Abstract
Ponds—small, isolated freshwater bodies—have vanished in large numbers during the last decades. Despite such great loss, the number of natural small water bodies has still remained quite high in Estonia. Nevertheless, many pond-related species, including amphibians such as the northern crested newt Triturus cristatus and the common spadefoot toad Pelobates fuscus—are in decline in Estonia, suggesting that the conditions of extant natural ponds might not be optimal. However, these conditions have never been examined. To halt the decline of these two pond-breeding species, more than 400 ponds have been constructed or restored from 2004 to 2014 in Estonia. In this study we compared 85 natural and 85 constructed ponds (which were created or restored especially for T. cristatus and/or P. fuscus) to find out: (i) what the main differences are between natural ponds and ponds specially created for threatened species; (ii) whether natural ponds provide breeding conditions for local amphibians; (iii) given the decline of T. cristatus and P. fuscus, what are the characteristics lacking in natural ponds, due to which they are not providing quality breeding habitats for these species. Whereas the constructed ponds were located in open habitats with mineral soils, the natural ponds were mainly in mires and forests, being thus more shaded. Amphibian diversity was higher in the constructed ponds and was positively related to the depth of the pond, the clarity of the water, the presence of slanting slopes, the absence of fish and the presence of nearby fields. T. cristatus preferred constructed ponds for reproduction, while the breeding site selection of P. fuscus was determined mainly by terrestrial habitat characteristics. Importantly, when the threatened species were removed from the sample, the diversity of common amphibians did not differ between natural and constructed ponds, suggesting that in our study sites natural water bodies act still as breeding sites for common species, but not for threatened ones. We conclude that pond construction is an important tool to halt the decline of threatened species, even in landscapes where natural ponds are still preserved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ponds, small, isolated freshwater bodies with ephemeral or permanent hydrology support a considerable number of unique and scarce species, thus playing a central role in maintaining high regional biodiversity (Semlitsch and Bodie 1998; Williams et al. 2004; Céréghino et al. 2008; Davies et al. 2008; Biggs et al. 2017). Ponds have been demonstrated to hold a higher macrophyte and macroinvertebrate diversity than ditches, lakes, rivers and/or streams (Davies et al. 2008; Martinez-Sanz et al. 2012; Bubíková and Hrivnák 2018). Despite their significant ecological values, pond ecosystems are threatened by a number of human activities, typically related to the loss of ponds’ historical function and changed land use (Wood et al. 2003; Brönmark and Hansson 2005; Oertli et al. 2005; Piha et al. 2007; Curado et al. 2011). Contamination, invasion of exotic species, increased nutrient loads and acid rain have been cited as the major threats to pond biodiversity (Brönmark and Hansson 2002), while reduced connectivity between individual ponds isolates populations of organisms with short migration distances (Sjögren-Gulve 1994; Rothermel 2004; Cushman 2006). During the 20th century more than 50% of ponds have disappeared in the European states (Hull 1997), while losses in agricultural landscapes have often been the greatest (Heath and Whitehead 1992).
For pond-breeding amphibians, small freshwater bodies are vital. Thus the destruction of these aquatic habitats in conjunction with overall habitat deterioration is considered to be one of the major causes of the worldwide decline in amphibians (Ficetola et al. 2015), which has escalated within the past 40 years (Semlitsch 2000; Beebee and Griffiths 2005). The fragmentation and isolation of the remaining ponds causes problems for amphibian population connectivity (for a review see Cushman 2006), mainly affecting the dispersal of juveniles (Rothermel 2004; Preisser et al. 2000). Also in Estonia the conditions of amphibian habitats, including small freshwater bodies, have degraded during the 20th century due to not only the changes in land use and the intensification of agriculture and drainage, but also by the abandonment of small-scale farming and the subsequent forestation of landscapes (Rannap et al. 2007; Suislepp et al. 2011; Rannap et al. 2015; Remm et al. 2015). These changes have caused a decrease in and range constriction of several amphibian species, including the common spadefoot toad (Pelobates fuscus) and the northern crested newt (Triturus cristatus). Both of these species currently have declining population trends in Europe (Eggert et al. 2006; IUCN 2009; Denoël 2012), including in Estonia (Red Data Book of Estonia 2008), and they are strictly protected within the European Union (EU Habitats Directive 92/43/EEC).
In order to compensate for the current loss and destruction of natural habitats, a large-scale restoration of amphibian breeding sites has been conducted in several countries (Denton et al. 1997; Petranka et al. 2007). Also in Estonia more than 400 ponds have been constructed for P. fuscus and T. cristatus in order to stop their further decline and strengthen their existing populations (Rannap et al. 2009a). At the same time, Estonian landscapes still hold a significant number of small natural water bodies. As natural ponds have largely been destroyed in several European countries and the existing ponds are usually anthropogenic in origin (Lemmens et al. 2013), the studies of amphibian breeding sites have often focused on constructed ponds (e.g., Beebee 1977; Ildos and Ancona 1994; Morand and Joly 1995; Chester and Robson 2013) or natural water bodies of particular type, such as beaver ponds (e.g., Stevens et al. 2006; Dalbeck et al. 2014; Zero and Murphy 2016), forest pools (e.g., Calhoun et al. 2003; Vuorio et al. 2013; Van Dyke et al. 2017), bog pools (Mazerolle et al. 2006), Alpine lakes (Tiberti et al. 2018), karst ponds (Cirovic et al. 2008) or coastal rock pools (Laurila 1998). However, amphibian diversity and species composition may differ in natural water bodies and man-made ponds (Hazell et al. 2004; Brown et al. 2012; Remm et al. 2015). Therefore, Estonia provides a unique opportunity to compare a variety of natural small water bodies with ponds specially constructed for threatened amphibians, in order to find out their potential differences and importance as breeding sites for amphibians, including threatened species. Although our previous studies have demonstrated that the aquatic habitat restoration and construction targeted to P. fuscus and T. cristatus has been successful (Rannap et al. 2009a; Soomets et al. 2016), the role of natural small water bodies as breeding sites for these species is still unknown. Therefore, in this study we explored the following research questions: (i) which characteristics distinguish natural and constructed ponds; (ii) whether the amphibian diversity differs in natural and constructed ponds and if so, which pond and landscape characteristics could explain it; (iii) which habitat features influence the breeding site selection of threatened species, P. fuscus and T. cristatus, and is there any difference between natural and constructed ponds as breeding sites for these species.
Methods
Study area
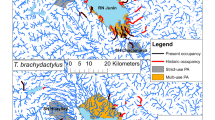
Our study was conducted within the range of T. cristatus and P. fuscus (Soomets et al. 2016; Estonian Nature Information Database), in areas where large-scale pond construction has been carried out specifically for these species since 2004 (Fig. 1; for a picture of a typical constructed pond see Online Resource 1). In northern Estonia the studied water bodies are located on the Pandivere Upland, which is the highest bedrock upland in Estonia (Fig. 1 area 1). The terrain is hilly moraine with clearly defined eskers that rise over 160 m a.s.l. Agricultural landscapes dominate the area; grasslands and conifer-dominated forests cover ca. 30%. The raised limestone topography causes intense filtration and karst processes, such that many temporary lakes are formed during the snowmelt and rainfall in the spring, but which typically dry out by late summer or early autumn (for a photo of the typical karst pond of the study area see Online Resource 2). In southern Estonia the study area is comprised of hilly, well-forested landscape with small scattered settlements and agricultural lands (Fig. 1 areas 3–6). Numerous lakes, beaver floods, swamps and depressions are found in these areas (for a photo of the typical beaver flooding of the study area see Online Resource 3). In eastern Estonia the study area covers the oldest and largest Estonian delta swamp and an island in Lake Peipsi (Fig. 1 area 2). The lentic water bodies include flooded marshes and depressions which can be found on sandy areas near human settlements or in the swamp (for a photo of a typical lentic water body of the study area see Online Resource 4).
As the pond construction was especially targeted to T. cristatus and P. fuscus, their habitat demands were taken into account (Rannap et al. 2009a). To increase colonization probabilities and preserve the existing populations of target species (Semlitsch 2000; Petranka and Holbrook 2006; Petranka et al. 2007), at least one pond was constructed within 200 m of an existing breeding pond of these species. The ponds constructed or restored for T. cristatus were located in landscapes with a mosaic of forests and semi-natural grasslands (Rannap et al. 2012). For P. fuscus semi-natural grasslands and small extensively used fields or vegetable gardens in sandy soils were considered (Rannap et al. 2013, 2015).
Sample selection
In order to compare constructed ponds (restored or created especially for threatened amphibians—T. cristatus and/or P. fuscus) with natural ones, we selected 85 from both pond types in regions where large-scale pond construction has been carried out (Fig. 1). For selecting natural ponds we used orthophotos of Estonian Land Board. We defined natural ponds as water bodies which have formed as a result of different natural processes, ranging from geological events to the activity of Eurasian beavers (Castor fiber). These water bodies were either temporary or permanent, with an area less than 1 ha and encompassed natural depressions, small karst lakes and beaver floods (see Online Resources 2, 3, 4). Each natural pond was located within a 500 m radius from the constructed ponds—a maximum dispersal rate of P. fuscus adults (Nöllert 1990; Nielsen and Dige 1995; Hels 2002) and a distance within which most movements of T. cristatus are made (Joly et al. 2001; Denoël et al. 2013). For the constructed ponds, we randomly selected 10–12 water bodies for each year in which ponds had been constructed, excluding the years 2004, 2008 and 2009, in which fewer than ten ponds had been constructed. The distance between two study ponds was ≥ 100 m. Mapinfo Professional 12.0 was used for the compilation of the sample. During the fieldwork, the preselected sample of natural ponds was corrected for as several ponds had dried out. We replaced the dried ponds with other adjacent small natural water bodies.
Fieldwork and data collection
The fieldwork was carried out in June 2015, during the larval period of all local amphibians in Estonia. Each water body was visited once and we dip-netted the ponds using a standard method (Skei et al. 2006). To detect the presence of amphibian larvae, a trained herpetologist dip-netted 15–30 min in each water body (the dip-netting effort increased proportionally with the area of the pond), covering all parts of the pond (the shore area, the area covered with vegetation and open water), also the bottom of the pond and the upper part of the water column were covered. In addition, the eggs of two newt species found in the area (T. cristatus and the smooth newt Lissotriton vulgaris) were searched for and the presence of adult individuals confirmed via visual observation. Since we only visited each water body once, it was possible that we missed species with extremely low larval abundances. However, if this was the case, the ponds were probably marginal, and not optimal breeding sites, which should not remarkably affect our results. The presence of fish in the pond was established using the combined data of visual observation and dip-netting. Given the difficulties in distinguishing the tadpoles of the pool frog Pelophylax lessonae and the edible frog P. esculentus (Adrados et al. 2010), we refer to those species collectively as Pelophylax sp.
We assessed altogether 25 aquatic and terrestrial features for each pond and its surroundings (Table 1), considering potentially important characteristics for larvae and adult amphibians (Nyström et al. 2002; Gustafson et al. 2011; Suislepp et al. 2011; Rannap et al. 2012, 2013, 2015; Denoël et al. 2013). In order to determine the distance to the closest pond with the target species, we used previous survey data (Rannap et al. 2015; Soomets et al. 2016) and the orthophotos of The Estonian Land Board and Estonian base map. The genera of dominant water plants were recorded. We measured the land cover within a 100 m and a 500 m radius of the pond—covering the average home-range and maximum migration distances of P. fuscus and T. cristatus (Nielsen and Dige 1995; Jehle and Arntzen 2000; Hels 2002; Denoël et al. 2013), using a spatial data calculator from the digital archive of the University of Tartu. In addition, the presence of sandy soils and vegetable gardens were recorded only in the field, because these landscape characteristics were missing in the spatial data calculator. To be able to identify these features, we chose a distance of 50 m from the pond.
Data analysis
In order to find out the differences between natural and constructed ponds, the characteristics of those two water body types were compared. The features with the normal distribution or with transformed values of normal distribution, were analysed using a t test. Otherwise the Mann–Whitney U test was used. Discrete parameters were analysed using the χ2 test. The p values were subjected to Bonferroni correction as a multiple-comparison correction, and the level of statistical significance was corrected accordingly. This correction has been considered suitable for our order of magnitude of variables (McDonald 2014), but the use of the correction might bring along a threat of false negatives.
As T. cristatus and P. fuscus were the target species for pond construction, the two pond types were also compared with regard to these species. We considered the pond as a breeding site if larvae or eggs of these species were found. Since we visited each pond just once, only presence/absence data was used for analysis. Ponds which were located outside the range of one of the target species (Soomets et al. 2016), were omitted from the analysis of that particular species. We did not find larvae of the target species in ponds with fish, thus, we considered the presence of fish as a limiting factor for breeding of T. cristatus (e.g., Skei et al. 2006; Rannap et al. 2012) and P. fuscus (e.g. Nyström et al. 2002; Rannap et al. 2013) and we omitted ponds hosting fish (natural ponds N = 10, created ponds N = 3) from the analyses of these species. In order to unify the data, the substrate of the pond ‘sand’ and ‘clay’ were grouped as ‘mineral’. In addition, as brown water was always clear, we reclassified the two water colour groups ‘brown’ and ‘clear’ into ‘clear’.
To find out which habitat characteristics determine the breeding of T. cristatus and P. fuscus we combined the multistep procedure of Hosmer and Lemeshow (2000) and the information-theoretic model selection approach (Burnham et al. 2011) to build multiple logistic regression models. We used the following steps of analysis: (1) performed univariate logistic regression analyses to estimate and test the effect of 25 variables one by one, using STATISTICA 8 software; (2) conducted a correlation analysis for the p ≤ 0.15 variables; (3) built 20 preliminary GLZ models of non-correlated variables with p ≤ 0.15 in univariate analyses using expert knowledge; (4) fitted selected models and used AIC model selection for models’ comparison in the program R 3.5.1. The variables with p ≤ 0.15 in univariate analyses were used in step 2 and 3 to retain variables that could gain significance in combination with other variables (Hosmer and Lemeshow 2000). As the variable ‘pond type’ contained multiple factors we did not include it into the GLZ models in step 3. When compiling the models, we followed the subsequent principles: (1) considering the importance of each variable in univariate analyses; (2) using expert knowledge to test for possible interactions and combinations; (3) including all factors which gained p ≤ 0.15 in univariate analysis into the set of models, since the importance of the correlating factors is revealed through the AIC value; (4) including only non-correlated factors to each GLZ model.
We determined amphibian diversity in each pond by the presence of their larvae, eggs or adults. For analysis, only presence/absence data was used. We built multiple logistic regression models using the program R 3.5.1. and following the procedure described above. For the ordering of the models we used MuMin package of R. As the data in the amphibian diversity analyses were underdispersed, we used Conway–Maxwell–Poisson distribution and Conway–Maxwell-distribution package of R in these analyses. In addition, we compiled a univariate model including all amphibian species without T. cristatus and P. fuscus (the target species of pond construction) and taking ‘pond type’ as an independent factor.
Results
The characteristics of natural versus constructed ponds
Natural and constructed ponds differed by 11 variables (Table 1). Natural ponds were larger and shallower with more gradually shelving slopes and a larger cover of macrophytes (< 1 m high water vegetation) than the constructed ponds. They also were more shaded and contained muddier sediment compared to the constructed ponds which were mostly sun-exposed and contained mineral sediment (clay or sand). Additionally, the natural ponds were often situated in mire landscapes and were surrounded mostly by forest.
Amphibian diversity
Seven species of amphibians were found in the studied ponds: L. vulgaris, T. cristatus, P. fuscus, the common toad (Bufo bufo), the common frog (Rana temporaria), the moor frog (R. arvalis) and green frogs (Pelophylax sp.) Among them, Pelophylax sp. were the most common, found in 72.4% of the ponds. T. cristatus and L. vulgaris were found considerably more often in constructed ponds than in natural ponds (Chi square test respectively: χ2 = 38.45, p < 0.001; χ2 = 33.72, p < 0.001), while R. temporaria and R. arvalis preferred to breed in natural ones (respectively: χ2 = 11.08, p < 0.001; χ2 = 4.61, p < 0.032; Fig. 2).
The amphibian diversity differed between natural and constructed ponds (N = 170; t = − 3.6; p < 0.001) with natural ponds having on average 2.4 ± 1.22 species (± SD, range 0–5) and constructed ponds 3 ± 1.16 species (± SD, range 1–6). When T. cristatus and P. fuscus were omitted from the amphibian data set, the factor ‘pond type’ lost its significance (N = 169; coef. = 0.07; p = 0.35). According to the univariate regression analysis, several pond and landscape characteristics influenced the amphibian diversity (Table 2). The best model for the prediction of amphibian diversity contained the following factors: field within 100 m radius from the pond (with a positive influence) and colour/transparency of water (clear water preferred), slope (slanting slopes preferred), and presence of fish (negative) (AIC = 533.75; Table 3). The next best model contained the same factors listed above plus the depth of the pond (deeper ponds preferred; Table 3).
Pelobates fuscus and Triturus cristatus
The larvae of P. fuscus were found in 19 ponds, of which six were natural and 13 constructed. However, there was no significant difference between natural and constructed ponds (N = 152; χ2 = 3.63; p = 0.057; Fig. 2). Importantly, no tadpoles were found in ponds with fish. In univariate regression analysis only a few factors appeared to have significant influence on the breeding of P. fuscus (Table 4). The top ranked multifactorial model for the prediction of larval presence (AIC = 107.8; Table 5) contained the following factors: sandy soil within a 50 m radius of the pond (positive influence), proximity to the nearest pond with the presence of P. fuscus (longer distances having a negative influence), grazing by the pond (having a positive influence), and percentage of mires within a 100 m around the pond (having a negative influence). However, five additional models remained within < 2 ∆AIC units from the top-ranking model, containing largely the same factors.
The eggs and larvae of T. cristatus were found in 55 ponds: 46 of these were constructed ponds and nine natural ponds (Fig. 2). No T. cristatus larvae nor eggs were found in ponds which contained fish. In contrast with P. fuscus, several landscape and pond characteristics appeared to have a significant influence on the presence of the species in a univariate regression analysis (Table 6). It revealed that T. cristatus prefers to breed in constructed ponds with clear water and mineral sediment, close to landscapes with mosaics of forest and open areas, and that it tends to avoid mires. The top ranked multifactorial model for the prediction of the presence of T. cristatus larvae and eggs contained the following factors: ‘region’ (the most suitable being Haanja and Seto regions) and ‘sediment’ (clay or sand preferred) (AIC = 132.9; Table 7). The next best model with an AIC value of 133.8 was a subset of the best model with the additional factor of ‘grassland within a 500 m radius of the pond’ (higher proportion with positive influence).
We found a positive correlation between the breeding of T. cristatus and the presence of certain macrophytes, such as Potamogeton (χ2 = 4.8; df = 1; p = 0.03) and Glyceria (χ2 = 4.6; df = 1; p = 0.03). The breeding was negatively correlated with the presence of Carex (χ2 = 5.0; df = 1; p = 0.03) (Fig. 3). Natural and constructed ponds differed in terms of the presence of those plant genera: there was more Potamogeton and Glyceria found in the constructed ponds (respectively: χ2 = 11.2, df = 1, p < 0.001; χ2 = 21.7, df = 1, p < 0.01) and more Carex in the natural ponds (χ2 = 13.2, df = 1, p < 0.01; Fig. 3).
Discussion
Natural and constructed ponds as breeding sites for amphibians
Our study demonstrated that amphibian diversity was higher in constructed or restored ponds than in small natural water bodies. However, previous studies comparing the amphibian diversity and population structure of natural and man-made ponds have yielded variable results depending on the concrete type of water body and the species studied (for a review see Brown et al. 2012). In our study, species richness was associated with deeper ponds with gradually shelving slopes and transparent water, absence of fish and presence of fields nearby. All aquatic habitat characteristics listed above have proven to be important for amphibians (e.g., Semlitsch 2002; Porej and Hetherington 2005; Rannap et al. 2012; Vojar et al. 2016; Miró et al. 2017; Remm et al. 2018). Constructed ponds were deeper having mainly transparent water and no fish, while natural ponds held more frequently fish, but also had more gradually shelving slopes. Whereas constructed ponds were created mainly on mineral soils, having clayish or sandy sediment that assures clear transparent water, natural water bodies were often located close to the mires, having peaty or muddy sediment. Transparent water indicates high oxygen and low nutrient levels of water bodies (Brönmark and Hansson 2005). Our study was also consistent with previous surveys, demonstrating that the presence of fish is a major limiting factor for pond-breeding amphibians (e.g. Joly et al. 2001; Hartel et al. 2007; Brown et al. 2012). The positive impact of the pond depth on amphibian species richness was probably through the length of the hydroperiod—deeper ponds retain water for longer (Beja and Alcazar 2003) and thus, favour usually more species (Oertli et al. 2002; Péntek et al. 2017). The positive effect of the fields (open habitat) in the vicinity of the breeding sites on amphibian diversity could derive from a general lack of such sun-exposed habitats caused by large-scale overgrowing/afforestation in Estonia, as demonstrated previously by Rannap et al. 2015. Importantly, the natural water bodies were mainly found in forested areas, while constructed ponds were located in open areas, often near extensively used fields.
Although the constructed ponds appeared to provide optimal breeding conditions for various amphibian species, the factor ‘pond type’ lost its significance when species of conservation concern—T. cristatus and P. fuscus—were removed from the analysis. Thus, the higher amphibian diversity in the constructed ponds is largely related to the habitat demands of these threatened species. Moreover, our results testified to the importance of natural ponds for common species. Rana temporaria and R. arvalis, the two Boreal Palearctic anurans occurring often in mire and forest habitats (Elmberg 1993; Suislepp et al. 2011; Remm et al. 2015, 2018), were found more often in natural water bodies than in constructed ponds. Thus, our study supports previous amphibian surveys, showing that a niche position is of decisive importance for rare species (Rannap et al. 2009b). The presence of resources which have become locally scarce, but which were considered when digging the ponds (e.g., pond’s exposure to the sun, presence of mineral soil and high quality terrestrial habitats) is vital for the survival of rare amphibians, whereas more common species can live and survive on the more widespread and accessible resources. In addition to providing breeding sites for amphibians, different kinds of small natural water bodies also provide essential habitat for invertebrates (Harthun 1999; Mazerolle et al. 2006; Colburn et al. 2007; Vaikre et al. 2018), zooplankton communities (Caramujo and Boavida 2010), wetland plants (Rhazi et al. 2012) and multiple other taxonomic groups.
Breeding site selection by Triturus cristatus and Pelobates fuscus
Our study confirmed that the pond construction has had a significant impact on the increase of the population of T. cristatus in Estonia (Rannap et al. 2009a; Soomets et al. 2016). The species bred mainly in the constructed ponds in the Haanja and Seto regions and was less commonly found in Karula (Fig. 1). The reason that the regions differ so much in their suitability as breeding habitat may be related to the soil types found in those regions. It has been previously known that T. cristatus occurs mainly on clay or loam soils and more rarely on sandy soils (Edgar and Bird 2006). In the Haanja and Seto areas the main soil types are clay or loam, while in Karula sandy soils are prevalent (Rooma and Voiman 2002). In addition to the region, the mineral sediment in the pond itself was important for T. cristatus. This amphibian’s preference for breeding in constructed ponds could therefore be related to the location of these ponds in mineral soils; the natural ponds were often situated in mires. Also the presence of grasslands in the average migration range of T. cristatus was essential for the species. Previous studies have indicated that T. cristatus prefers a mosaic of (deciduous) forests and grasslands as terrestrial habitat (Rannap et al. 2009b, 2012; Gustafson et al. 2011; Miró et al. 2017) or open landscapes (Denoël et al. 2013) and avoids mires (Gustafson et al. 2011). However, demands for specific types of terrestrial habitat vary across the species’ range (Miró et al. 2017; Skei et al. 2006; Vuorio et al. 2013), hence the need to take into account the specific preferences of local populations in habitat management (Miró et al. 2017).
In addition, our study confirmed that the breeding of T. cristatus can be predicted by presence of two macrophyte genera—Potamogeton and Glyceria, which occurred more frequently in constructed than in natural ponds. Also previous studies have demonstrated that these plant genera have a positive influence on the breeding site selection of T. cristatus (Gustafson et al. 2006; Miaud 1995; Sztatecsny et al. 2004), as this species prefers leaves of plants that can be easily folded (Miaud 1994). Both plant species have underwater leaves which T. cristatus uses for egg-laying (R. Magnus personal observations). As the predator pressure on the eggs of T. cristatus is relatively high and egg survival varies between 3 and 16%, folding their eggs within the leaves of these macrophytes provides protection from the predation of water invertebrates and other newts (Miaud 1994, 1995).
The presence of P. fuscus tadpoles in the pond was mainly related to the soil and landscape characteristics in the surroundings of the breeding site. Considering the results of previous studies, the landscape features and soil type in the vicinity of the breeding site are important for P. fuscus (Eggert 2002; Nyström et al. 2007; Rannap et al. 2013; Carisio et al. 2014). The species bred considerably more often in ponds which were located in sandy soils. It is known that the adult individuals stay close to their breeding sites (Hels 2002) and (due to their nocturnal lifestyle) spend most of their time dug into the soil (Székely et al. 2017). Thus the availability of sandy soils which can easily be dug by the toad is essential for the species (Eggert and Guyétant 1999; Eggert 2002). In addition, the existence of P. fuscus tadpoles in the pond could also be predicted by land cover types in the vicinity of the breeding site—open areas with grazing were preferred, whereas forested and mire areas avoided, which is corroborated by previous studies (Kauri 1947; Eggert 2002; Eggert et al. 2006; Rannap et al. 2015). Similarly to T. cristatus, the terrestrial habitat preferences of P. fuscus also vary across its range—the species inhabits mostly open agricultural areas in Western-Europe, although at the edges of its range it also occurs in forested areas (Nyström et al. 2002; Rannap et al. 2013). Additionally, the presence of water bodies with conspecifics near the breeding sites of P. fuscus was positively correlated with the occurrence of its tadpoles. A clustered configuration of water bodies increases the probability of successful breeding and secures ecological connectedness and long-term survival of meta-populations (Semlitsch 2000; Petranka et al. 2007). As discussed earlier in this paper, a number of natural ponds have been destroyed due to drainage; others do not possess qualities essential for amphibians to breed in (Suislepp et al. 2011), thus natural water bodies which provide high-quality breeding sites for amphibians are scarce in modern Estonian landscapes (Rannap et al. 2009a; Remm et al. 2015).
Both, T. cristatus and P. fuscus avoided ponds with fish. The negative impact of fish on T. cristatus breeding has been demonstrated in earlier studies (Morand and Joly 1995; Reshetnikov 2003; Rannap et al. 2012; Miró et al. 2017). Fish influence the abundance of the species during different phases of the newt’s development. While larger fish eat the adults and larvae of the newt, smaller fish feed on its eggs and larvae (Denoël and Ficetola 2008). In contrast with other newt species, T. cristatus does not have a morphological or life-history plasticity which would be related to the presence of predators (Schmidt and Van Buskirk 2001). In case of P. fuscus, its larvae also constitute easy prey for fish due to their lengthy developmental time and nektonic behaviour.
Implications for management
The results of our study demonstrated that within the Estonian range of P. fuscus and T. cristatus natural ponds have been preserved mainly in forested and mire areas and rarely occur in open areas with mineral soils. Therefore, the construction of ponds in such landscapes has proven to be effective for restoring habitats, especially when threatened species were considered. The construction of special ponds for amphibians helps to provide specific conditions for threatened species with particular habitat demands (see also Rannap et al. 2009a). Additionally, these ponds contribute to maintaining overall amphibian diversity and providing quality habitat for aquatic invertebrates (Soomets et al. 2017).
In the management of constructed ponds, the introduction of fish should be avoided and some of the tall and dense vegetation and bushes (bulrush, willows) removed in order to avoid the pond becoming overgrown and shaded. If the ponds are constructed for specific target species, the location of these ponds is crucial, considering the current distribution range of the species as well as its habitat requirements. For P. fuscus, the presence of sandy soils and the avoidance of forests and mires in the vicinity of breeding sites is vital, whereas for T. cristatus, the availability of mineral soil and grasslands near the breeding pond is important.
Although natural ponds did not provide optimal breeding conditions for the threatened amphibians, they were still used by common species inhabiting forest landscapes, such as Rana arvalis and R. temporaria. It is therefore of high importance to protect natural ponds that still have an intact hydrology, are open to the sun and do not contain fish. Moreover, there are some minor factors, which can be easily changed with management practices (e.g., removal of bushes, ditch blocking), that could guarantee suitable breeding conditions for amphibians in natural ponds. In some cases the restoration of small water bodies which have been altered by ditching could also be considered (Suislepp et al. 2011; Remm et al. 2018).
Given the substantial differences between the characteristics of constructed and natural small water bodies, a variety of ponds might function as a cluster in the landscape, preserving amphibian meta-populations and supplementing one another in case of changing environmental conditions (e.g., climate, predation). Thus, the construction of ponds is important even in landscapes where natural small water bodies have still been preserved. To specify how these water bodies of different types supplement each other in the maintenance of amphibian populations, additional studies covering multiple years would be needed.
References
Adrados L, Rannap R, Briggs L (2010) Field guide of amphibians in Estonia. University of Tartu, Tallinn (in Estonian)
Beebee TJC (1977) Habitats of the British Amphibians (1): chalk uplands. Biol Conserv 12:279–293
Beebee TJC, Griffiths RA (2005) The amphibian decline crisis: a watershed for conservation biology? Biol Conserv 125:271–285
Beja P, Alcazar R (2003) Conservation of Mediterranean temporary ponds under agricultural intensification: an evaluation using amphibians. Biol Conserv 114:317–326
Biggs L, von Fumetti S, Kelly-Quinn M (2017) The importance of small waterbodies for biodiversity and ecosystem services: implications for policy makers. Hydrobiologia 793:3–39
Brönmark C, Hansson LA (2002) Environmental issues in lakes and ponds: current state and perspectives. Environ Conserv 29:290–307
Brönmark C, Hansson LA (2005) The biology of lakes and ponds. Oxford University Press, Oxford
Brown DJ, Street GM, Nairn RW, Forstner MRJ (2012) A place to call home: amphibian use of created and restored wetlands. Int J Ecol. https://doi.org/10.1155/2012/98987
Bubíková K, Hrivnák R (2018) Comparative macrophyte diversity of waterbodies in the central European landscape. Wetlands 38:451–459
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioural ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 64:23–35
Calhoun AJK, Walls TE, Stockwell SS, McCollough M (2003) Evaluating vernal pools as a basis for conservation strategies: a Maine case study. Wetlands 23:70–81
Caramujo MJ, Boavida MJ (2010) Biological diversity of copepods and cladocerans in Mediterranean temporary ponds under periods of contrasting rainfall. J Limnol 69:64–75
Carisio L, Sacchi R, Seglie D, Sindaco R (2014) Habitat selection in a fossorial toad Pelobates fuscus insubricus (Amphibia: Pelobatidae): Does the soil affect species occurrence? Acta Herpetol 9:51–59
Céréghino R, Biggs J, Oertli B, Declerck S (2008) The ecology of European ponds: defining the characteristics of a neglected freshwater habitat. Hydrobiologia 597:1–6
Chester ET, Robson BJ (2013) Anthropogenic refuges for freshwater biodiversity: their ecological characteristics and management. Biol Conserv 166:64–75
Cirovic R, Radovic D, Vukov TD (2008) Breeding site traits of European newts (Triturus macedonicus, Lissotriton vulgaris, and Mesotriton alpestris: Salamandridae) in the Montenegrin karst region. Arch Biol Sci 60:459–468
Colburn EA, Weeks SC, Reed SK (2007) Diversity and Ecology of Vernal Pool Invertebrates. In: Calhoun AJK, DeMaynadier PG (eds) Science and conservation of vernal pools in Northeastern North America: ecology and conservation of seasonal wetlands in Northeastern North America. CRC Press, Boca Raton, pp 105–126
Curado N, Hartel T, Arntzen JW (2011) Amphibian pond loss as a function of landscape change—a case study over three decades in an agricultural area of northern France. Biol Conserv 144:1610–1618
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240
Dalbeck L, Janssen J, Völsgen SL (2014) Beavers (Castor fiber) increase habitat availability, heterogeneity and connectivity for common frogs (Rana temporaria). Amph Rept 35:321–329
Davies BR, Biggs B, Williams P, Whitfield M, Nicolet P, Sear D, Bray S, Maund S (2008) Comparative biodiversity of aquatic habitats in European agricultural landscapes. Agric Ecosyst Environ 125:1–8
Denoël M (2012) Newt decline in Western Europe: highlights from relative distribution changes within guilds. Biodivers Conserv 21:2887–2898
Denoël M, Ficetola GF (2008) Conservation of newt guilds in an agricultural landscape of Belgium: the importance of aquatic and terrestrial habitats. Aquat Conserv 18:714–728
Denoël M, Perez A, Cornet Y, Ficetola GF (2013) Similar local and landscape processes affect both a common and a rare newt species. PLoS ONE 8:1–11
Denton JS, Hitchings SP, Beebee TJC, Gent A (1997) A recovery program for the natterjack toad (Bufo calamita) in Britain. Conserv Biol 11:1329–1338
Edgar P, Bird DR (2006) Action Plan for the Conservation of the Crested Newt Triturus cristatus Species Complex in Europe. Council of Europe, Strasbourg
Eggert C (2002) Use of fluorescent pigments and implantable transmitters to track a fossorial toad (Pelobates fuscus). Herpetol J 12:69–74
Eggert C, Guyétant R (1999) Age structure of a spadefoot toad Pelobates fuscus (Pelobatidae) population. Copeia 4:1127–1130
Eggert C, Cogalniceanu D, Veith M, Dzukic G, Taberlet P (2006) The declining Spadefoot toad, Pelobates fuscus (Pelobatidae): paleo and recent environmental changes as a major influence on current population structure and status. Conserv Genet 7:185–195
Elmberg J (1993) Threats to boreal frogs. Ambio 4:254–255
Estonian nature information database, https://infoleht.keskkonnainfo.ee/. Accessed 1 Sep 2018
EU Habitats Directive 92/43/EEC, Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31992L0043. Accessed 1 Sep 2018
Ficetola GF, Rondinini C, Bonardi A, Baisero D, Padoa-Schioppa E (2015) Habitat availability for amphibians and extinction threat: a global analysis. Divers Distrib 21:302–311
Gustafson DH, Pettersson CJ, Malmgren JC (2006) Great crested newts (Triturus cristatus) as indicators of aquatic plant diversity. Herpetol J 16:347–352
Gustafson DH, Malmgren JC, Mikusinski G (2011) Terrestrial habitat predicts use of aquatic habitat for breeding purposes—a study on the great crested newt (Triturus cristatus). Ann Zool Fenn 48:295–307
Hartel T, Nemes S, Cogălniceanu D, Öllerer K, Schweiger O, Moga CI, Demeter L (2007) The effect of fish and aquatic habitat complexity on amphibians. Hydrobiologia 583:173–182
Harthun M (1999) Der Einfluss des Bibers (Castor fiber albicus) auf die Fauna (Odonata, Mollusca, Trichoptera, Ephemeroptera, Diptera) von Mittelgebirgsbächen in Hessen (Deutschland). Limnologica 29:449–464
Hazell D, Hero J, Lindenmayer D, Cunningham R (2004) A comparison of constructed and natural habitat for frog conservation in an Australian agricultural landscape. Biol Conserv 119:61–71
Heath DJ, Whitehead A (1992) A survey of pond loss in Essex, South-East England. Aquat Conserv 2:267–273
Hels T (2002) Population dynamics in a Danish metapopulation of spadefoot toads Pelobates fuscus. Ecography 25:303–313
Hosmer DW, Lemeshow S (2000) Applied logistic regression, 2nd edn. Wiley, New York
Hull A (1997) The Pond Life Project: a model of conservation and sustainability. In: Boothby J (ed) British Pond Landscape, Proceedings of the UK Conference of the Pond Life Project. Liverpool, pp 101–109
Ildos AA, Ancona N (1994) Analysis of amphibian habitat preferences in a farmland area (Po plain, northern Italy). Amph Rept 15:307–316
IUCN 2009 Red list of threatened species: http://www.iucnredlist.org/. Accessed 1 Sep 2018
Jehle R, Arntzen JW (2000) Post-breeding migrations of newts (Triturus cristatus and T. marmoratus) with contrasting ecological requirements. J Zool 251:297–306
Joly P, Miaud C, Lehmann A, Grolet O (2001) Habitat matrix effect on pond occupancy in newts. Conserv Biol 15:239–248
Kauri H (1947) Die Verbreitung der Amphibien und Reptilen in Estland. Scripta Societatis Litterarum Esthonicae in Suecia, Ser. A. Stockholm
Laurila A (1998) Breeding habitat selection and larval performance of two anurans in freshwater rock-pools. Ecography 21:484–494
Lemmens P, Mergeay J, De Bie T, Van Wichelen J, De Meester L, Declerck SAJ (2013) How to maximally support local and regional biodiversity in applied conservation? Insights from pond management. PLoS ONE 8:e72538
Martinez-Sanz C, Cenzano CSS, Fernández-Aláez M, Garcia-Criado F (2012) Relative contribution of small mountain ponds to regional richness of littoral macroinvertebrates and the implications for conservation. Aquat Conserv 22:155–164
Mazerolle MJ, Poulin M, Lavoie C, Rochefort L, Desrochers A, Drolet B (2006) Animal and vegetation patterns in natural and man-made bog pools: implications for restoration. Freshw Biol 51:333–350
McDonald JH (2014) Handbook of biological statistics, 3rd edn. Sparky House Publishing, Baltimore
Miaud C (1994) Role of wrapping behavior on egg survival in three species of Triturus (Amphibia: Urodela). Copeia 2:535–537
Miaud C (1995) Oviposition site selection in three species of European newts (Salamandridae) genus Triturus. Amph Rept 16:265–272
Miró A, O’Brien D, Hall J, Jehle R (2017) Habitat requirements and conservation needs of peripheral populations: the case of the great crested newt (Triturus cristatus) in the Scottish Highlands. Hydrobiologia 792:169–181
Morand A, Joly P (1995) Habitat variability and space utilization by the amphibian communities of the French Upper-Rhone floodplain. Hydrobiologia 300(301):249–257
Nielsen SM, Dige T (1995) A one season study of the common spadefoot, Pelobates fuscus. Memoranda Soc Fauna Flora Fennica 71:106–108
Nöllert A (1990) Die Knoblauchkröte Pelobates fuscus. Die Neue Brehm Bücherei, Wittenberg Lutherstadt
Nyström P, Birkedal L, Dahlberg C, Brönmark C (2002) The declining spadefoot toad Pelobates fuscus: calling site choice and conservation. Ecography 25:488–498
Nyström P, Hansson J, Månsson J, Sundstedt M, Reslow C, Broström A (2007) A documented amphibian decline over 40 years: possible causes and implications for species recovery. Biol Conserv 138:399–411
Oertli B, Joye DA, Castella E, Juge R, Cambin D, Lachavanne JB (2002) Does size matter? The relationship between pond area and biodiversity. Biol Conserv 104:59–70
Oertli B, Biggs J, Céréghino R, Grillas P, Joly P, Lachavanne JB (2005) Conservation and monitoring of pond biodiversity: introduction. Aquat Conserv 15:535–540
Péntek AL, CsabaFV Zsuga K, Horváth Z (2017) Metacommunity dynamics of amphibians in years with differing rainfall. Aquat Ecol 51:45–57
Per Sjögren-Gulve (1994) Distribution and extinction patterns within a Northern metapopulation of the pool frog, Rana lessonae. Ecology 75:1357–1367
Petranka JW, Holbrook CT (2006) Wetland restoration for amphibians: should local sites be designed to support metapopulations or patchy populations? Restor Ecol 14:404–411
Petranka JW, Harp EM, Holbrook CT, Hamel JA (2007) Long-term persistence of amphibian populations in a restored wetland complex. Biol Conserv 138:371–380
Piha H, Louto M, Merila J (2007) Amphibian occurrence is influenced by current and historic landscape characteristics. Ecol Appl 17:2298–2309
Porej D, Hetherington TE (2005) Designing wetlands for amphibians: the importance of predatory fish and shallow littoral zones in structuring of amphibian communities. Wetl Ecol Manag 13:445–455
Preisser EL, Kefer JY, Lawrence JD, Clark TW (2000) Vernal pool conservation in Connecticut: an assessment and recommendations. Environ Manage 26:503–513
Rannap R, Lõhmus A, Jakobson K (2007) Consequences of coastal meadow degradation: the case of the natterjack toad (Bufo calamita) in Estonia. Wetlands 27:390–398
Rannap R, Lõhmus A, Briggs L (2009a) Restoring ponds for amphibians: a success story. Hydrobiologia 634:87–95
Rannap R, Lõhmus A, Briggs L (2009b) Niche position, but not niche breadth, differs in two coexisting amphibians having contrasting trends in Europe. Divers Distrib 15:692–700
Rannap R, Lõhmus A, Linnamägi M (2012) Geographic variation in habitat requirements of two coexisting newt species in Europe. Acta Zool Acad Sci H 58:69–86
Rannap R, Markus M, Kaart T (2013) Habitat use of the common spadefoot toad (Pelobates fuscus) in Estonia. Amph Rept 34:51–62
Rannap R, Kaart T, Iversen LL, Briggs L, Vries W (2015) Geographically varying habitat characteristics of a wide-ranging amphibian, the common spadefoot toad (Pelobates fuscus), in Northern Europe. Herpetol Conserv Biol 10:904–916
Red Data Book of Estonia 2008. Commission for Nature Conservation of the Estonian Academy of Sciences. http://www.envir.ee/sites/default/files/elfinder/article_files/eesti_punane_raamat_2008.pdf. Accessed 1 Sep 2018
Remm L, Lõhmus A, Rannap R (2015) Temporary and small water bodies in human-impacted forests: an assessment in Estonia. Boreal Environ Res 20:603–619
Remm L, Vaikre M, Rannap R, Kohv M (2018) Amphibians in drained forest landscapes: conservation opportunities for commercial forests and protected sites. For Ecol Manag 428:87–92
Reshetnikov AN (2003) The introduced fish, rotan (Perccottus glenii), depresses populations of aquatic animals (macroinvertebrates, amphibians, and a fish). Hydrobiologia 510:83–90
Rhazi L, Grillas P, Saber ER, Rhazi M, Brendonck L, Waterkeyn A (2012) Vegetation of Mediterranean temporary pools: a fading jewel? Hydrobiologia 689:23–36
Rooma I, Voiman V (2002) Estonian soils. Estonian Encyclopedia, 11, Tallinn (in Estonian)
Rothermel BB (2004) Migratory success of juveniles: a potential constraint on connectivity for pond-breeding amphibians. Ecol Appl 14:1535–1546
Schmidt BR, Van Buskirk J (2001) Verhalten, Wachstum und Morphologie von Kammolch-Larven in der An- und Abwesenheit von Libellenlarven. RANA Sonderheft 4:179–191
Semlitsch RD (2000) Principles for management of aquatic-breeding amphibians. J Wildl Manag 64:615–631
Semlitsch RD (2002) Critical elements for biologically based recovery plans of aquatic-breeding amphibians. Conserv Biol 16:619–629
Semlitsch RD, Bodie JR (1998) Are small, isolated wetlands expendable? Conserv Biol 12:1129–1133
Skei JK, Dolmen D, Rønning L, Ringsby TH (2006) Habitat use during the aquatic phase of the newts Triturus vulgaris (L.) and T. cristatus (Laurenti) in central Norway: proposition for a conservation and monitoring area. Amph Rept 27:309–324
Soomets E, Rannap R, Lõhmus A (2016) Patterns of assemblage structure indicate a broader conservation potential of focal amphibians for pond management. PLoS ONE 11:e0160012. https://doi.org/10.1371/journal.pone.0160012
Soomets E, Lõhmus A, Rannap R (2017) Brushwood removal from ditch banks attracts breeding frogs in drained forests. For Ecol Manage 384:1–5
Stevens CE, Paszkowski CA, Scrimgeour GJ (2006) Older is better: beaver ponds on Boreal streams as breeding habitat for the wood frog. J Wildl Manag 70:1360–1371
Suislepp K, Rannap R, Lõhmus A (2011) Impacts of artificial drainage on amphibian breeding sites in hemiboreal forests. For Ecol Manage 262:1078–1083
Székely D, Cogălniceanu D, Székely P, Denoël M (2017) Out of the ground: two coexisting fossorial toad species differ in their emergence and movement patterns. Zoology 121:49–55
Sztatecsny M, Jehle R, Schmidt B, Arntzen JW (2004) The abundance of premetamorphic newts (Triturus cristatus, T. marmoratus) as a function of habitat determinants: an a priori model selection approach. Herpetol J 14:89–97
Tiberti R, Bogliani G, Brighenti S, Iacobuzio R, Liautaud K, Rolla M, von Hardenberg A, Bassano B (2018) Recovery of high mountain Alpine lakes after the eradication of introduced brook trout Salvelinus fontinalis using non-chemical methods. Biol Invasions. https://doi.org/10.1007/s10530-018-1867-0
Vaikre M, Remm L, Rannap R, Voode M (2018) Functional assemblages of macroinvertebrates in pools and ditches in drained forest landscape. Wetlands 38:957–964
Van Dyke F, Berthel A, Harju SM, Lamb RL, Thompson D, Ryan J, Pyne E, Dreyer G (2017) Amphibians in forest pools: does habitat clustering affect community diversity and dynamics? Ecosphere 8:e01671. https://doi.org/10.1002/ecs2.1671
Vojar J, Doležalová J, Solský M, Smolová D, Kopecký O, Kadlec T, Knapp M (2016) Spontaneous succession on spoil banks supports amphibian diversity and abundance. Ecol Eng 90:278–284
Vuorio V, Heikkinen RK, Tikkanen O-P (2013) Breeding success of the threatened great crested newt in boreal forest ponds. Ann Zool Fenn 50:158–169
Williams P, Whitfield M, Biggs J, Bray S, Fox G, Nicolet P, Sear D (2004) Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol Conserv 115:329–341
Wood PJ, Greenwood MT, Agnew MD (2003) Pond biodiversity and habitat loss in the UK. Area 35:206–216
Zero VH, Murphy MA (2016) An amphibian species of concern prefers breeding in active beaver ponds. Ecosphere 7:e01330. https://doi.org/10.1002/ecs2.1330
Acknowledgements
We thank E. Soomets, L. Remm, T. Kaasiku for help in the field, A. Lõhmus for constructive comments to the analysis and Sonya Thayer for language correction.
Funding
The study was financially supported by the Estonian Research Council (grants no IUT 34-7, 9051, PRG314, PUT1363) and EU LIFE + project LIFE08NAT/EE/000257(DRAGONLIFE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.




Appendix
Appendix
Rights and permissions
About this article
Cite this article
Magnus, R., Rannap, R. Pond construction for threatened amphibians is an important conservation tool, even in landscapes with extant natural water bodies. Wetlands Ecol Manage 27, 323–341 (2019). https://doi.org/10.1007/s11273-019-09662-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-019-09662-7