Abstract
Non-Saccharomyces yeasts are metabolically active during grape must fermentations and can contribute with enzymes and metabolites to enhance the complexity and to define the final wine aroma. Nowadays, the use of non-Saccharomyces yeasts in combination with Saccharomyces cerevisiae is a state-of-the art strategy to improve wine composition and/or wine sensory properties. The present paper deals with the new yeast strains of Metschnikowia fructicola and S. cerevisiae, that were selected as representatives of the yeast microbiota isolated from grapes and grape juice of Aglianico cultivar. S. cerevisiae was utilized both as single strain starter and in combination with M. fructicola in experimental fermentations of Aglianico must. The dynamic of yeast populations was evaluated during the fermentation process analyzing the wine volatile compounds profile. The volatile compounds were identified by SPME-GC/MS. The results, showed that the multiple indigenous yeast starter was able to modulate the volatile compounds profiles and improve the aromatic complexity of wine, with a higher content of esters and terpenes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vitis vinifera Aglianico is a very old red grape variety of Campania, a Southern Italy region. This cultivar is a later-maturing grape able to adapt to different environments and managements of vine vigor (D’Agata 2014; Alba et al. 2011), producing a DOCG (Controlled and Guaranteed Designation of Origin) wine. Nowadays the winery global industry uses the selected commercial yeasts (LSA) to minimize risks in the winemaking process, because they give the possibility to standardize the procedures and ensure the quality of the final product. The LSA yeasts belong to Saccharomyces genus in particular to Saccharomyces species “sensu stricto”. The widespread use of such starter cultures often resulted in a loss of wine sensory properties and flattening aromatic characteristics that differentiate wines of diverse cultivars. For this reason, the research is focused on the selection of new autochthonous yeast cultures that can be isolated from vineyard, grapes, grape juice, winery and winery equipment. The microbiology of wine and the origin of indigenous wine yeasts have been extensively studied (Fleet 2003; Brysch-Herzber and Seidel 2015; Boscaino et al. 2014). In particular, the yeasts from vineyard and grapes-bunches belong to non-Saccharomyces group are characterized by low fermentative activity (Ciani et al. 2010), while those from wineries and their equipments are clustered in the Saccharomyces group, that is distinguished for an higher fermentative vigor (Bisson and Walker 2015). The non-Saccharomyces yeasts belong to the Hanseniaspora, Pichia, Rhodotorula, Metschnikowia, Candida, Hansenula, Kloeckera genera and are able to start the alcoholic fermentation process, producing, in the first phase of the process, ethylic alcohol and different metabolites and enzymes, responsible for the aromatic complexity of the wine. When the alcohol reach 4°–5° their fermentation activity decreases and the Saccharomyces yeasts action becomes predominant. Different studies have established, the positive role of non-Saccharomyces species during the winemaking process, demonstrating that they can improve the efficiency of the starter increasing the bouquet complexity and leading to a better chemical and sensory properties of the wine (Tofalo et al. 2016; Varela 2016). The biochemical transformation of flavor-inactive grape juice constituents into aromatic components has come out, as the fundamental action mechanism of non-Saccharomyces yeasts. In fact, through enzymes as glycosidases and esterases, they can substantially impact on wine aroma and flavor, facilitating a greater expression of grape varietal character (Fia et al. 2005). In addition, during the fermentation process, the winery yeasts besides producing ethanol, also release other compounds such as glycerol, esters, higher alcohols, aldehydes, acids, terpenes and ketones. These molecules, known as secondary metabolites, determine the final quality of the product. The quantitative level of these compounds is determined by the different yeast species utilized and, within the same species, between biotypes (Robinson et al. 2014). In particular, yeast belonging to Metschnikowia genus shows greater production of esters during fermentation as a consequence of their specific metabolism (Suarez-Lepe and Morata 2012). The native yeasts are selected for characteristics that, over the fermentative vigor, alcoholigenous power, tolerance to the antioxidant and antimicrobial compounds and minimal foam production, are relative to the liberation of a balanced array of flavor metabolites, without undesirable excess of volatile compounds (Ribéreau et al. 2006).
These yeasts, enclosed in the starters, lead to a final product enriched in those aromatic compounds which enhance the character of the wine. The aim of the present work was the evaluation of the impact on the aromatic profile of wines, of autochthonous yeast strains isolated from Aglianico grapes and used as starters. In particular an autochthonous S. cerevisiae, also in combination with a strain of M. fructicola, was utilized and the aromatic profile of different wine samples was evaluated through the identification of the volatile compounds by Solid Phase Micro Extraction-Gas Chromatography/Mass Spectrometry (SPME-GC/MS technique).
Materials and methods
Identification of yeast strains
Two autochthonous yeast strains (AGYP37, AGYP28) isolated in a previous screening from the native microflora of Aglianico grapes and musts (Sorrentino et al. 2012) and stored in the ISA-CNR collection has been selected as potential starter to realize an experimental Aglianico grape juice winemaking process. The yeast strains were identified through API 20C AUX assays (bioMérieux Italia Spa, Italy) confirming the results, at species level with the analysis of the D1/D2 domain of 26S rDNA sequence. The genetic region was PCR amplified directly from individual yeast colony following the protocol, as described by Arroyo-Lopez et al. (2006). The standard primers utilized were the commonly referred in literature as NL1 and NL4 (O’Donnel 1993).
Assessment of technological characters of yeast strains
The selected yeasts were characterized for different technological features, such as SO2 resistance and fermentation power, as described by Boscaino et al. (2015), and for ethanol resistance. Briefly, to YPD agar (1% yeast extract, 2% peptone, 2% glucose and 2% agar, kept melted at 45 °C) were added different aliquots of ethanol to obtain the final concentrations between 14 and 17% (v/v). Twenty microliters of broth-culture overgrowth for 48 h were spotted onto YPD plates and the growth was observed after 24 and 48 h at 25 °C. The presence of a microbial coating in correspondence of the spot showed resistance ability of yeast strain. The culture of each selected yeast was scaled up to 100 mL and the growth was carried out at 28 °C in shake-flasks at 200 rev/min in an orbital shaker (New Brunswick Scientific Co., Inc., USA) for 24 h. The obtained yeast suspension was used to inoculate (at 1% v/v) 10 L of YPD liquid medium in a pilot plant fermenter (B-Braun Biostat C—10 L). The obtained biomass was stored at 4 °C.
Aglianico vinification
The winemaking process was carried out at cellar of the Agrarian Technical Institute “F. De Sanctis” in Avellino, Italy. Aglianico grapes were harvested, destemmed, pressed and then fermented with their skins. The chemical parameters of Aglianico must were: 23° Brix, pH 3.23, total acidity 9.52 g L−1 and assimilable nitrogen content 316 mg L−1. Potassium metabisulfite (MBK) (Sigma-Aldrich, Gmb H) was added to grape juice at a final concentration of 100 mg L−1, followed by 10 mg hL−1 of Trenolin® Rouge DF pectolitic enzyme (Erbslöh Geisenheim AG, Gmb H) and warming the mix at 18 °C for 18 h. The grape must was separated in tanks of 50 L, the first one was co-inoculated with mixed selected autochthonous yeast (AGYP37 and AGYP28) [ST1], (3 × 106 CFU mL−1); the second with a single yeast strain (AGYP37) [ST3], while the last, was inoculated with 0.3 g L−1 (3.1 × 1010 CFU g−1) of dry S. cerevisiae var. cerevisiae [ST5], (I.C.V. D254, LALVIN) (Lallemand inc) previously rehydrated according to manufacturer instructions (control trial) (every fermentation was conducted in duplicated n = 2). The inocula were dispersed in the grape juice mass by stirring. Every day in the tanks were carried out three punching down, namely the breaking of the marc hat by immersion, to facilitate a leaching from the marcs of trapped yeasts and re-oxygenation of the mass. The fermentation processes were carried out at room temperature and monitored at different stages (0, 4, 8 and 10 days).
At the beginning of the fermentation a mobilisator and a nutrient (0.2 g L−1 VitaDrive® and Vitamon® Combi from Erbslöh, Gmb H, respectively) were added. The fermentation process was completed in 11 days, when the residual sugars concentration reached a value lower than 2 g L−1 hence, 60 mg L−1 of MBK was added. On the experimental wines the analytical analysis, as titrable acidity, volatile acidity, pH, total sulfure dioxide, alcohol strength, density and total dry matter, were carried out following the OIV procedures (2015) (Table 1).
Monitoring of yeasts population
Grape juice samples were collected in sterile containers before inoculation and brought to the laboratory to determinate the yeast microbiome. Likewise, to evaluate the performance of selected yeasts starter during Aglianico vinification, samples in the initial, middle or tumultuous and late phase of process were analyzed. To enumerate the yeast CFU, 100 μL of proper decimal dilution of must samples was spread onto plates of two different agar media: WL (Wallersteins Nutrient agar) and Lysine-agar (Oxoid Spa, Italy) and the plates were incubated at 28 °C for 5 days. WL medium allowed the yeast identification by colony morphology and dye uptake, while, the selective Lysine agar allowed to distinguish Saccharomyces from non-Saccharomyces yeasts as reported (Cavazza et al. 1992).
Solid phase microextraction gas chromatography/mass spectrometer analysis of volatile compounds (SPME-GC/MS)
Volatile compounds were determined by solid phase microextraction coupled with gas chromatography/Mass Spectrometry (SPME-GC/MS). All reagents were purchased from Sigma-Aldrich (Sigma-Aldrich, GmbH).
The SPME fiber (PDMS-100 μm, polydimethylsiloxane) was conditioned according to the manufacturer’s recommendations prior to its first use. To a 20 mL Headspace vial was added 5 mL of wine samples, 3 g of NaCl and octan-3-ol, in hydro-alcoholic solution (1/1, v/v) at 100 μg L−1, as Internal Standard. The solution was homogenized with a vortex shaker and then loaded onto a Gerstel autosampling device. The program consisted in swirling the vial at 250 rpm for 5 min at 40 °C, inserting the fiber into the headspace for 30 min at 40 °C as the solution was swirled again, and in transferring the fiber to the injector for desorption at 240 °C for 30 min.
Gas chromatography analysis were carried out using a 7890 Agilent GC system coupled to an Agilent 5975 inert quadrupole Mass Spectrometer (MS) equipped with a Gerstel MPS2 autosampler. The capillary column employed was a HP-Innowax (Agilent technologies) (30 m × 0.25 mm i.d. × 0.50 μm film thickness) and the carrier gas was Helium. Splitless injections were used. The initial oven temperature was set to 40 °C for 1 min. The temperature was increased in four steps: 40–60 °C at 2 °C min−1; 60–150 °C at 3 °C min−1, 150–200 °C at 10 °C min−1 and 200–240 °C at 25 °C min−1; the final temperature was maintained for 7 min. The injector, the quadrupole, the source and the transfer line temperature were maintained at 240 °C, 150 °C, 230 °C and 200 °C, respectively. Electron ionization mass spectra in full-scan mode were recorded at 70 eV electron energy in the range 40–300 amu. Identification was achieved matching mass spectra obtained from the sample with those from pure standards injected in the same conditions and by comparing the mass spectra presents in the Wiley07/NIST 98 libraries and the Retention Index present in database or in the literature. Afterwards, to calculate the RI value of these compounds, the n-alkanes (C5–C25) were also analyzed under the same conditions using GC–MS.
Quantification was performed by using the relative concentration in μg L−1 of the Internal Standard, calculated as the ratio between each compound area and the internal standard area. The samples were analyzed in triplicate and blank runs were made by using an empty vial every two analysis (Boscaino et al. 2015).
Odor activity value (OAV)
To evaluate the contribution of a chemical compound to the wine aroma the odor activity value (OAV) was determined. OAV is an indicator of the importance of a specific compound to the aroma of a sample. It was calculated as the ratio between the concentration of an individual compound and the perception threshold described in the literature (Jiang et al. 2013).
Statistical analysis
The mean and standard deviation were calculated for each experimental parameter. Differences among the experimental wines were determined for each volatile compound by analysis of variance, Duncan’s test, and the results were considered significant if the associated p values were below 0.05. Statistical data processing was performed using XLSTAT (Addinsoft XLSTAT v 2015.1.02). Principal Component Analysis (PCA) was carried out using Tanagra 1.4 software.
Results and discussion
Technological characterization of yeast strains
Two selected indigenous yeasts preserved in the ISA-CNR collection belonging to Metschnikowia and Saccharomyces genera were employed in the present grapes juice Aglianico winemaking process. These yeast strains, named AGYP28 and AGYP37, were assigned to the species S. cerevisiae and M. fructicola, respectively, through the analysis of the D1/D2 domain of 26S rDNA sequence (Sorrentino et al. 2012). The yeast strains were characterized for different technological features, such as SO2 and ethanol resistance, and fermentation power. In particular, the strain (AGYP37) S. cerevisiae showed the best resistance to SO2 concentrations tested (100 mg L−1 and 250 mg L−1), the greatest fermentative power with higher CO2 production (15 g CO2/100 mL) and alcoholigenous power (12°). The strain (AGYP28) M. fructicola showed the ability to resist at SO2 concentration of 100 mg L−1, a fermentative power with CO2 production (5 g CO2/100 mL) and a low alcoholigenous power (3°) (Sadoudi et al. 2012; Ciani et al. 2010). As regards the resistance to ethanol the two selected yeasts showed a different behavior: while AGYP37 was resistant to concentrations 14–15%, the AGYP28 strain has not been able to show any resistance.
Yeast population
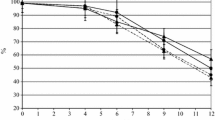
The enumeration of the growth of native yeast starters during Aglianico vinification was carried out over the initial, middle and late phases of the process. The starters were prepared utilizing the autochthonous S. cerevisiae alone (ST3) or in combination with M. fructicola (ST1). The results, reported in the Fig. 1, showed a similar pattern between the experimental starters (ST1 and ST3) and the commercial yeast (ST5) used as a control.
The yeast counts (Fig. 1a, b), obtained from seeding on different culture media, demonstrated that the order of magnitude reached by the populations of S. cerevisiae and Metschnikowia spp. during the alcoholic fermentation, was 107 and 108 CFU mL−1 in the middle-phase decreasing to about 105 CFU mL−1 at the end of fermentation (11 days), for all experimental native starters. On the contrary, the commercial yeast (ST5) after 11 days, still showed a high microbial load, (about 106 CFU mL−1), indicating that, a longer fermentation time was needed to complete the process. The counts on Lysine medium (Fig. 1b), allowed to define the order of magnitude of indigenous yeasts belonging to Metschnikowia spp. (included in ST1 in combination with S. cerevisiae), because this is a component of non-Saccharomyces group able to assimilate nitrogen from lysine (Cavazza et al. 1992). Moreover, a lot of colonies showed a red colour at the bottom (data not shown). This characteristic is typical of Metschnikowia genus (Kurtzman and Droby 2001). The obtained data highlighted the evolution of the microbiota during the alcoholic fermentation and showed the loss of yeast loads simultaneously with the accumulation of ethyl alcohol, which is a toxic metabolite, especially for non-Saccharomyces strains, as reported by several papers (Rossouw and Bauer 2016; Fleet 2003). Interestingly these vinifications experiments demonstrated that the cultures of indigenous yeasts (Fig. 1), utilized as starters both in combination (ST1) or alone (ST3), were faster to complete the fermentation process, compared to commercial yeasts (ST5). These findings suggested that the use of these experimental autochthonous starters could be of great interest from an economic point of view because it allow a reduction of processing time and at the same time an enrichment of the wine with peculiar metabolites (Capozzi et al. 2015; Tufariello et al. 2014; Comitini et al. 2011). The choice of M. fructicola as selected yeast in this work was linked to its higher frequency and relative abundance on grapes than the sister species M. pulcherrima (Kurtzman and Droby 2001), that together with Hanseniaspora uvarum, is the most abundant non-Saccharomyces species on grapes (Rossouw and Bauer 2016; Fleet 2003).
Volatile composition of wine
The SPME-GC/MS analysis of experimental wines allowed the identification and quantification of forty compounds belonging to six groups of volatile molecules (Table 2). In this work were quantified 15 esters, 11 alcohols, 4 acids, 4 C13-norisoprenoids, 4 terpenes and 2 volatile phenols. Many of these volatile compounds are commonly found in wine and are derived from grapes, yeast fermentation and vinification process.
The Ethyl esters and acetates were quantitatively the largest group (~ 55%) of the volatile compounds in Aglianico wines (ST1; ST3; ST5). The Ethyl esters were mainly synthesized during yeast fermentation by enzymatic grape precursors and by ethanolysis of acylCoA that is formed during fatty acid synthesis or degradation. Their concentrations are influenced by yeast strain, fermentation temperature, aeration degree and sugar content. Both esters and acetates have a key role in the whole wine aroma impressing a positive contribution by distinct sensory notes such as the sweet-fruity, sweet balsamic and grape like ones (Rapp 1998). The acetates are produced from the reaction of acetyl-CoA with higher alcohols derived from the degradation of amino acids or carbohydrates (Perestrelo et al. 2006).
The wine ST1 obtained by co-inoculations of M. fructicola and S. cerevisiae had higher amount of esters and acetates respect to that ST3 obtained by mono-culture of the selected indigenous S. cerevisiae and the control wine (ST5). In particular, the following compounds responsible for different types of aroma such as the fruity (ethyl butanoate, ethyl hexanoate and ethyl 2-methylbutanoate), apple (ethyl isovalerate) and banana odour (isoamylacetate) contributed significantly to the aroma of the wine with a OAV > 1 (Fig. 2); these compound concentrations were higher in the wine ST1 compared to ST3 (significantly different p < 0.05) and the control wine (except isoamyl acetate p > 0.05).
The Alcohols class represents about the 36% of the total volatiles and includes isobutanol, isoamyl alcohol, 2-phenylethanol, 1-hexanol. Isobutanol, isoamyl alcohol, 2-phenylethanol; they are, among the aromas, released as secondary products of yeasts metabolism. These compounds could be synthesized through either the anabolic pathway from glucose, or the catabolic pathway from their corresponding amino acids (valine, leucine or iso-leucine and phenylalanine respectively). Another compound related to the catabolic pathway is methionol, produced from the amino acid methionine (Perestrelo et al. 2006). Among the alcoholic compounds the isoamyl alcohol, responsible for the fusel odor, was at the highest concentrations in all the wine samples followed by 2-phenylethanol that confers a rose flavor. The importance of these compounds is also related to their role as precursors for the formation of esters (Soles et al. 1982), that are very important for wine aroma. However, all the alcohols concentration are below its odor threshold.
The Acids class represented about the 5% of the total volatiles and includes acetic, hexanoic, octanoic and decanoic acids. These molecules, enzymatically produced during fermentation, constitute an important group of aroma compounds that can contribute with cheese, fatty and rancid notes. Usually, the presence of C6–C10 fatty acids is related to the appearance of negative odors, they are very important for aromatic equilibrium in wine, because they are opposed to the hydrolysis of the corresponding esters. Only the octanoic acid (cheese aroma) had a concentration higher than its odor threshold (OAV > 1) giving negative sensory notes in ST1 and ST5 wines but not in ST3 wine.
The C13-norisoprenoids, important as aromas in many foods and fragrances, derive from carotenoids, and are commonly found in nature. Four norisoprenoids comprising the isomers vitispirane I and vitispirane II, β-damascenone and trimethyl dihydronapthalene (TDN) were identified in these wines and represented about the 1.2% of the total volatiles. In all the wines, β-damascenone (rose aroma) and TDN had a concentration higher than its odor threshold (OAV > 1).
Terpenes are present in free and glycosylated form in grapes. During alcoholic and malolactic fermentation, the content of free terpenes often increases due to the β-glucosidase activity of yeasts (Gil et al. 1996). The ability of non-Saccharomyces yeast to release terpenes from glycosidic precursors has been described by several authors (Maturano et al. 2012; Ferreira et al. 2001) and the amount of the enzymatic hydrolysis is dependent on the yeast strain and the chemical structure of the substrate. In fact the ST1 wine, obtained with M. fructicola have a higher concentration of terpenes (linalol, 4-terpineol, nerolidol and β-citronellol) than ST3 and ST5 wines (Table 2).
In the group of volatile phenols, ethyl-phenols are particularly important because they negatively contribute to the final quality of wine, being responsible for the ‘phenolic’, ‘animal’ and ‘stable’ off-odors found in certain red wines. In this work we identified two volatile phenols, 4-ethyl phenol and 4-ethyl guaiacol that contributed to wine flavor at normal levels with a stable, sweaty saddles and smoky spicy odor respectively. Usually their production may be associated with Brettanomyces spoilage yeasts and below certain levels (140 and 620 µg L−1 for 4-ethyl-guaiacol and 4-ethyl phenol, respectively) these compounds can also positively contribute to the aroma of red wines (Weldegergis et al. 2011).
The ST1 wine obtained with M. fructicola/S. cerevisiae co-culture had the most interesting aromatic profile characterized by high concentrations of fatty acids (hexanoic acid and octanoic acid), ethyl esters and acetates (ethyl acetate, ethyl butanoate, ethyl 2-methylbutanoate, isoamyl acetate, ethyl hexanoate) and terpenol compounds, with elevated levels of linalool, 4-terpineol, nerolidol, β-citronellol. These data could be the consequence of a positive synergistic effect between the two yeasts included.in the fermentation starter. A similar positive interactions between two yeasts has been reported for M. pulcherrima/S. cerevisiae co-culture but never investigated for M. fructicola/S. cerevisiae (Ciani and Comitini 2015; Sadoudi et al. 2012).
Odor activity value (OAV)
In order to assess the influence of the compounds identified on wine samples, Odor Activity Value (OAV) was calculated by dividing the concentration of each compound by its odor threshold. Only the compounds with OAV higher than 1 individually contribute to the wine aroma; compounds with OAV lower than 1 could also contribute to the aroma character through the additive effect with molecules possessing similar structure or odor. Moreover, substances with similar OAVs can improve the wine aroma through synergy with other compounds (Vilanova et al. 2010; Guth 1997). The Fig. 2 showed the volatile compounds with an OVA > 1: ethyl butanoate, ethyl 2-methylbutanoate, ethyl isovalerate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, octanoic acid, β-damascenone, TDN and linalool that were the predominant odorants and constituted the global aroma of Aglianico wine. As can be noted the trend was similar for the three wine even if the wine ST1 with co-culture of M. fructicola/S. cerevisiae, had a higher content of volatile compounds that contribute to the aroma with floral and fruity notes.
Principal component analysis (PCA)
The principal component analysis performed on the wine volatile compounds composition was showed in Fig. 3. PCA gives a pictorial relationship among wines based on their volatile concentration. It makes easy the interpretation of multivariate analysis and in this study was used to identify the volatile compounds that best discriminated among wines. The first two principal components, PC1 and PC2, accounted for 97% of total variance (74% and 23% respectively). Wines clustered, according to starter composition, in three different quadrants. The ST1 wine was positioned in the second quadrant and it was mainly characterized by monoterpenes (β-citronellol, nerolidol, 4-terpineol, linalol), C13-norisoprenoids (vitispirane isomers, TDN), ethyl esters (ethyl acetate, ethyl octanoate) and methionol. The ST3 wine was positioned in the first quadrant and was mainly characterized by phenols (4-ethylguaiacol, 4-ethylphenol), some ethyl esters (ethyl 2-methylbutanoate, ethyl dodecanoate, ethyl decanoate, ethyl 9-decenoate, diethylsuccinate) and C13-norisoprenoids (β-damascenone); while the wine control (ST5) was located in the third quadrant and was principally characterized by isoamyl alcohol, 2-phenylethanol and acetic acid.
This representation shows that the presence of M. fructicola in ST1 respect the S. cerevisiae alone (ST3), contributes to the aroma of the wine through volatile compounds with fruity and floral notes (some esters and terpenes). This result suggests, again, the positive synergic effect between the two yeast strains (S. cerevisiae/M. fructicola) used in this work.
Conclusion
The present study confirmed that the non-Saccharomyces yeasts, generally considered as spoilage yeasts, represent tools of great value for the winemaking industry. Moreover, was also evidenced that, the wine-related environments represent a reserve of biodiversity that might give new input to quality and aromatic complexity of the wine. In fact the results obtained by using in co-culture a M. fructicola and a S. cerevisiae yeasts isolated from grapes and grape juice of Aglianico, demonstrated that the multiple indigenous yeast starter was able to modulate the volatile compounds profiles and improve the aromatic complexity of wine, with a higher content of esters and terpenes.
References
Alba V, Anaclerio A, Gasparro M, Caputo AR, Montemurro C, Blanco A, Antonacci D (2011) Ampelographic and molecular characterisation of Aglianico accessions (Vitis vinifera L.) collected in Southern Italy. S Afr J Enol Vitic 32(2):164–173
Arroyo-Lopez FN, Duran-Quintana MC, Ruiz-Barba JL, Querol A, Garrido-Fernandez A (2006) Short communication: use of molecular methods for the identification of yeast associated with table olives. Food Microbiol 23:791–796
Bisson LF, Walker GA (2015) The microbial dynamics of wine fermentation. In: Holzapfel W (ed) Advances in fermented foods and beverages improving quality, technology and health benefits. Woodhead Publishing, Cambridge, pp 434–476
Boscaino F, Sorrentino A, Ionata E, La Cara F, Volpe MG (2014) Evaluation of autochthonous selected yeasts from grapes and cellar in winemaking of Aglianico vine. Bioprospect J 24(3):66–70
Boscaino F, Cutri G, Volpe MG, Blaiotta G, Sorrentino A (2015) Evolution of polyphenols, volatile aroma compounds and natural yeast flora of Coda di Volpe white grape. Chem Eng Trans 43:7–12
Brysch-Herzber M, Seidel M (2015) Yeast diversity on grapes in two German wine growing regions. Int J Food Microbiol 214:137–144
Capozzi V, Garofalo C, Chiriatti MA, Grieco F, Spano G (2015) Microbial terroir and food innovation: the case of yeast biodiversity in wine. Microbiol Res 181:75–83
Cavazza A, Grando MS, Zini C (1992) Detection of the microflora in musts and wines (Rilevazione della flora microbica di mosti e vini). Vignevini 9:17–20
Ciani M, Comitini F (2015) Yeast interactions in multi-starter wine fermentation. Curr Opin Food Sci 1:1–6
Ciani M, Comitini F, Mannazzu I, Domizio P (2010) Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res J 10:123–133
Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M (2011) Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol 28:873–882
D’Agata I (2014) Native wine grapes of Italy. University of California Press, Berkeley, p 355
Ferreira AM, Climaco MC, Faia AM (2001) The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components—a preliminary study. J Appl Microbiol 91:67–71
Fia G, Giovani G, Rosi I (2005) Study of β-glucosidase production by wine-related yeasts during alcoholic fermentation. A new rapid fluorimetric method to determine enzymatic activity. J Appl Microbiol 99:509–517
Fleet GH (2003) Yeast interactions and wine flavour. Int J Food Microbiol 86:11–22
Gil JV, Mateo JJ, Jiménez M, Pastor A, Huerta T (1996) Aroma compounds in wine as influences by apiculate yeasts. J Food Sci 61:1247–1249
Guth H (1997) Quantitation and sensory studies of character impact odorants of different white wine varieties. J Agric Food Chem 45:3027–3032
Jiang B, Xi Z, Luo M, Zhang Z (2013) Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res Int 51:482–489
Kurtzman CP, Droby S (2001) Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst Appl Microbiol 24:395–399
Maturano YP, Rodríguez Assaf LA, Toro ME, Nally MC, Vallejo M, Castellanos de Figueroa LI, Combina M, Vazquez F (2012) Multi-enzyme production by pure and mixed cultures of Saccharomyces and non-Saccharomyces yeasts during wine fermentation. Int J Food Microbiol 155(1–2):43–50
O’Donnel K (1993) Fusarium and its near relatives. In: Reynold DR (ed) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. Taylor JW, CAB International Wallingford, Wallingford, pp 225–233
OIV (2015) Compendium of international methods of wine and must analysis. International Organization of Vine and Wine, Paris
Perestrelo R, Fernandes A, Albuquerque FF, Marques JC, Camara JS (2006) Analytical characterization of the aroma of Tinta Negra Mole red wine: identification of the main odorants compounds. Anal Chim Acta 563:154–164
Rapp A (1998) Volatile flavour of wine: correlation between instrumental analysis and sensory perception. Nahrung J 42:351–363
Ribéreau P, Glories Y, Maujean A, Dubourdieu D (2006) The chemistry of wine, in handbook of enology, the chemistry of wine, stabilization and treatments, 2nd edn. Wiley, Chichester
Robinson AL, Boss PK, Solomon PS, Trengove RD, Heymann H, Ebeler SE (2014) Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am J Enol Vitic 65:1–24
Rossouw D, Bauer FF (2016) Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol 55:32–46
Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyer D, Gallardo-Chacón JJ, Ballester J, Vichi S, Guérin-Schneider R, Caixach J, Alexandre H (2012) Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol 32:243–253
Soles RM, Ough CS, Kunkee RE (1982) Ester concentration differences in wine fermented by various species and strains of yeasts. Am J Enol Viticult 33:94–98
Sorrentino A, Boscaino F, Cozzolino R, Volpe MG, Ionata E, La Cara F (2012) Autochthonous fermentation starters for the production of Aglianico wines CET 27:211–216
Suarez-Lepe JA, Morata A (2012) New trends in yeast selection for winemaking. Trends Food Sci Technol 23:39–50
Tofalo R, Patrignani F, Lanciotti R, Perpetuini G, Schirone M, Di Gianvito P, Pizzoni D, Arfelli G, Suzzi G (2016) Aroma profile of Montepulciano d’Abruzzo wine fermented by single and co-culture starters of autochthonous Saccharomyces and non-Saccharomyces yeasts. Front Microbiol 12:10. https://doi.org/10.3389/fmicb.2016.00610
Tufariello M, Chiriatti MA, Grieco F, Perrotta C, Capone S, Rampino P, Tristezza M, Mita G, Grieco F (2014) Influence of autochthonous Saccharomyces cerevisiae strains on volatile profile of Negroamaro wines. LWT-Food Sci Technol 58:35–48
Varela C (2016) The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl Microbiol Biotechnol 100:9861–9874
Vilanova M, Genisheva Z, Masa A, Oliveira JM (2010) Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem J 95:240–246
Weldegergis BT, Crouch AM, Górecki T, de Villiers A (2011) Solid phase extraction in combination with comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry for the detailed investigation of volatiles in South African red wines. Anal Chim Acta 701:98–111
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boscaino, F., Ionata, E., La Cara, F. et al. Impact of Saccharomyces cerevisiae and Metschnikowia fructicola autochthonous mixed starter on Aglianico wine volatile compounds. J Food Sci Technol 56, 4982–4991 (2019). https://doi.org/10.1007/s13197-019-03970-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03970-9