applications
Of the different mechanisms of microbial interaction with chromium biosorption and bioreduction are those that have been considered for the development of biotechnological strategies for the removal of Cr from the environment. As shown in Table 3, several systems have been implemented for use in different types of reactors for Cr removal, using monocultures or biomass of bacterial or fungal strains, as well as consortia of different bacteria or fungi. The use of microbial consortia in some of the systems relies on the notion that consortia of microorganisms are metabolically superior for removing metals and are more suitable for field applications (Joutey et al. 2015). Table 3 shows the results of an ex situ study for Cr(VI) bioremediation using a native consortium of SRB carried out in an up-flow anaerobic sludge bed bioreactor, which showed a 90 % removal efficiency by reduction of 50 mg L−1 Cr(VI) (Qian et al. 2016). Table 3 also highlights the use of a consortium of bacteria (Acinetobacter junii, Escherichia coli, B. subtilis) incubated in a continuous packed bed column reactor, which improved the Cr(VI)-reducing capability as compared to that of a batch reactor. In several Cr-contaminated environmental water matrices with 100 mg L−1 Cr(VI), the consortium showed 55–65 % efficiency of Cr(VI) removal by adsorption (Samuel et al. 2013).
Another biotechnological process of interest is based on the bacterial cells and enzymes immobilised in different polymer matrices such as agar layers, polyhydroxyalkanoate granules, calcium alginate, alginate beads, and poly(vinyl alcohol) alginate, which have proven to be effective for Cr(VI) reduction. Table 3 shows the performance of the ChromeBac™ system, based on the use of Acinetobacter haemolyticus EF369508, which was immobilised onto rubber wood sawdust inside a bioreactor; after 90 % of the initial Cr(VI) (30–60 mg L−1) was reduced, the residual Cr(VI) was reduced to between 1.0 and 1.5 mg L−1 by immobilised chromate reductase alginate beads packed in a flow-through column (Ishak et al. 2016).
Two types of bioreactors, a stirred tank bioreactor and a concentric tube airlift bioreactor, were compared for the removal efficiency of Cr by the filamentous fungus Trichoderma viride, and the results showed that the airlift bioreactor might be a promising alternative, especially when shear-sensitive microorganisms are used (Morales-Barrera and Cristiani-Urbina 2006) (Table 3). The ability of the yeast Candida tropicalis to remove Cr was tested in artificially contaminated soils in a microcosm system to simulate natural environmental conditions, showing reduction of 72.2 % of 40 mg L−1 Cr(VI) (Bahafid et al. 2013). An A. niger strain isolated from a tannery was used in an airlift reactor for the treatment of tanning wastewater, leading to maximum removal efficiency by adsorption of 88 % of an initial Cr(III) concentration of 1300 mg L−1 (Sepehr et al. 2012a). These results are even better than those obtained (removal efficiency of 72 %) with the same organism using a stirred tank reactor (Sepehr et al. 2012b). The use of a consortium of Cr-resistant fungi immobilised in a support material in a stirred tank bioreactor filled with wastewater from a tannery achieved an overall removal efficiency of 99.9 % of the total Cr present (Sharma and Malaviya 2016).
Other systems with potential applications in chromium removal
As mentioned above, the application of immobilised cells in Cr bioremediation has proven to be satisfactory in some cases. This process has also been favoured along with the development of nanotechnology. Pang et al. (2011) demonstrated that carbon nanotubes impregnated into Ca-alginate beads, which were used to immobilise Pseudomonas aeruginosa cells, markedly enhanced the stability of the enzyme and the process of Cr(VI) reduction. Another process related to nanotechnology is the mobilisation and immobilisation of metal ions by bacteria at the nanometer scale. In this regard, a process for remediating Cr-contaminated sites has been developed by applying metal-reducing bacteria in combination with nano-minerals with a size ranging from 10 to 50 nm [siderite (FeIICO3)], which were obtained from acid mine drainage and act as electron donors for the catalysis of Cr(VI) reduction and immobilisation (Cr(III)-containing precipitates) (Seo and Roh 2015).
In relation to cell-immobilisation processes, Robins et al. (2013) applied genetic engineering techniques to develop a system for Cr(VI) remediation based in an immobilised chromate reductase. They constructed a fusion of the E. coli nemA gene and the polyhydroxyalkanoate synthase gene phaC from Ralstonia eutropha, which enabled the high-level biosynthesis of functionalised polyhydroxyalkanoate granules displaying stable and active NemA chromate reductase on their surface. They proposed that this system offers good promise as an economic solution for ex situ Cr(VI) remediation. In the same sense, Barak et al. (2006) obtained an E. coli strain that produces a mutant enzyme termed ChrR6. This enzyme was obtained by a directed evolution approach through the error-prone polymerase chain reaction technique, and showed 200-fold greater chromate-reducing activity than the wild-type E. coli ChrR enzyme. Collectively, these studies demonstrate that enzymes from genetically modified bacteria represent a promising approach for the bioremediation of Cr(VI)-contaminated wastewater over a wide range of environmental conditions.
Concluding remarks
Bacteria and fungi display diverse mechanisms of interactions with Cr. The uptake of chromate through the sulphate transporter is a common process in bacteria and fungi; however, these organisms appear to differ with respect to the function of the chromate transporter homologous proteins ChrA/ChrR. Both bacteria and fungi show Cr(VI)-reducing capability, although the pathways of enzymatic and non-enzymatic Cr(VI) reduction have been predominantly studied in bacteria, and the location and identity of the enzymes and metabolic processes involved have been elucidated. Nevertheless, a few studies have described Cr(VI)-reducing activities and metabolic processes in fungi. Bacteria and fungi have the ability to accumulate Cr in the biomass, and the cell wall functional groups involved in Cr binding have been determined in several cases. Cr biosorption and bioreduction are the primary mechanisms that have attracted attention for the development of biotechnological processes for Cr removal. Several systems have implemented the use of different types of reactors for Cr removal using monocultures or consortia of bacteria or fungi. The application of immobilised microbial cells combined with the development of nanomaterials is facilitating development of processes for Cr remediation. In this context, studies have been performed with recombinant microorganisms overexpressing the chromate reductase and other enzymes.
References
Abigail EA, Samuel MS, Chidambaram R (2015) Hexavalent chromium biosorption studies using Penicillium griseofulvum MSR1 a novel isolate from tannery effluent site: Box–Behnken optimization, equilibrium, kinetics and thermodynamic studies. J Taiwan Inst Chem E 49:156–164
Ahmad WA, Zakaria ZA, Khasim AR et al (2010) Pilot-scale removal of chromium from industrial wastewater using the ChromeBac™ system. Bioresour Technol 101:4371–4378
Annamalai K, Nair AM, Chinnaraju S, Kuppusamy S (2014) Removal of chromium from contaminated effluent and simultaneously green nanoparticle synthesis using Bacillus subtilis. Malaya J Biosci 1:13–18
Arévalo-Rangel DL, Cárdenas-González JF, Martínez-Juárez VM, Acosta-Rodríguez I (2013) Hexavalent chromate reductase activity in cell free extracts of Penicillium sp. Bioinorg Chem Appl 2013:1–6
Bahafid W, Joutey NT, Sayel H et al (2013) Bioaugmentation of chromium-polluted soil microcosms with Candida tropicalis diminishes phytoavailable chromium. J Appl Microbiol 115:727–734
Balaji R, David E (2016) Isolation and characterization of Cr(VI) reducing bacteria and fungi their potential use in bioremediation of chromium containing tannery effluent (Ambur and Ranipet, Vellore dist, Tamilnadu). Adv Res J Life Sci 2:1–4
Bankar AV, Kumar AR, Zinjarde SS (2009) Removal of chromium (VI) ions from aqueous solution by adsorption onto two marine isolates of Yarrowia lipolytica. J Hazard Mater 170:487–494
Barak Y, Ackerley DF, Dodge CJ et al (2006) Analysis of novel soluble chromate and uranyl reductases and generation of an improved enzyme by directed evolution. Appl Environ Microb 72:7074–7082
Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B (2012) A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J Hazard Mater 223:1–12
Barsainya M, Chandra P, Singh DP (2016) Investigation of Cr(VI) uptake in saline condition using psychrophilic and mesophilic Penicillium sp. Int J Curr Microbiol Appl Sci 5:274–288
Bencheikh-Latmani R, Obraztsova A, Mackey MR, Ellisman MH, Tebo BM (2007) Toxicity of Cr(III) to Shewanella sp. strain MR-4 during Cr(VI) reduction. Environ Sci Technol 41:214–220
Cervantes C, Campos-García J, Devars S et al (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Chang F, Tian C, Liu S et al (2016) Discrepant hexavalent chromium tolerance and detoxification by two strains of Trichoderma asperellum with high homology. Chem Eng J 298:75–81
Chatterjee S, Ghosh I, Mukherjea KK (2011) Uptake and removal of toxic Cr(VI) by Pseudomonas aeruginosa: physico-chemical and biological evaluation. Curr Sci India 101:645–652
Cheung KH, Gu JD (2007) Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeterior Biodegrad 59(1):8–15
Coreño-Alonso A, Solé A, Diestra E, Esteve I, Gutiérrez-Corona JF, Reyna López GE, Fernández FJ, Tomasini A (2014) Mechanisms of interaction of chromium with Aspergillus niger var tubingensis strain Ed8. Bioresour Technol 158:188–192
Dey S, Paul AK (2016) Evaluation of chromate reductase activity in the cell-free culture filtrate of Arthrobacter sp. SUK 1201 isolated from chromite mine overburden. Chemosphere 156:69–75
Dhal B, Thatoi HN, Das NN et al (2013) Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J Hazard Mater 250:272–291
Díaz-Pérez C, Cervantes C, Campos-García J et al (2007) Phylogenetic analysis of the chromate ion transporter (CHR) superfamily. FEBS J 274:6215–6227
Dogan NM, Doganli GA, Dogan G et al (2015) Characterization of extracellular polysaccharides (EPS) produced by thermal Bacillus and determination of environmental conditions affecting exopolysaccharide production. Int J Environ Res 9:1107–1116
Dong G, Wang Y, Gong L et al (2013) Formation of soluble Cr(III) end-products and nanoparticles during Cr(VI) reduction by Bacillus cereus strain XMCr-6. Biochem Eng J 70:166–172
Flores-Alvarez L, Corrales-Escobosa A, Cortés-Penagos C et al (2012) The Neurospora crassa chr-1 gene is up-regulated by chromate and its encoded CHR-1 protein causes chromate sensitivity and chromium accumulation. Curr Genet 58:281–290
Gu Y, Xu W, Liu Y et al (2015) Mechanism of Cr(VI) reduction by Aspergillus niger: enzymatic characteristic, oxidative stress response, and reduction product. Environ Sci Pollut R 22:6271–6279
Habibul N, Hu Y, Wang YK et al (2016) Bioelectrochemical chromium (VI) removal in plant-microbial fuel cells. Environ Sci Technol 50:3882–3889
Holland SL, Avery SV (2009) Actin-mediated endocytosis limits intracellular Cr accumulation and Cr toxicity during chromate stress. Toxicol Sci 111:437–446
Ishak AF, Karim NA, Ahmad WA et al (2016) Chromate detoxification using combination of ChromeBac™ system and immobilized chromate reductase beads. Int Biodeterior Biodegrad 113:238–243
Joshi PM, Juwarkar AA (2009) In vivo studies to elucidate the role of extracellular polymeric substances from Azotobacter in immobilization of heavy metals. Environ Sci Technol 43:5884–5889
Joutey NT, Sayel H, Bahafid W, El Ghachtouli N (2015) Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev Environ Contam Toxicol 233:45–69
Kathiravan MN, Rani RK, Karthick R et al (2010) Mass transfer studies on the reduction of Cr(VI) using calcium alginate immobilized Bacillus sp. in packed bed reactor. Bioresour Technol 101:853–858
Khambhaty Y, Mody K, Basha S, Jha B (2009) Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus niger. Chem Eng J 145:489–495
Krishna KR, Philip L (2005) Bioremediation of Cr(VI) in contaminated soils. J Hazard Mater 121(1):109–117
Latha S, Vinothini G, Dhanasekaran D (2015) Chromium [Cr(VI)] biosorption property of the newly isolated actinobacterial probiont Streptomyces werraensis LD22. Biotech 5:423–432
Liu H, Huang J, Zhang S et al (2015) Chromate interaction with the chromate reducing actinobacterium intrasporangium chromatireducens Q5-1. Geomicrobiol J 32:616–623
Madhavi V, Reddy AVB, Reddy KG et al (2013) An overview on research trends in remediation of chromium. Res J Recent Sci 2:71–83
Marandi R (2011) Biosorption of hexavalent chromium from aqueous solution by dead fungal biomass of Phanerochaete crysosporium: batch and fixed bed studies. Can J Chem Eng Technol 2:8–22
Mohan D, Pittman CU (2006) Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J Hazard Mater 137(2):762–811
Mohite PT, Kumar AR, Zinjarde SS (2015) Biotransformation of hexavalent chromium into extracellular chromium (III) oxide nanoparticles using Schwanniomyces occidentalis. Biotechnol Lett 2015:1–6
Morales-Barrera L, Cristiani-Urbina E (2006) Removal of hexavalent chromium by Trichoderma viride in an airlift bioreactor. Enzyme Microb Technol 40:107–113
Mukherjee K, Saha R, Ghosh A et al (2013) Chromium removal technologies. Res Chem Intermediat 39(6):2267–2286
Naik UC, Srivastava S, Thakur IS (2012) Isolation and characterization of Bacillus cereus IST105 from electroplating effluent for detoxification of hexavalent chromium. Environ Sci Pollut Res Int 19:3005–3014
Ozturk S, Aslim B (2008) Relationship between chromium(VI) resistance and extracellular polymeric substances (EPS) concentration by some cyanobacterial isolates. Environ Sci Pollut Res 15:478–480
Pang Y, Zeng GM, Tang L et al (2011) Cr(VI) reduction by Pseudomonas aeruginosa immobilized in a polyvinyl alcohol/sodium alginate matrix containing multi-walled carbon nanotubes. Bioresour Technol 102:10733–10736
Park D, Yun YS, Park JM (2005) Use of dead fungal biomass for the detoxification of hexavalent chromium: screening and kinetics. Process Biochem 40:2559–2565
Park D, Yun YS, Park JM (2006) Mechanisms of the removal of hexavalent chromium by biomaterials or biomaterial-based activated carbons. J Hazard Mater 137(2):1254–1257
Park D, Lim SR, Yun YS et al (2007) Reliable evidences that the removal mechanism of hexavalent chromium by natural biomaterials is adsorption-coupled reduction. Chemosphere 70:298–305
Paul ML, Samuel J, Chandrasekaran N et al (2012) Comparative kinetics, equilibrium, thermodynamic and mechanistic studies on biosorption of hexavalent chromium by live and heat killed biomass of Acinetobacter junii VITSUKMW2, an indigenous chromite mine isolate. Chem Eng J 187:104–113
Poljsak B, Pócsi I, Raspor P et al (2010) Interference of chromium with biological systems in yeasts and fungi: a review. J Basic Microb 50:21–36
Polti MA, Amoroso MJ, Abate CM (2011) Intracellular chromium accumulation by Streptomyces sp. MC1. Water Air Soil Pollut 214:49–57
Qian J, Wei L, Liu R et al (2016) An exploratory study on the pathways of Cr(VI) reduction in sulfate-reducing up-flow anaerobic sludge bed (UASB) reactor. Sci Rep 6:23694
Ramírez-Díaz M, Díaz-Pérez C, Vargas E et al (2008) Mechanisms of bacterial resistance to chromium compounds. Biometals 21:321–332
Ramrakhiani L, Majumder R, Khowala S (2011) Removal of hexavalent chromium by heat inactivated fungal biomass of Termitomyces clypeatus: surface characterization and mechanism of biosorption. Chem Eng J 171:1060–1068
Rath BP, Das S, Mohapatra PKD et al (2014) Optimization of extracellular chromate reductase production by Bacillus amyloliquefaciens (CSB 9) isolated from chromite mine environment. Biocatal Agric Biotechnol 3:35–41
Robins KJ, Hooks DO, Rehm BH et al (2013) Escherichia coli NemA is an efficient chromate reductase that can be biologically immobilized to provide a cell free system for remediation of hexavalent chromium. PLoS One 8:e59200
Romo-Rodríguez P, Acevedo-Aguilar FJ, Lopez-Torres A et al (2015) Cr(VI) reduction by gluconolactone and hydrogen peroxide, the reaction products of fungal glucose oxidase: cooperative interaction with organic acids in the biotransformation of Cr(VI). Chemosphere 134:563–570
Samuel J, Pulimi M, Paul ML et al (2013) Batch and continuous flow studies of adsorptive removal of Cr(VI) by adapted bacterial consortia immobilized in alginate beads. Bioresour Technol 128:423–430
Samuel MS, Abigail MEA, Ramalingam C (2015) Isotherm modelling, kinetic study and optimization of batch parameters using response surface methodology for effective removal of Cr(VI) using fungal biomass. PLoS One 10:e0116884
Seo H, Roh Y (2015) Biotransformation and its application: biogenic nano-catalyst and metal-reducing-bacteria for remediation of Cr(VI)-contaminated water. J Nanosci Nanotechnol 15:5649–5652
Sepehr MN, Nasseri S, Zarrabi M et al (2012a) Removal of Cr(III) from tanning effluent by Aspergillus niger in airlift bioreactor. Sep Purif Technol 96:256–262
Sepehr MN, Zarrabi M, Amrane A (2012b) Removal of CR (III) from model solutions by isolated Aspergillus niger and Aspergillus oryzae living microorganisms: equilibrium and kinetic studies. J Taiwan Inst Chem E 43:420–427
Sharma S, Malaviya P (2016) Bioremediation of tannery wastewater by chromium resistant novel fungal consortium. Ecol Eng 91:419–425
Shroff KA, Vaidya VK (2012) Effect of pre-treatments on the biosorption of Chromium (VI) ions by the dead biomass of Rhizopus arrhizus. J Chem Technol Biotechnol 87:294–304
Shuhong Y, Meiping Z, Hong Y et al (2014) Biosorption of Cu(2+), Pb(2+) and Cr(6+) by a novel exopolysaccharide from Arthrobacter ps-5. Carbohydr Polym 101:50–56
Silva B, Figueiredo H, Quintelas C et al (2012) Improved biosorption for Cr(VI) reduction and removal by Arthrobacter viscosus using zeolite. Int Biodeterior Biodegrad 74:116–123
Singh R, Bishnoi NR (2015) Biotransformation dynamics of chromium (VI) detoxification using Aspergillus flavus system. Ecol Eng 75:103–109
Somasundaram V, Philip L, Bhallamudi SM (2009) Experimental and mathematical modeling studies on Cr(VI) reduction by CRB, SRB and IRB, individually and in combination. J Hazard Mater 172:606–617
Sukumar C, Janaki V, Kamala-Kannan K, Shanthi K (2014) Biosorption of chromium(VI) using Bacillus subtilis SS-1 isolated from soil samples of electroplating industry. Clean Technol Environ Policy 16:405–413
Thatoi H, Das S, Mishra J et al (2014) Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J Environ Manage 146:383–399
Tripathi M, Garg SK (2013) Co-remediation of pentachlorophenol and Cr6+ by free and immobilized cells of native Bacillus cereus isolate: spectrometric characterization of PCP dechlorination products, bioreactor trial and chromate reductase activity. Process Biochem 48:496–509
Viti C, Marchi E, Decorosi F, Giovannetti L (2014) Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Microbiol Rev 38:633–659
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wrobel K, Escobosa ARC, Ibarra AAG et al (2015) Mechanistic insight into chromium (VI) reduction by oxalic acid in the presence of manganese (II). J Hazard Mater 300:144–152
Xie P, Hao X, Mohamad OA et al (2013) Comparative study of chromium biosorption by Mesorhizobium amorphae strain CCNWGS0123 in single and binary mixtures. Appl Biochem Biotechnol 169:570–587
Yahya SK, Zakaria ZA, Samin J et al (2012) Isotherm kinetics of Cr(III) removal by non-viable cells of Acinetobacter haemolyticus. Colloids Surf B Biointerfaces 1:362–368
Zawadzka AM, Crawford RL, Paszczynski AJ (2007) Pyridine-2,6-bis(thiocarboxylic acid) produced by Pseudomonas stutzeri KC reduces chromium(VI) and precipitates mercury, cadmium, lead and arsenic. Biometals 20:145–158
Ziagova M, Dimitriadis G, Aslanidou D et al (2007) Comparative study of Cd(II) and Cr(VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour Technol 98:2859–2865
Acknowledgments
This work was funded by CONACyT, México, Project CB-2014-01 registry number 239575 and by DAIP Universidad de Guanajuato, México, Project FO-DAI-05.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutiérrez-Corona, J.F., Romo-Rodríguez, P., Santos-Escobar, F. et al. Microbial interactions with chromium: basic biological processes and applications in environmental biotechnology. World J Microbiol Biotechnol 32, 191 (2016). https://doi.org/10.1007/s11274-016-2150-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2150-0