Abstract
Background, aim, and scope
Chromium(VI) resistance and its association with extracellular polymeric substance (EPS) concentration in cyanobacteria was investigated. Increased EPS concentration was associated with Cr(VI) resistance. The most resistant isolate, Chroococcus sp. H4, secreted the most EPS (427 mg/L).
Materials and methods
EPS concentration of the two most resistant isolates (Chroococcus sp. H4 and Synechocystis sp. S63) was investigated following exposure to 15 and 35 ppm Cr(VI). The composition of EPS produced by Chroococcus sp. H4 following exposure to 10 ppm Cr(VI) was analyzed using high-performance liquid chromatography. Control EPS was composed of glucose (99%) and galactronic acid (1%); in the presence of 10 ppm Cr(VI), EPS composition changed to glucose (9%), xylose (75%), rhamnose (14%), and galacturonic acid (2%).
Results and discussion
Results indicated that (1) exposure to elevated concentrations of Cr(VI) affected the composition of EPS produced by Chroococcus sp. H4, and (2) there was a correlation between Cr(VI) resistance and EPS concentration in some cyanobacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background, aim, and scope
Chromium is a heavy metal used extensively in industry, and high levels contaminate both terrestrial and aquatic habitats. Toxic effects of chromium on bacteria and algae have been reviewed by Wong and Trevors (1988). Many microorganisms are capable of secreting high molecular-mass polymers that can either be released into the surrounding environment [extracellular polysaccharides (EPS), exopolysaccharides] or remain attached to the cell surface (capsular polysaccharides). These polysaccharides are believed to protect bacterial cells from desiccation, heavy metals, or other environmental stresses by producing biofilms, thus enhancing the cells chances of colonizing special ecological habitats (Kazy et al. 2002). EPSs have found a wide range of applications in industries. Generally, they may replace polysaccharides used in the food industry as thickeners, stabilizers, emulsifiers, bodying agents, foam enhancers, and gelling agents (Ruas-Madeido et al. 2002). Cyanobacteria are important organisms in water that have a cell surface consisting of polysaccharides, proteins, and lipids that may act as a basic binding site of heavy metals. Studies on exopolysaccharides from cyanobacteria have dealt largely with chemical composition and their protective role (Panof et al. 1988). This study was designed to determine the correlation between metal resistance and EPS concentration of some cyanobacterial isolates isolated from different freshwaters of Turkey and to investigate the effect of Cr(VI) concentrations on EPS concentration, and in particular, on monosaccharide composition of EPS.
2 Materials and methods
Samples were isolated from different freshwaters of Turkey and isolates were maintained in BG-11 medium [NaNO3, 15; K2HPO4, 0.4; MgSO4·7H2O, 0.75; CaCl2·2H2O, 0.36; citric acid, 0.06; iron(III) ammonium citrate, 0.06; Na2–EDTA, 0.01; Na2CO3, 0.2 g/L, 1 mL; trace elements solution, (H3BO3, 61; MnSO4·H2O, 169; ZnSO4·7H2O, 287; CuSO4·5H2O, 2.5; (NH4)6Mo7O24·4H2O, 12.5 mg/L) pH 6.8; Rippka et al. 1979] for Cr(VI) resistance and for EPS concentration. Cultures were incubated at 22°C to 25°C with light/dark cycle of 12/12 h by using an incubator shaker (MINITRON) for 12 days (light intensity period: 12,000 μmol m−2 s−1). Cr(VI) resistance of cyanobacterial cultures was investigated by determining chlorophyll-a (Hirschberg and Chamovitz 1994) every 48 h, during 12 days. Five, 15, and 35 ppm Cr(VI) concentrations were prepared by dissolving K2Cr2O7 salt (Merck) in distilled water. Also, flasks containing medium lacking Cr(VI) were inoculated in the same manner to serve as controls. The EC50 was determined by probit analysis (Finney 1971). EPS was extracted by the modified procedure of Cérantola et al. (2000). Total carbohydrate contents of the EPS samples were determined by the method of Dubois et al. (1956) using glucose as a reference standard. The monosaccharide composition of freeze-dried exopolysaccharides samples was determined with high-performance liquid chromatography (HPLC; VARIAN ProStar) by using Metacarb 87H column (300 × 7.8 mm, Cat No. 5210). Monomer analyses of EPS were carried out by Middle East Technical University, Central Laboratory, Molecular Biology and Biotechnology R&D Center. To determine the effect of Cr(VI) on EPS concentration, Chroococcus sp. H4 and Synechocystis sp. S63 were incubated with 15 and 35 ppm Cr(VI) concentrations for 5 days, and final biomass of the isolates were equaled by determining chlorophyll-a. EPS was isolated as described earlier. Results of each representative experiment were analyzed by ANOVA. P values smaller than 0.05 are considered significant.
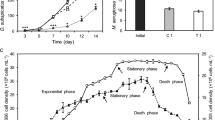
3 Results
Ten cyanobacterial isolates from different freshwaters of Turkey were obtained. The isolates, original habitats, EC50, and EPS values are listed in Table 1. The EC50 values of the ten strains ranged from 1.5 to 10.7 ppm. Chroococcus sp. H4 (EC50 of 10.7 ppm) was the isolate most resistant to Cr(VI). Microcystis sp. S80 (EC50 of 1.5 ppm) was the most sensitive to Cr(VI). EPS concentration of the ten strains ranged from 427 to 108 mg/L. Chroococcus sp. H4 (427 mg/L) and Synechocystis sp. S63 (418 mg/L) were high EPS-producing isolates. Microcystis sp. S80 (108 mg/L) was low EPS-producing isolate. The sugar monomer make-up was characterized of the EPSs produced by Chroococcus sp. H4 with and without exposure to Cr(VI) and quantified the monomer content by HPLC. In the absence of Cr(VI) exposure, the EPSs were composed mainly of glucose (99%) and a very small amount of galacturonic acid (1%). Following Cr(VI) exposure, EPSs were composed mainly of xylose (75%) and small amounts of glucose (9%), rhamnose (14%), and galacturonic acid (2%). Mannose, galactose, arabinose, ribose, and glucuronic acid were not detected in the EPSs produced by Chroococcus sp. H4 with or without Cr(VI) exposure. Final biomasses of isolates (H4 and S63) were equaled before determining the effect of Cr(VI) on EPS concentration. A significant and regular increase in the concentration of EPS by both Chroococcus sp. H4 (ANOVA; F 2,3 = 25.92; P = 0.013) and Synechocystis sp. S63 (ANOVA; F 2,3 = 22.58; P = 0.016) was observed. EPS concentrations of Chroococcus sp. H4 at control, 15, and 35 ppm Cr(VI) were determined as 477 ± 2, 482 ± 2, and 510 ± 6 mg/L respectively and 457 ± 6, 497 ± 8, 525 ± 5 mg/L for Synechocystis sp. S63.
4 Discussion
In this study, Cr(VI)-resistant isolates produced high amounts of EPS, and sensitive isolates produced low amounts of EPS. Kazy et al. (2002) reported that EPS production by a copper-resistant isolate of Pseudomonas aeruginosa was considerably higher than its copper-sensitive counterpart. The monosaccharide most frequently found in the cyanobacterial EPSs is glucose (in more than 90% of the polymers; Bar-Or and Shilo 1987). Glucose was the most common monosaccharide detected in this study, but the ratio of glucose to other monosaccharides decreased following cyanobacteria exposure to Cr(VI), Chroococcus sp. H4 in particular. Conversely, xylose was a major sugar in Chroococcus sp. H4 treated with Cr(VI), but xylose was not detected in the controls. Additionally, only one acidic sugar was found in both control and Cr(VI)-treated Chroococcus sp. H4. Priester et al. (2006) also detected differences in the monosaccharide composition of EPS in Cr(VI)-exposed Pseudomonas putida. Differences in the monosaccharide composition and values of EPS may promote heavy-metal resistance in these microorganisms. The EPS concentration from both isolates (H4 and S63) was considerably higher than control and was observed to have a good correlation between Cr(VI) exposure and EPS concentration in both isolates. A similar correlation was detected by Fang et al. (2002). In sulfate-reducing bacterial biofilms, exposure to trivalent Cr resulted in a nearly 82% increase in extracellular carbohydrate. Many different environmental stresses increase production of extracellular carbohydrates (Priester et al. 2006). Here, it was shown that Cr(VI) is an important stress factor that increases EPS concentration in cyanobacteria.
5 Conclusions
Very little comparative information about the effect of Cr(VI) on EPS concentration and composition is available, especially in case of practical applications, indicating the need for more research in this area. Ultimately, our results suggest a potential to exploit EPS-producing cyanobacteria for the production of a wide range of biopolymers suited to various industrial and environmental applications such as gelling agents and heavy-metal biosorption.
References
Bar-Or Y, Shilo M (1987) Characterization of macromolecular flocculants produced by Phormidium sp strain J-1 and by Anabaenopsis circularis. PCCs. Appl Environ Microb 53:2226–2230r
Cérantola S, Bounéry JD, Segonds C, Marty N, Montrozier H (2000) Exopolysaccharide production by mucoid and non-mucoid strains of Burkholderia cepacia. FEMS Microbiol Lett 185:243–246
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric methods for determination of sugars and related substances. Anal Chem 28:350–356
Fang HHP, Xu LC, Chan KY (2002) Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Res 36:4709–4716
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge
Hirschberg J, Chamovitz D (1994) Carotenoids in cyanobacteria. In: Bryant D (ed) The molecular biology of cyanobacteria. Kluwer, Dordrecht, pp 559–579
Kazy SK, Sar P, Singh SP, Sen AK, D’Souza SF (2002) Extracellular polysaccharides of a copper-sensitive and copper-resistant Pseudomonas aeruginosa strain: synthesis, chemical nature and copper binding. World J Microb Biot 18:583–588
Panof JM, Priem B, Morvan H, Joset F (1988) Sulphated exopolysaccharides produced by two unicellular strains of cyanobacteria, Synechocystis sp. PCC 6803 and 6714. Arch Microbiol 150:558–563
Priester JH, Olson SG, Webb SM, Neu MP, Hersman LE, Holden PA (2006) Enhanced exopolymer production and chromium stabilization in Pseudomonas putida unsaturated biofilms. Appl Environ Microb 72(3):1988–1996
Rippka R, Deruelles J, Waterbury JB, Herdman MR, Stanier Y (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Ruas-Madiedo P, Hugenholtz J, Zoon P (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12:163–171
Wong PT, Trevors JT (1988) Chromium toxicity to algae and bacteria. In: Nriagu J, Nieboer E (eds) Chromium in the natural and human environments. Wiley, New York, pp 305–315
Acknowledgment
This research was supported by the Gazi University B.A.P. (05/2005-31).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Lee Young
Rights and permissions
About this article
Cite this article
Ozturk, S., Aslim, B. Relationship between chromium(VI) resistance and extracellular polymeric substances (EPS) concentration by some cyanobacterial isolates. Environ Sci Pollut Res 15, 478–480 (2008). https://doi.org/10.1007/s11356-008-0027-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-008-0027-y