Abstract
Atrazine, a herbicide used for controlling broadleaf weeds, has been one of the predominant pollutants constituting 80–90% of detection frequency in the samples collected from rivers, estuaries, oceans, sediments, agricultural lands, and crops. The fate of atrazine is highly unpredictable depending on the physio-chemical, physiological and geographical conditions. Range of metabolites such as deethylatrazine (DEA), deisopropyl atrazine (DIA), and didealkylatrzine (DDA) are formed as a result of biotic as well as abiotic degradation process in the environment following cyanuric acid, ammelide, CO2 and NH3 are formed as final products. Atrazine degraded products has shown more hazardous nature than the parent compound, atrazine. Atrazine is banned in Italy, India, Germany and European union but widely used in China, Australian, Canadian and US agriculture. To date, reviews evaluating the assimilation of synerigistic treatment technologies and comparative degration mechanism have not been highlighted. This work focuses on (1) the spatiotemporal distribution of atrazine and its metabolites globally and the factors governing it (2) provides an in-depth discussion about the various studies showing the toxicity of atrazine in microbes, cattle, human, terrestrial and aquatic organisms; (3) discusses the contaminants of emerging concern which are continuously replacing atrazine like terbuthylazine and their intermediate compounds posing more risk to wildlife and humans; (4) summarises the different treatment technologies which have been predominantly applied for the removal of atrazine in water and soil systems and also discusses the synergistic or mutualistic aspects of treatment methods in degrading atrazine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Developed in 1958, atrazine is a herbicide used prominently for controlling broadleaf weeds and grasses in crops like corn, maize, millets, and sugarcane. Being a herbicide, it works by inhibiting the photosynthetic electron transport chain in plants (Rodríguez-González et al., 2017). The molecular formula of atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine) is C8H14ClN5, it’s melting point varies between 173 °C-175°C. It has low biodegradability and solubility ranges between 30-33 mg L−1 at 20 °C (Hong et al., 2022). Atrazine (ATZ) such as simazine, and terbuthylazine (TBA) belong to the chlorotriazine class of herbicides. Soil adsorption coefficient (KOC) and half-life (DT50) are fundamentals in assessing pesticide persistence and dissipation risk in different environment matrices (Góngora-Echeverría et al., 2019). Adsorption of atrazine to soil constituents is moderate with Kd values ranging in between 0.4—3.1 L kg−1(Salazar-Ledesma et al., 2018). The half-life of atrazine with no previous application on soil was 14.5 days while with previous history application the half-life reduced to 2.3 days suggesting widespread application over time in soil leads the development of atrazine degrading bacteria (Barrios et al., 2019). It is a weak base, its pKa is 1.7. If the pH of soil solution comes around pKa leading to migration of atrazine into groundwater or surface water (Salazar-Ledesma et al., 2018). In aquatic ecosystems half-life ranges from a few months to several years (Tulcan et al., 2021).
The ubiquitous nature of herbicides and their metabolites in different environments is posing a risk to human health and other living organism. The annual usage of atrazine is approximately 70,000–90,000 tons worldwide (Zhang et al., 2018). Canada, China and USA are the major consumers of atrazine. However, owing to toxicity issues, atrazine was banned in Italy and Germany in 1991 (Scherr et al., 2017). Even after 20 years of ban of atrazine by European union, atrazine and its by-product, deethylatrazine presence in groundwater was detected above the threshold value of 0.1 µg L−1in Germany (Vonberg et al., 2014). Several reports are available across countries like USA, China, and Spain where atrazine was frequently detected crossing the regulatory levels, exceeding the toxicological end points for organisms indicating the prevalence of atrazine at high concentrations (de Araújo et al., 2022).
The permissible limits of atrazine and its metabolites (diaminochlorotriazine, desethylatrazine, hydroxyatrazine, deisopropylatrazine, and atrazine mercapturate) are 5 µg L−1 as per WHO guidelines (Dehghani et al., 2022) and 3 µg L−1 in the United states while in Europe it is 0.1 µg L−1. Several reports are available in literature where the atrazine and its metabolite concentrations are above the permissible limits.The lipophilic nature of atrazine leads to bioaccumulation in the exposed organisms and the food chain. The Liphophilicity of atrazine, Log D at pH 7.4 is 2.20 representing more water soluble nature of this compound whereas for atrazine degraded products Log D values are even more smaller indicating the presence of more polar groups in the chemical structure (Furtado et al., 2019). Atrazine has been reported to be mutagenic, genotoxic, and morphotoxic in different exposed organisms (de Oliveira et al., 2020). Several epidemiological studies have shown that atrazine, even at lower than the maximum contaminant level (MCL), causes alteration in the functioning and formation of different parts of the brain, reproductive system, neuronal cell development, and neuroendocrine developmental process (Abass et al., 2021; Sadeghnia et al., 2021; Shan et al., 2021; Stradtman & Freeman, 2021). Some metabolites of atrazine such as desethylatrazine and deisopropylatrazine have been reported to have similar toxicity and structure to that atrazine, while hydroxyatrazine shows different toxicological properties from atrazine (Geng et al., 2013). Terbuthylazine (TBA), a triazine family herbicide have been classified in carcinogen category 3 by European Food Safety Authority (EFSA) (Bottoni et al., 2013). It has already replaced atrazine, causing frequent detection in most of the EU countries, including Italy, Spain and Portugal (Álvarez et al., 2016; Bottoni et al., 2013). It has been detected in pristine marine ecosystem of North Sea, Mediterranean basin achieving the status of chemical of emerging concern because of its persistence and hazardous nature even at lower concentrations (Brumovský et al., 2017; Mai et al., 2013; Navarro et al., 2004). At cellular and animal organism level, TBA induces toxicological risk as it causes low-level DNA instability, DNA cross-links and cytotoxic effects (Želježić et al., 2018).
Several processes for the mineralization of Atrazine in aqueous and soil system have been reported (Arar et al., 2023; Liu et al., 2023; Tuğaç et al., 2023; Wang et al., 2022; Zhang et al., 2023). The treatment technologies include bioremediation, biological adsorption, oxidation, reduction, immobilization, nanofiltration, and adsorption (Gao et al., 2018; Khandarkhaeva et al., 2017; Moeini et al., 2019). Also, conventional drinking water treatment methods such as chlorination have limited degradation capacity for atrazine removal.
Although several reviews on atrazine treatment have been published (Mili et al., 2022; Rostami et al., 2021; Singh et al., 2018) few have discussed the occurrence, toxic effects, and advanced oxidation processes (AOPs). Hybrid approaches to the remediation of atrazine have also not been discussed. This review discusses the spatio-temporal distribution of atrazine in several countries, and also an overview of the toxicity in different organisms at different stages of their growth. In addition, this review discusses the various treatment processes, new technologies, and their efficiency in remediating atrazine from the water and soil environment.
2 Occurrence
Due to greater application, atrazine presence is detected in biotic and abiotic components like water, sediments, different organisms, and food (Yin et al., 2020). Due to its symmetrical molecular structure, hydrophobic nature, and low solubility, it retains in the aqueous environment for a longer time (de Souza et al., 2020).
2.1 Occurrence in the Water Resources
Atrazine has been applied for more than 60 years in several countries worldwide, with an estimated use of 60,000–70,000 tons annually (Y. Xue et al., 2021a, b). The frequent detection of atrazine in coastal bay areas worldwide implies that the continuous application on agricultural lands also leads to transportation and leaching of atrazine in open seas due to various geographical, environmental, and climatic factors (Nowell et al., 2018). The half-life period of atrazine ranges between 41 and 237 days in freshwater, with an average of 159 days, but in saltwater, it varies between 3–190 days (Almasi et al., 2020). In a study conducted in the seawater of Xiangshan Harbor, the concentration of atrazine was between 3.99 and 73.0 ng L−1, which was higher than the maximum permissible limit for coastal areas in the European union (Y. Xue et al., 2021a, b). In the southern Baltic Sea area, the mean value of atrazine was 2.2 ng L−1 (Fisch et al., 2021). Atrazine was also detected in Bohai (31.2–112.20 ng L−1 range), yellow sea, the Liaodong Peninsula (23.3 ng L−1 mean concentration) and Laizhou Bay (6.8- 83.0 ng L−1 range) (Xie et al., 2019). On the Portuguese coast, the concentration of atrazine was detected around 35.3 ng L−1, which was related to medium risk in the sea (Sousa et al., 2020). In the Jiaozhou Bay of eastern China, the spatial distribution of atrazine and its degraded primary and secondary metabolite products were compared in water, suspended particulate sediment and surface sediment in an estuary-to-bay system. Deisopropylhydroxy-atrazine (DIHA), Hydroxy- atrazine (HyA), and Deethylhydroxy-atrazine (DEHA) in seawater were estimated with concentrations around 80.77 ng L−1, 12.54 ng L−1, 15.11 ng L−1, and 4.95 ng L−1, respectively. Higher atrazine concentrations was detected in the sea water near the coast then in the estuary and the bay showing the settlement of contaminants in sediments during transportation with seawater. (Ouyang et al., 2021). In the Jiaozhau Bay, atrazine and its metabolites was observed, reaching maximum in August, suggesting that difference in temperature and other climatic factors affects the transportation as well as degradation of atrazine and metabolites (Z. Wang et al., 2021a, b). In the ctalmochita River basin, atrazine detection was higher in the spring season as monitored for the year (2011–2015), stating that season plays a role in fluctuating the atrazine level (Bachetti et al., 2021) In the St. Lawrence Estuary and Gulf aquatic environments, predominantly atrazine was found at a high range of 34 ng L−1. (Picard et al., 2021).
The continuous application of pesticides on agricultural lands makes them enter into surface water through continuous leaching and surface runoff. Proactive precautionary measures already taken as European union banned the use of atrazine as it exceeded permissible drinking water concentrations limits of 0.1 µg/L (van Rensburg et al., 2022b). In the drinking water treatment plants in Brazil, atrazine as well as its metabolite HA, DIA and DEA had a detection frequency of 100% from 2 to 2744 ng L−1 (Vizioli et al., 2023). In rural areas of Nigeria, the atrazine levels ranged from 10 to 80 µg L−1 when assessed in drinking water (Loureiro et al., 2023). Atrazine was detected at the highest concentration in more than 99% of samples taken from water bodies near agricultural land in the Mid-Atlantic area with concentrations up to 1.9 µg L−1, indicating atrazine was a major pollutant (Zhu et al., 2021). In Paranoa lake, during the rainy season, surface water samples detect the concentration of atrazine at a range of 1.2 ± 0.9 to 5.5 ± 0.4 ng L−1 (Bachetti et al., 2021). Atrazine and its metabolites were also equally detected in Danube River, Hungary River, Brittany River (France), the Arno River, Klodnica River (Poland), Spanish Duero River, Miño River, Ebro River, Scheldt River (Netherland), Tagus River (Portugal), the concentration ranges from 18.1 to 105.5 ng L−1 with a mean value of 54.4 ng L − 1 (Triassi et al., 2022).
Atrazine presence was evaluated by the U.S. EPA National Lake assessment,the 2021 U.S. Department of agriculture cropscape and the global HydroLAB HydroLAKES databases where atrazine was detected in 32% of the U.S. waterbodies with a mean concentration of a 0.17 µg L−1 (Beaulieu et al., 2020). In the lower Gangetic River basins (WBB) of West Bengal, India, Atrazine (0.95 – 3.93 µg L−1) was detected at a very high concentration, surpassing the maximum level by 46 times (Duttagupta et al., 2020; Organization, 2004). Currently, TBA, an alternative to atrazine is the most detected herbicide in the natural waters of Spain due to its continuous and increased usage (Herrero-Hernández et al., 2017). Table 1, 2, 3, 4, 5, 6, 7, and 8.
2.2 Occurrence in Agricultural Land
Atrazine is a persistent organic pollutant whose half-life can range from 2 to 57 weeks in environment, depending upon soil characteristics, application history and various other factors whereas its metabolites can reside up to 4 months post-application (Dehghani et al., 2022) (Y. Zhang et al., 2021a, b). The persistence and soil interaction of atrazine is governed by the concentrations of dissolved organic matter (DOM) and soluble soil organic matter (SOM). Atrazine is predominantly sorbed by SOM whereas presence of humic acids,clay minerals play a huge role in migration, degradation, availability, sorption and accumulation of atrazine in soils.Clay minerals and smectites soil particularly have higher potential of atrazine sorption (Salazar-Ledesma et al., 2018). In a study done on agricultural land of Brazil on red and yellow soil for 70 days, atrazine shows a half-life of 10 days in sunlight while in shadow the half life is 19 days (de Paula et al., 2016). It has been reported that atrazine undergoes many transformations depending on geographical, agricultural, temperature conditions and climatic factors (Srivastava et al., 2017) (Yu et al., 2020). Atrazine was detected at deeper layers of soil, where the corn root was unable to reach, showing the ease of migration and adsorption in the soil system (de Oliveira et al., 2019). The distribution of atrazine in major aquatic bodies and soils around the world is depicted in Table 1. Another study reported that even after 20 years of application in agricultural soil of Germany and Belgium atrazine was detected at 19.5 g ha−1 (Jablonowski et al., 2011). In a study done for the adsorption–desorption characteristic of atrazine in three soils, atrazine sorption capacity ranked as paddy soil > alluvial soil > laterite stating sorption is governed by the presence of sorption sites on the soil surfaces irrespective of atrazine concentration (Yue et al., 2017).
In the cultivated corn field in Croatia, when atrazine was applied at a concentration of 1 kg ha−1 in soil, it remained detected until 5 months of corn harvesting (Stipičević et al., 2015). We summarized and described how the contaminant atrazine enters the food web, hampering the exposed organism's life cycle at different stages in Fig. 1.
3 Toxicity of Atrazine on Non-Target Organisms
The causal effect of Atrazine and its metabolite on the living system depends upon the time the organism has been exposed, the residual concentration of the metabolite in the living system, and physiochemical, physiological, geographical, as well as biological factors. Atrazine has shown genotoxic and mutagenic effects on yeasts and plants, while chromosomal aberrations and DNA damage have been reported in mammalian cell lines.
3.1 Inhibitory Effects on Mammals
Atrazine has been observed to have broad implications on the human body as it’s continuous presence in drinking water makes it more pernicious to multiple organs affecting the human body's reproductive, neuronal, and immunological cells (Surmeier et al., 2017). Being an endocrine-disrupting chemical, it plays an interwined role in targeting the reproductive and neuroendocrine system. (Stradtman & Freeman, 2021).
Atrazine exposure is associated with many neurodegenerative problems, including Parkinson’s disease, schizophrenia, and attention deficit disorder (P. Li et al., 2021a, 2021b). Substantia nigra pass compacta, and striatal dopaminergic neurons are most prone to atrazine toxicity as it has caused degeneration of dopaminergic neurons in the substantia nigra. (Li et al., 2020) (P. Li et al., 2021a, 2021b). Phosphatidycholine and CDP- choline are important metabolites associated with neurotransmitter synthesis and transmission, lipid transportation and bile acid secretion (Kennelly et al., 2018; McDaniel et al., 2002; Trousil et al., 2014). Atrazine exposure decreased the production of phosphatidylcholine and CDP-choline indicating the altered metabolism of these compounds(Yin et al., 2020). It has been observed in mice that Perinatal exposure, even to a lower dose of atrazine (1.4 mg kg−1), has been associated with abnormal behavior patterns linked with brain dopamine and serotonin disruption (Lin et al., 2014). Exposing rats to atrazine has caused alteration in the expression levels of proteins like transferrin receptor (TFR), divalent metal transporter1 (DMT1), Hephaestion (HEPH), and ferroportin1(fpn1), which are essential regulators of Fe transporter maintaining homeostasis of the mid brain (B. Li et al., 2021a, b). In the zebrafish, atrazine exposure has shown adverse effects on the development of primary and secondary sexual characters in males and females, affecting LH surge, delay in mammary gland development, delayed vaginal opening, and GnRH release (Gonadotropin releasing hormone) (Stradtman & Freeman, 2021).
Atrazine has been shown a significant decrease in methylated CPG (meCPG) proteins, histone 3 lysine, and 9 methylated (H3K9me3) proteins, causing altered enzymatic expression levels overall decreasing the cell growth rate in human kidney cell line (HEK293T) (Sánchez et al., 2020). At the genetic level, metabolomics studies indicate that atrazine alters the expression of various anti-apoptotic Bcl-2, Lc3-II, Mtorc1, TNF-α, IL6, BEX 2, and GSH genes, causing a change in the expression of several proteins (Li et al., 2020). To understand the toxicity and underlying mechanism involved, various toxicology tests have been performed on human SH-SY5Y cell lines showing decreased viable neuroblastoma cells and increased MCF7 cell proliferation (Sogos et al., 2021); (Lu et al., 2022). Figure 2a represents the adverse effects of atrazine exposure on different organs of the human body, disturbing regular physiological activity, while 2b depicts the adverse outcome on the health of pregnant women when exposed to atrazine, causing many abnormalities during and post partum.
Atrazine exposure showed metabolic alterations in folate biosynthesis, affecting purine and pyrimidine synthesis and suppressing various physiological processes like cell division (Lu et al., 2022). In metabolomics study determining the alteration in metabolic pathways, the metabolites synthesis is getting affected has been linked to oxidative stress, downregulation of gene expression with an increase in gluconeogenesis and reduced oxidative phosphorylation and ATP synthesis mechanism such as glycolysis and citric acid cycle. (Lin et al., 2014); (Yin et al., 2020). Intoxication occurred in cattle when ten of 40 cows died because of, multi-organ mitochondrial dysfunction and oxidative stress indicating acute toxicity (Props et al., 2021). Atrazine mercapturate (metabolite) presence was detected in urine samples of corn farmers', but no major association has been detected between the atrazine metabolite and the three markers of oxidative stress that are malondialdehyde (MDA), 8-hydroxy-2’-deoxyguanosine (8-OHdG), and 8-isoprostaglandin-F2α (8-isoPGF). MDA is created when Reactive oxygen species reacts with polyunsaturated fatty acids, 8-hydroxy-2’-deoxyguanosine (8-OHdG) is a marker of oxidative injury causing lesion in DNA (Muniz et al., 2008) while (8-isoPGF) is a prostaglandin compound produced during non-enzymatic lipoprotein peroxidation (Lee et al., 2006). However when atrazine mercapturate measured above limit of detection (LOD) an association with 8-OHdG was observed (Lerro et al., 2017). The no observed adverse effect level (NOAEL) and lowest observed adverse effect level (LOAEL) for infants and children are considered at 6.25 mg/kg/day and 12.5 mg/kg/day for short-term oral dietary exposure. Meanwhile for females aged (13–50 years), US EPA NOAEL is 10 mg/kg/day whereas the LOAEL is 70 mg/kg/day (Stradtman & Freeman, 2021).
3.2 Inhibitory Effects on Aquatic Organisms
Atrazine has been shown to induce histopathological, hematological, reproductive, carcinogenic, and genotoxic changes in aquatic organisms. In the aquatic system, the half-life of atrazine is about 168 days (Stradtman & Freeman, 2021). we summarized the inhibitory aspects of atrazine on various aquatic and non-aquatic organisms exposed at a specific concentration and periods in Table 2. Atrazine has been shown to cause oxidative damage in microalgae, studied by calculating antioxidant response and oxidative stress in algae (Castro et al., 2021). Atrazine and its metabolites desethyl atrazine and de isopropyl atrazine are phytotoxic, directly affecting various phytoplankton (Yang & Zhang, 2020). Office of environmental health hazard management (OEHHA) officially declared atrazine had endocrine disruptor activity mainly in reproduction toxicity (Zheng et al., 2017). Reproductive toxicity has been observed to cause population declines in various aquatic organisms, including amphibians, gastropods, shellfish, fishes, and crustaceans (Lopes‐Lima et al., 2017).
At a concentration range of (0.02-20 mg L−1), abnormalities ranging from axial shortening, tail flexure, and facial edema have been noticed in Anaxyrus americanus, Lithobates pipiens, and Lithobates sylvaticus frogs (Rohr & McCoy, 2010). The continuous leaching of pesticides in aquatic ecosystems shows the highest bioaccumulation in fishes in the food chain (Yang et al., 2021). Meanwhile, the gonadal intersex abnormality is observed due to atrazine toxicity in fishes, wherein an egg yolk precursor protein, the vitellogenin gets abnormal. This vitellogenin protein is responsible for improper sex development, abnormal steroid levels, fertility, and reproductive problems in aquatic organisms (Rohr & McCoy, 2010). Atrazine reduces the expression of vitellogenin protein in the female crayfish ovaries and hepatopancreas forming smaller oocytes (Silveyra et al., 2018). In estuarine crabs, it has been seen that atrazine exposure has decreased the growth of ovaries (Silveyra et al., 2017). In fathead minnows, an atrazine concentration range of (0.5–50 g L−1) downregulated vitellogenin protein, decreasing oocyte maturation (Ali et al., 2018). At a concentration range of 2.5 g L−1 atrazine, feminine characteristics were observed in male Xenopus laevis and increased production of testicular oocytes was observed on consecutive exposure of 7 days (Ali et al., 2018).
The embryotoxicity of atrazine and its degraded products (DE isopropyl, atrazine, and desethyl atrazine) was studied in zebrafish, observing mortality, hatching, and edema at 24, 48, 72 and 96 h post fertilization (hpf) showing retardation in hatching at 96 hpf and pericardial edema just at 48 hpf at varying concentrations (Zheng et al., 2017). Atrazine exposure of 30–300 µg L−1 disturbs the swimming pattern of larval zebrafish (Tai et al., 2021). In common carp, mortality was found at an atrazine concentration of 0.3–300 g L−1 when exposed for 33 days (Blahova et al., 2020). Increased axial malformations were reported when tadpoles were exposed to atrazine (Hanson et al., 2019). Over six days, exposure to 0.003 mg L−1 atrazine in goldfish has been shown to cause severe stress on circadian rhythm (Ren et al., 2019). Atrazine exposure has been shown to cause detrimental effects on oxidative stress, protein carbonyl level, cell injury, and lysosomal stability by generating free radicals when exposed for 7 days in marine blue mussels (Shaw et al., 2019). However, in green mussels, exposure of atrazine did not affect immunity and differentiation in the male and female sexes (Juhel et al., 2017). In oysters, high concentrations of atrazine exposure induce GST (glutathione s-transferase), further activating antioxidant enzymes in different organs (Geret et al., 2013).
3.3 Inhibitory Effects on Microbiota
Various studies have shown the effects of atrazine on regulating parameters like growth activity (phytohormones), biochemical functions, cell physiology, cell morphology and molecular activity, and microbial communities (Shahid et al., 2019). It has been reported that atrazine causes decreased plant growth-promoting traits in Azotobacter vinelandii strain AZ6, decreasing IAA (Indole 3 acetic acid) production, phosphate solubilization, and phenolate siderophore production, causing oxidative damage and membrane destruction (Shahid et al., 2019).
Gut microbiota is vital in maintaining gut and overall health in humans and animals. The presence of atrazine in food and crops has significantly disturbed gut microbes (Luo et al., 2021). It has also been reported that atrazine has altered the microbiome composition, decreasing Acetobacter aceti and Rhodospirillales compared to Lactobacillus acidophilus and other genera impacting the gut microbiome of Drosophila melanogaster (Brown et al., 2021). Atrazine either increases or has no significant effect on soil microbial biomass, as it has been reported that the respiration rate increases, boosting the soil microbial metabolic rate (Bonfleur et al., 2015). In a study on the effects of atrazine on the benthic microbial nutrient assessment by analyzing nutrient absorption and remineralization of phosphate, ammonium, and nitrate, atrazine has been shown not to have a significant impact on nutrient cycling (Elias & Bernot, 2014). When atrazine concentration (2-10 mg kg−1) was applied, microbial diversity decreased from 2.59 to 2.23, showing Microvirga species., Haplosporidium species., and Sphingopyxis species., absence compared to the control test (Chen et al., 2015). Applying atrazine has increased bacterial strains of Betaproteobacteria belonging to Methylophilicea and Nitrosomonadacea family (Briceño et al., 2010). At the genetic level, various changes have been detected when exposed to atrazine, causing the downregulation of some genes and affecting cell morphology and physiology (Brown et al., 2021). In mice, abundant Rodentibacter pneumotropicus has been reported when exposed to atrazine, which might cause ailments like conjunctivitis and autophagy signaling disturbance in the liver, causing abnormal enzyme cascades (Liu et al., 2021).
3.4 Inhibitory Effects on Non Target Aquatic and Crop Plants
The various physiochemical properties of soil, such as its binding capacity, phytotoxicity, and biotoxicity, determine the bioavailability, migration, and half-life of herbicides in the soil ecosystem and subsequently in the plants also (Y. Zhang et al., 2021a, b). Wolffia brasiliensis, an aquatic plant, showed a mortality rate of 16% at 11.2, 36.5, and 118.0 mg L−1 concentrations of atrazine, indicating the herbicide risk in the aquatic environment (Pereira et al., 2019). In Arabidopsis thaliana, plant atrazine interactions have shown several energy changes, causing mitochondrial dysfunction, increased ROS, and PSII inhibition (Alberto et al., 2017). When in carrots, cucumber, lettuce, onion, perennial ryegrass, and tomato, rate-response trends were observed, causing photosynthesis starvation in plants due to the generation of reactive oxygen species capable of degrading cell membranes (Brain & Hoberg, 2016). When exposed to more tolerant plants or to lower herbicide concentrations, this deleterious effect caused by reactive oxygen species reduces the photosynthetic rate of plants, reducing the accumulation of dry matter and altering the normal development of plant growth. In seagrass, Halophila ovalis exposure of herbicides has reduced the photosynthetic ability to block the electron transport chain by binding to protein in the thylakoid membrane and displacing the plastoquinone affecting the synthesis of ATP and NADPH (Wilkinson et al., 2015).
4 Atrazine and the Transformed Compounds
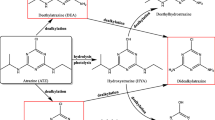
Atrazine belongs to the chloro s triazine class of compounds constituting atrazine, simazine, propazine, and chlorinated by-products. The metabolites formed as a result of the natural degradation of atrazine in the environment are de-ethyl ATZ (DEA), de-ethyldeisopropyl hydroxy ATZ (DEDIHA), de-isopropyl ATZ (DIA), di-dealkyl ATZ (DDA), hydroxy ATZ (HA), atrazine mercapturate (AM), desethylterbuthylazine (DET), de-ethylhydroxy ATZ (DEHA) and de-isopropylhydroxy ATZ (DIHA). The primary degraded products formed as a result of Atrazine degradation are deethylatrazine (DEA), deisopropylatrazine (DIA), and didalkylatrazine (DDA), show equal or more toxicity than atrazine (Bhatti et al., 2022). The occurrence, distribution, and degradation of atrazine and its metabolites depend on various parameters like seasonal variation, solar radiation, temperature, precipitation, dissolved oxygen, salinity, wind, sea currents, pH, the mode of transportation, and geographical area (Y. Xue et al., 2021a, b) (Bhatti et al., 2022). After the mineralization of atrazine and the intermediate metabolites, the final products consist of ammelide, cyanuric acid, CO2, and NH3. Table 3 depicts the different physio-chemical natures of atrazine and its metabolites formed due to the biodegradation of atrazine.
The potential of desethylterbuthylazine (DET) as a continuous leachate is notable (Bozzo et al., 2013) as its leaching potential (Gustafson, 1989) or Groundwater Ubiquity score (GUS) was estimated at 3.07 (Hertfordshire, 2017), showing higher leaching potential in soil. (Jian et al., 2021), whereas in seawater of Xiangshan Harbor atrazine and its metabolites detection fraction ranges ATZ (89.7%), DIA (4.6%), DEA (0%) and DDA (5.7%) (Y. Xue et al., 2021a, b). In soils, DEA (2700–3200 mgL−1) and DIA (670–980 mgL−1) showed more mobility as well as leachate fractions than the parent compound, ATZ (30–33 mgL−1), explaining the presence of less alkyl groups on the s-triazine rings increasing the polarity (Bhatti et al., 2022). In a study, it was corroborated that the parent compound (ATZ) degrades faster than its metabolites (DEA, DIA) as the presence of more alkyl subunits in atrazine enhanced the hydrolytic degradation suggesting metabolites may be more recalcitrant than the parent compound (Bhatti et al., 2022).
A study showed that the acute, carcinogenic, and mutagenic toxicity of atrazine and its metabolites pose a continuous threat to organism’s lives (Li et al., 2014). In other significant findings, it was found that not only ATZ but its metabolites, DACT, DIA, and DEA have the same or even more inhibitory effects on developmental and locomotory stages of zebrafish constituting even at 300 µg L−1 exposure of ATZ causes no inhibitory effects but 100 µg L−1 and 300 µg L−1 of DIA and DEA induces developmental toxicity (Liu et al., 2016). Another study showed that the concentration of atrazine and the degraded by-products in different aquatic organisms in Xiangshon harbor seawater shows that benthic organisms Ditrema and Black sea bream have increased bioconcentration of ATZ, posing low risk to fish and invertebrate (Y. Xue et al., 2021a, b). For soil species, springtail and earthworm, metabolites DEA and DIA pose unacceptable hazards, while for avian and mammalian species, it poses moderate to mild threats (Bhatti et al., 2022). Among the 15 metabolites formed due to atrazine degradation, the significant metabolites formed are DEA and DIA by the N dealkylation of the parent molecule's ethyl or isopropyl side chains (Lin et al., 2008). Figure 3 depicts the primary and secondary metabolites formed through different metabolic pathways.
5 Atrazine Remediation
Scopus database was used to search for the literature on the degradation of atrazine using the keywords (ATRAZINE AND DEGRADATION) OR (REMOVAL) OR (TREATMENT) OR (CONTROL). A total of 7,317 documents from the year 2012 to the current year (2023) were obtained out of which 3,585 documents were found to be relevant. VOS VIEWER network visualization tool was used to better understand the various treatment technologies applied and also the upcoming technologies. The threshold value selected was 5 showing the number of times the keyword is reoccurring. Figure 4 a shows the co-occurrence map. The different classes of Clusters show the topic area studied for the degradation of atrazine. A total of 15 clusters were observed out of which 6 clusters were found to be distinct and each is represented by a different colour. Figure 4 corroborates the research focus of the article. The map shows the diversity and uniqueness of degradation methods for the atrazine pollutant and their inter relatedness. Cluster 1 (dark green) Fig. 4 (a) focusses on advanced oxidation processes (AOPs) covering ozonation, UV, photocatalysis, hydroxide radical, UV/H2O2. Cluster 2 (dark brown) Fig. 4 (b) focusses on sorption, desorption and adsorption based on biochar, activated carbon as well as the related kinetic and mechanistic studies. Cluster 3 (dark orange) Fig. 4 (c) highlights biodegradation aspect of atrazine removal including mineralization, encapsulation, bioaugmentation involving different bacterial communities. Cluster 4 (light blue) highlights the monitoring of pollutant and its transformed products in surface water, drinking water using gc–ms, ic-ms/ms analysis. Cluster 5 (yellow) highlights the histopathological and immunotoxicity of atrazine in different organisms. Cluster 6 (dark red) covers the efficacy of atrazine in controlling the growth as well its effect on different crops. According to this cluster analysis, biodegradation of atrazine, AOPs, and toxicity have been the major areas of research focus. VOS VIEWER overlay visualization also points out to the recent application of synergistic removal techniques like photoelectrocatalysis, photo – fenton etc. and also the new classes of catalyst applied for AOPs.
5.1 Microbial Degradation of Atrazine
Microbial remediation has been extensively used for atrazine removal from contaminated soils as it has a low application cost and is less toxic to the environment (Rehan et al., 2014). The efficiency and mechanism of atrazine degradation depend on the soil microbial community and their physiology. Dichlorination, dealkylation, and deamination are the main degradation pathways utilized by bacteria for atrazine degradation. Some bacteria break atrazine through dichlorination, replacing the OH group with a chlorine atom. Hydroxyatrazine formed was further degraded into N- isopropylammelide or N-ethylammelide proceeding through hydrolytic deamination reactions, producing cyanuric acid's final degraded product. (Rostami et al., 2021). Gram-negative and Gram-positive bacteria follows different degradation pathways. Gram-positive bacteria degrade atrazine following a hydrolysis reaction catalyzed by enzymes such as TrzN, (triazine hydrolase) while gram-negative bacteria catalyze the reaction by the enzyme AtzA (atrazine chlorohydrolase) playing an essential role in the breakdown process. (Huang et al., 2017). Paenarthrobacter ureafaciens ZF1 a Gram + bacteria has the potential of degrading atrazine (99.3%) (100 mg kg −1) from soil while in liquid it could completely remove atrazine within 2 h (Zhang et al., 2022). Bacillus pumilus and Bacillus subtilis, which are gram + bacteria could, successfully degrade 95% and 98% of atrazine respectively (Zhu et al., 2022). Figure 5, shows the degradation pathway utilized by gram negative bacteria involving atrazine chlorohydrolase (AtzA) enzyme, hydroxydechloroatrazine ethylaminohydrolase (atzB), and N-isopropylammelide isopropylaminohydrolase (atzC).
New techniques, like microbial encapsulation, have been developed for treating soil contaminated with atrazine. (Rostami et al., 2021). It has also been observed that the microbial strain Bacillus velezensis MHNK1 producing surfactin lipopeptide resulted in 100 ± 1.20% degradation within 4 days. Presence of atrazine degrading genes and surfactin are potential source in removing atrazine (Jakinala et al., 2019). It has been shown that bioaugmentation has enhanced the atrazine-degrading efficiency of constructed wetland. Pseudomonas and Arthrobacter sp. were dominant among the atrazine degrading microbial community because of high adaptability and atrazine degrading capability in the constructed wetland (Zhao et al., 2019). Co-culture of Arthobacter sp. DNS10 and Enterobacter sp. P1 degraded 99.18 ± 1.00% of atrazine as compared to 38.57 ± 7.39% by the single microbial strain DNS10. The expression of the atrazine degradation-associated genes trzN, atzB, and atzC was also more as compared to single microbial strain treatment (Jiang et al., 2019). We summarized the different microbial strains used for treatment of atrazine and the biodegradation efficiency in Table 4.
5.2 Fungal Remediation
Fungi, an essential constituent of the soil ecosystem, play a significant role in degrading atrazine after bacteria. Fungi degrade or transform recalcitrant compounds into biotransformed products which are further broken down by other soil microorganisms (Maqbool et al., 2016). The plasmidial genes in bacteria atzA, atzB, and atzC encode the various enzymes breaking the compound through several metabolic pathways. Gene atzA follows dichlorination and s-triazine ring cleavage, gene atzB (hydroxyatrazine N-ethylamino hydrolase enzyme) proceeds the reaction by hydrolytic conversion of hydroxyatrazine to N-isopropylammelide whereas atzC (N-isopropylammelide isopropylaminohydrolase) catalyses the hydrolysis of N-substituted amino dihydroxy-s-triazines as well as N-isopropylammelide to cyanuric acid and isopropylamine (Fan & Song, 2014b). Further, genes encoding atzD metabolises cyanuric acid whereas atzE and atzF hydrolase cyanuric acid further yielding carbon dioxide and ammonia each of three moles (Fan & Song, 2014b). So far, these proteins have not been characterized in fungi. Enzyme atzA has been reported to catalyse the chlorohydrolysis of atrazine, deisopropylatrazine (DIA) and desethylatrazine(DEA), but not desethyldeisopropylatrazine (DEDIA) although this enzyme has been described only in bacteria, some researchers identified the chlorohydrolysis products from atrazine breakdown by fungus (Lopes et al., 2020). Conversly, Fungi have a complex set of hydrolytic and oxidative enzymes with N- dealkylation, deamination steps, or both forming DEA, DIA following diverse metabolic pathway (Esparza-Naranjo et al., 2021). Studies have shown that mycorrhizal and nonmycorrhizal fungi were associated with degrading atrazine. However, ericoid mycorrhizal fungi showed the best mineralization capacity, highlighting that the degradation depends upon herbicide and fungus, irrespective of fungal ecotype (mycorrhizal or free-living) (Donnelly et al., 1993).
Arbuscular mycorrhizal fungi (AMF), which forms a symbiotic association with the plants, has been observed to play a great role in removing atrazine. In studies it has been reported that atrazine removal efficiency was upto 74.65% in Medicago sativa mycorrhizal stating atrazine degradation rate is higher in mycorrhizal treatments than those in non-mycorrhizal treatments (Song et al., 2016). During the contaminants degradation process by AMF, the exudates secretion by fungi may change the dynamics of rhizosphere soil.
microbial activity affecting the rate of atrazine dissipation in the soil (Fan et al., 2020). Lignolytic enzymes produced by white rot fungi have been classified into three types of peroxidases-manganese, lignin peroxidases,
and laccases. Ligninolytic enzymes applied under the controlled or symbiotic association of deutromycetes with soil bacteria have shown complete mineralization of atrazine (Chan-Cupul et al., 2016; Jin et al., 2016). Fungi follow the intracellular and extracellular enzymatic degradation pathways wherein Basidiomycetes and ascomycetes follow the extracellular enzymatic ligninolytic complex degradation pathway leading to high degradation efficiency of atrazine (Deshmukh et al., 2016; Fan & Song, 2014a). Figure depicts the degradation of atrazine favours N-dealkylation of ethylamine and/or isopropylation Figure 6.
The mechanism N-desethylation and N-deisopropylation showing breakdown pathway of atrazine and subsequent metabolites in pleurotus ostreatus fungus adapted from (Lopes et al., 2020)
5.3 Phytoremediation
Phytoremediation is a widely used technique in which plants remove pollutants through degradation, uptake, evaporation, and by increasing the rhizobacterial communities. Plants with dense root systems help to reduce soil contaminants by decreasing the transport rate of herbicides in the soil, thereby acting as a natural filter in the environment(Chellaiah & Yule, 2018). Phytoremediation capacity is mainly shown by vascular plants, macrophytes, and some gramineous plants. Potentiality of some plants in remediating atrazine is well depicted because of its fast growth even in adverse conditions, large biomass exhibiting large specific surface area with ample macromolecules, and fibrous dense root system. The mechanism is generally associated with absorption, accumulation, and detoxification through enzymatic activity of glutathione-S-transferase (GST) as well as bacterial assisted rhizodegradation (Loureiro et al., 2023; McKnight et al., 2022; Zhang et al., 2023). The biosorption approach includes the absorption either by submerged roots and by the leaves.Plants secretion of root exudates provides energy source and favourable niche for rhizospheric bacterial communities increasing the activity of the microbial population by which the breakdown of herbicide is enhanced (Zhang et al., 2023). In a study on Cyperus alternifolius plant exposed to atrazine concentration at 20mgL−1, the phytoremediation efficiency was 91.28 ± 6.35% (Ameri Siahouei et al., 2020). Other findings showed that an increase of three times of Typha latifolia rhizomes causes an increase in the degradation rate of atrazine at twice the rate showing the result that terbuthylazine and its metabolite get accumulated in plant tissues (Papadopoulos & Zalidis, 2019). Macrophytes potamogeton crispus and Myriophyllum spicatum absorbed atrazine and DEDIA from the sediment, remediating atrazine from the sediment and water (H. Li et al., 2019a, b). Plants like eucalyptus, Hymenaea coubaril, and Cecropia hololeuca showed phytoremediation efficiency in the Quartzarenic Neosol soil (Heemann et al., 2018). In a study, it was found that the two treatments BR (Bean Rhizobium) and BRT (bean Rhizobium—Trichoderma) significantly caused the removal of 20 mg of atrazine from 50 g of soil (Madariaga-Navarrete et al., 2017). The plant species Iris versicolor has the potential to phytoremediate atrazine by 58.7% after 112 days of treatment, with no plant death reported. However, stunted growth or leaf injury was reported suggesting plants could produce more above-ground biomass to recover from herbicide contamination. (McKnight et al., 2022). The U.S environmental protection agency (2006) suggested less than 1µgL−1 of chronic atrazine for non-vascular plant species. However, metabolization of atrazine by crop plants are yet to be explored.
Prairie grasses have shown promising phytoremediation efficiency in removing atrazine which gets degraded into its metabolite, which later is accumulated in leaves at approximately (60 – 80)% (Madariaga-Navarrete et al., 2017). Another study showed that the Maize plant had more potential to accumulate atrazine in its tissues. (Sánchez et al., 2020). With the inoculation of Cannabis indica with Funneliformis mosseae, removal percentage rate of atrazine increased from 68.064% to 95.670%, indicating a viable phytoremediation approach for in situ remediation (Dong et al., 2016). A study found that maize plants planted with Pennisetum clandestinum degraded 45% of atrazine in about 80 days, showing Zea mays have the potential for phytoremediation of soils contaminated with atrazine (Ibrahim et al., 2013).
5.4 Advanced Oxidation Process
Advanced oxidation processes are chemical processes that include a set of chemical treatments, like ozone, hydroxide radicals, and UV irradiation. AOPs treatments are based on releasing active radicals like O2º¯,·OH, and SO4 −2 breaking the contaminants into small inorganic molecules. Oxidants like ozone, oxygen, hydrogen peroxide, sulfite ion, and catalysts like titanium oxide, metal oxides, and various compounds in the synergistic effect produce the radicals.
5.4.1 Photocatalytic Degradation
Photocatalysis is one of the most powerful technology used to treat organic contaminants due to its powerful mineralizing and oxidizing capacity (Xu et al., 2013). Photocatalytic degradation is associated with the formation of e¯—h + pair (Eq. 1) when irradiated with light, having a wavelength equal to or more than the bandgap energy on the semiconductor photocatalyst (Poonia et al., 2022). Further, the generated electrons and holes react with water or oxygen to form superoxide, hydrogen peroxide, hydroxyl radicals (Eq. 3,4), and hydroperoxyl radicals. Reactive oxygen species (ROS) generated plays a ubiquitous role in degrading atrazine ( Eq. 5,6 and 7) using an array of photocatalysts such as metal oxides (TiO2, ZnO) and sulfides (ZnS, CuS) (Poonia et al., 2022) and nano or mesoporous compounds (BaTiO3, Bi2MoO6) (Sobahi & Amin, 2021) (Sharma et al., 2019). A study showed that when an aqueous solution of atrazine was photolyzed (λ = 254 nm) under the optimum condition of low pressure (LP/UV/H2O2), about 90% of ATZ got degraded in one hour (Li et al., 2012). Various studies have proved that Titania is an effective photocatalyst in the degradation of atrazine, where titania film is applied at the surface of quartz crystal, making degradation two times higher than noncoated titania films (Zhang et al., 2013). It has also been observed that the doped TiO2 with the lowest nitrogen loading degraded atrazine at higher rate (Samsudin et al., 2015). Another study evaluated the photocatalytic activity of N- TiO2/ZSP (ZnS-based phosphors microparticle) to remove atrazine under UVA light radiation (Sacco et al., 2015). It has also been observed that AC/g-C3N4 composites with peroxymonosulphate enhance the atrazine's photodegradation efficiency by 57.90%, mainly due to the efficient charge carrier distinguishing ability of the composite and greater absorption capacity (Dikdim et al., 2019). Table 5 describes the degradation of atrazine by different photocatalysts.
5.4.2 Ozonation Process
Ozone is a powerful oxidizing agent that proved to be a powerful alternative yielding a higher degradation rate than conventional oxidation (Glaze et al., 1987). O3/UV removes the contaminants by producing hydroxy radicals (Eq. 8and 10) as the formed H2O2 (Eq. 9) acts as a promoter in ozonation process maintaining the redox cycle reaction. Table 6 summarizes the present scenario; along with the ozonation process, catalysts are added, enhancing the degradation rate of contaminants. MnCe-CM oxides showed the best efficiency with 99.99% atrazine degradation in 40 min, showing that the novel MnCe-CM has dual functions of filtration and catalytic ozonation (He et al., 2022) Plasmon-enhanced catalytic ozonation with silver doped spinel ferrite has shown excellent degradation efficiency compared with ozonation and catalytic ozonation processes (Yang & Wu, 2022). A study demonstrated that synthetic 4A zeolite showed promising results in removing atrazine at a rate of 87.5% in 6 min (Su et al., 2022). In MnOx/biochar and FeOx/biochar, the ozonation efficiency of ATZ increased to 83% and 100%, respectively, reducing the acute toxicity of atrazine from 38.3% to 6.3% (Tian et al., 2021).
5.4.3 Persulfate Oxidation
Persulfate-based remediation technology is one of the most reliable techniques. Light, (Eq. 11) heat, metal ions, and carbon compounds can easily activate persulfate to form SO4¯ radicals (Diao et al., 2021). These compounds enhance the oxidative degradation (Eq. 12) of atrazine in different environments. In this process, the Sulfate and hydroxy radicals react faster with atrazine, forming DIA and DEA (Lutze et al., 2015). In the persulfate advanced oxidation process, catalysts enhance the degradation capability shown in Table 7. A study found that the Co3O4 catalyst at high peroxymonosulphate (PMS) concentration removed about 20 µM ATZ with 2.0 mM PMS and 0.4 g/L Co3O4 at pH 6.0 (Fan et al., 2017). The Boron-doped diamond (BDD) anode used to activate persulfate (PS) demonstrated that degradation of ATZ got increased by 78.2% with the rise in the current density and quantity of PS (not more than 1.0 mM). However, the mechanism underlying these processes needs to be studied more (Bu et al., 2018). It was observed that persulfate activation with the mass ratio of 5:1 for nanoscale zero-valent iron to graphene showed the highest potential for atrazine catalytic degradation, removing 92.1% of atrazine within 21 min, showing degradation efficiency increased with the rise in persulfate concentration (Wu et al., 2018).
5.4.4 Fenton Treatment
Fenton treatment is an advanced oxidation process of ferrous salt (Fe2+) and H2O2 called Fenton’s reagent degrading organic contaminants. In the Fenton oxidation–reduction reaction, the free radicals are generated (Eq. 13 and 14) upon the reaction with iron ions with H2O2. Hydrogen peroxide acts as an oxidizer and catalyst, accelerating the redox cycle (Fe2+/Fe3+) and increasing toxicants' degradation efficiency rate. Fe/TiO2 heterogenous Fenton and visible light photocatalytic activity showed atrazine degradation of 10 mg−L at pH 3 (Yang et al., 2020). In a study, the ability of the photo Fenton process on the atrazine degradation was demonstrated, showing a mineralization rate of 62.5% in about 60 min (Benzaquén et al., 2012). Atrazine gets degraded at the rate of 20%, 60%, and 70% respectively under fenton, UV-A photo fenton and UV-C photo fenton treatments (De Luca et al., 2013). In the visible TaON (Tantalum) Fenton-like system, the degradation rate was higher, but dissolved oxygen reversed the degradation efficiency as the residue of atrazine left was 10% and 15% at 60 min in the absence and presence of DO, respectively (Zhang et al., 2014).
6 Challenges and Future Perspectives
In most countries, atrazine is the maximum percent of detected herbicide in different sampling sites across rivers, estuaries, bays, and agricultural lands. The physiochemical properties of this compound make it less degradable, enhancing the retention time in the environment. The herbicide is banned in some countries, but its presence crossing the minimum residual level is continuously detected in rivers, soils, groundwater, tap water, and surface water, posing a severe threat to aquatic and non-aquatic organisms, microbes, and mammals. It is an area of concern when multiple exposures occur. There is always a potential for multigenerational effects in an organism's different developmental stages, making it more hazardous to living forms.
Some of the critical future research perspectives are given below:
-
Bioremediation is currently the most reliable technique for the degradation of atrazine.However, the removal of atrazine from soils require a consortia of microbes or fungi for better remediation. Bioaugmented mixtures have already proved to be of more excellent value in remediating herbicides.
-
Recently, bioorganic fertilizers have been proven to reduce the phytotoxicity of atrazine, and future research should lay more importance on the behavior of atrazine and its metabolites in different environments.
-
Selecting green compounds/ biomaterials that are budget-friendly and cause no harm to the environment should be the focus of further research. Chemical methods come with many challenges risking soil and groundwater ecosystems. Thus, sustainable and cost-friendly biomaterials are ideal for atrazine degradation.
-
Most phytoremediation studies are done under controlled conditions. Few studies have been based on natural circumstances like field based, so to understand the effects of treatment, field-based mitigation studies should be done extensively.
-
The combination effects of the two treatment technologies should be the idea of future research implementing sustainability and low capital. AOPs have shown potential for the degradation of Atrazine-contaminated waters and soils. However, many reports have not observed the complete degradation of Atrazine to cyanuric acid. Enhancing the methods available for the complete degradation of Atrazine is necessary.
-
Most toxicity studies are done on aquatic organisms. Studies on the toxicity of atrazine on terrestrial organisms are not vigorous, although it has been directly exposed to soil microbes/organisms first, so researchers can target more on studying the toxicological inhibitory aspects of atrazine and its degraded products on terrestrial organisms.
7 Conclusion
The long shelf life of atrazine in different environments has put an enormous threat to every life form it has been exposed to, but more concerning is the intermediate metabolites being produced over time in the environment. Further research should be made to asses the ecotoxicological risk of atrazine metabolites and the substituted compounds (TBA) and its metabolites, due to the accumulation of atrazine in different plants, animals, water bodies through different routes. In most European countries, including Spain, Italy, and Portugal, atrazine has been replaced by more calcitrant compounds like terbuthylazine(TBA), which have more natural retention capacity and induce more hazards to living organisms even at lower doses. This implies that the atrazine ban is not foreseeable in countries like China, India, the USA, and some EU countries. Fungal/ microbial culture intervention have broad application prospects in soil as well as water. More understanding and knowledge of the interaction of adsorbents and microbial or fungal strains will facilitate more sorption and mineralization of atrazine. Some widely applied atrazine-contaminated soil/ aqueous treatment technologies were discussed, including bioremediation, phytoremediation, and AOPs. AOPs application time and degradation rate are comparatively faster, and potential associated risks need further assessment. Among the various strategies incorporated, some challenges still need to be focused on for pilot scale or in-situ application of AOPs.
Data Availability
The articles analyzed during the current study are available in the literature and listed in the references.
References
Abass, K., Pelkonen, O., & Rautio, A. (2021). Chloro-s-triazines-toxicokinetic, Toxicodynamic, Human Exposure, and Regulatory Considerations. Current Drug Metabolism, 22(8), 645. https://doi.org/10.2174/1389200222666210701164945
Alberto, D., Couée, I., Sulmon, C., & Gouesbet, G. (2017). Root-level exposure reveals multiple physiological toxicity of triazine xenobiotics in Arabidopsis thaliana. Journal of Plant Physiology, 212, 105–114. https://doi.org/10.1016/j.jplph.2017.01.013
Ali, J. M., Knight, L. A., D’Souza, D. L., & Kolok, A. S. (2018). Comparing the effects of atrazine and an environmentally relevant mixture on estrogen-responsive gene expression in the northern leopard frog and the fathead minnow. Environmental Toxicology and Chemistry, 37(4), 1182–1188. https://doi.org/10.1002/etc.4069
Almasi, H., Takdastan, A., Jaafarzadeh, N., Babaei, A. A., Birgani, Y. T., Cheraghian, B., Saki, A., & Jorfi, S. (2020). Spatial distribution, ecological and health risk assessment and source identification of atrazine in Shadegan international wetland. Iran. Marine Pollution Bulletin, 160, 111569. https://doi.org/10.1016/j.marpolbul.2020.111569
Álvarez, P. M., Quiñones, D. H., Terrones, I., Rey, A., & Beltrán, F. J. (2016). Insights into the removal of terbuthylazine from aqueous solution by several treatment methods. Water Research, 98, 334–343.
Ameri Siahouei, R., Zaeimdar, M., Moogouei, R., & Jozi, S. A. (2020). Potential of Cyperus alternifolius, Amaranthus retroflexus, Closia cristata and Bambusa vulgaris to phytoremediate emerging contaminants and phytodesalination; Insight to floating beds technology. Caspian Journal of Environmental Sciences, 18(4), 309–317.
Arar, M., Bakkour, R., Elsner, M., & Bernstein, A. (2023). Microbial hydrolysis of atrazine in contaminated groundwater. Chemosphere, 322, 138226.
Bachetti, R. A., Urseler, N., Morgante, V., Damilano, G., Porporatto, C., Agostini, E., & Morgante, C. (2021). Monitoring of atrazine pollution and its spatial-seasonal variation on surface water sources of an agricultural river basin. Bulletin of Environmental Contamination and Toxicology, 106(6), 929–935. https://doi.org/10.1007/s00128-021-03264-x
Barrios, R. E., Gaonkar, O., Snow, D., Li, Y., Li, X., & Bartelt-Hunt, S. L. (2019). Enhanced biodegradation of atrazine at high infiltration rates in agricultural soils. Environmental Science: Processes & Impacts, 21(6), 999–1010.
Beaulieu, M., Cabana, H., Taranu, Z., & Huot, Y. (2020). Predicting atrazine concentrations in waterbodies across the contiguous United States: The importance of land use, hydrology, and water physicochemistry. Limnology and Oceanography, 65(12), 2966–2983. https://doi.org/10.1002/lno.11568
Benzaquén, T. B., Isla, M. A., & Alfano, O. M. (2012). Quantum efficiencies of the photo-Fenton degradation of atrazine in water. Water Science and Technology, 66(10), 2209–2216.
Bhatti, P., Duhan, A., Pal, A., Beniwal, R. K., Kumawat, P., & Yadav, D. B. (2022). Ultimate fate and possible ecological risks associated with atrazine and its principal metabolites (DIA and DEA) in soil and water environment. Ecotoxicology and Environmental Safety, 248, 114299.
Blahova, J., Dobsikova, R., Enevova, V., Modra, H., Plhalova, L., Hostovsky, M., Marsalek, P., Mares, J., Skoric, M., & Vecerek, V. (2020). Comprehensive fitness evaluation of common carp (Cyprinus carpio L) after twelve weeks of atrazine exposure. Science of The Total Environment, 718, 135059. https://doi.org/10.1016/j.scitotenv.2019.135059
Bonfleur, E. J., Tornisielo, V. L., Regitano, J. B., & Lavorenti, A. (2015). The effects of glyphosate and atrazine mixture on soil microbial population and subsequent impacts on their fate in a tropical soil. Water, Air, & Soil Pollution, 226(2), 1–10. https://doi.org/10.1007/s11270-014-2190-8
Bottoni, P., Grenni, P., Lucentini, L., & Caracciolo, A. B. (2013). Terbuthylazine and other triazines in Italian water resources. Microchemical Journal, 107, 136–142.
Bozzo, S., Azimonti, G., Villa, S., Di Guardo, A., & Finizio, A. (2013). Spatial and temporal trend of groundwater contamination from terbuthylazine and desethyl-terbuthylazine in the Lombardy Region (Italy). Environmental Science: Processes & Impacts, 15(2), 366–372.
Brain, R. A., & Hoberg, J. (2016). Recovery of terrestrial plants in vegetative vigor and seedling emergence tests from exposure to atrazine. Environmental Toxicology and Chemistry, 35(5), 1284–1296. https://doi.org/10.1002/etc.3298
Briceño, G., Jorquera, M., Demanet, R., Mora, M., Durán, N., & Palma, G. (2010). Effect of cow slurry amendment on atrazine dissipation and bacterial community structure in an agricultural Andisol. Science of the Total Environment, 408(14), 2833–2839. https://doi.org/10.1016/j.scitotenv.2010.03.014
Brown, J. B., Langley, S. A., Snijders, A. M., Wan, K. H., Morris, S. N. S., Booth, B. W., Fisher, W. W., Hammonds, A. S., Park, S., & Weiszmann, R. (2021). An integrated host-microbiome response to atrazine exposure mediates toxicity in Drosophila. Communications Biology, 4(1), 1–12. https://doi.org/10.1038/s42003-021-02847-y
Brumovský, M., Bečanová, J., Kohoutek, J., Borghini, M., & Nizzetto, L. (2017). Contaminants of emerging concern in the open sea waters of the Western Mediterranean. Environmental Pollution, 229, 976–983.
Bu, L., Zhu, S., & Zhou, S. (2018). Degradation of atrazine by electrochemically activated persulfate using BDD anode: Role of radicals and influencing factors. Chemosphere, 195, 236–244. https://doi.org/10.1016/j.chemosphere.2017.12.088
Cao, P., Quan, X., Zhao, K., Chen, S., Yu, H., & Niu, J. (2020). Selective electrochemical H2O2 generation and activation on a bifunctional catalyst for heterogeneous electro-Fenton catalysis. Journal of Hazardous Materials, 382, 121102. https://doi.org/10.1016/j.jhazmat.2019.121102
Castro, M. S., Barbosa, F. G., Guimarães, P. S., Martins, C. D. M. G., & Zanette, J. (2021). A scientometric analysis of ecotoxicological studies with the herbicide atrazine and microalgae and cyanobacteria as test organisms. Environmental Science and Pollution Research, 28(20), 25196–25206. https://doi.org/10.1007/s11356-020-12213-w
Challis, J. K., Cuscito, L. D., Joudan, S., Luong, K. H., Knapp, C. W., Hanson, M. L., & Wong, C. S. (2018). Inputs, source apportionment, and transboundary transport of pesticides and other polar organic contaminants along the lower Red River, Manitoba, Canada. Science of the Total Environment, 635, 803–816.
Chan-Cupul, W., Heredia-Abarca, G., & Rodríguez-Vázquez, R. (2016). Atrazine degradation by fungal co-culture enzyme extracts under different soil conditions. Journal of Environmental Science and Health, Part B, 51(5), 298–308.
Chellaiah, D., & Yule, C. M. (2018). Effect of riparian management on stream morphometry and water quality in oil palm plantations in Borneo. Limnologica, 69, 72–80. https://doi.org/10.1016/j.limno.2017.11.007
Chen, Q., Yang, B., Wang, H., He, F., Gao, Y., & Scheel, R. A. (2015). Soil microbial community toxic response to atrazine and its residues under atrazine and lead contamination. Environmental Science and Pollution Research, 22(2), 996–1007. https://doi.org/10.1007/s11356-014-3369-7
Cheyns, K., Calcoen, J., Martin-Laurent, F., Bru, D., Smolders, E., & Springael, D. (2012). Effects of dissolved organic matter (DOM) at environmentally relevant carbon concentrations on atrazine degradation by Chelatobacter heintzii SalB. Applied Microbiology and Biotechnology, 95(5), 1333–1341. https://doi.org/10.1007/s00253-011-3741-1
de Araújo, E. P., Caldas, E. D., & Oliveira-Filho, E. C. (2022). Pesticides in surface freshwater: A critical review. Environmental Monitoring and Assessment, 194(6), 1–25. https://doi.org/10.1007/s10661-022-10005-y
de Oliveira, L. A., Grecco, K. L., Tornisielo, V. L., & Woodbury, B. L. (2019). Atrazine movement in corn cultivated soil using HYDRUS-2D: A comparison between real and simulated data. Journal of Environmental Management, 248, 109311.
de Oliveira, J. S. P., Vieira, L. G., Carvalho, W. F., de Souza, M. B., de Lima Rodrigues, A. S., Simões, K., De Silva, D., & d. M., dos Santos Mendonça, J., Hirano, L. Q. L., & Santos, A. L. Q. (2020). Mutagenic, genotoxic and morphotoxic potential of different pesticides in the erythrocytes of Podocnemis expansa neonates. Science of the Total Environment, 737, 140304. https://doi.org/10.1016/j.scitotenv.2020.140304
de Paula, R. T., de Abreu, A. B. G., de Queiroz, M. E. L., Neves, A. A., & da Silva, A. A. (2016). Leaching and persistence of ametryn and atrazine in red–yellow latosol. Journal of Environmental Science and Health, Part B, 51(2), 90–95.
de Souza, R. M., Seibert, D., Quesada, H. B., de Jesus Bassetti, F., Fagundes-Klen, M. R., & Bergamasco, R. (2020). Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Safety and Environmental Protection, 135, 22–37.
De Luca, A., Dantas, R. F., Simoes, A. S., Toscano, I. A., Lofrano, G., Cruz, A., & Esplugas, S. (2013). Atrazine Removal in Municipal Secondary Effluents by Fenton and Photo-Fenton Treatments. Chemical Engineering & Technology, 36(12), 2155–2162.
Dehghani, M., Gharehchahi, E., Jafari, S., Moeini, Z., Derakhshan, Z., Ferrante, M., & Conti, G. O. (2022). Health risk assessment of exposure to atrazine in the soil of Shiraz farmlands. Iran. Environmental Research, 204, 112090.
Deshmukh, R., Khardenavis, A. A., & Purohit, H. J. (2016). Diverse metabolic capacities of fungi for bioremediation. Indian Journal of Microbiology, 56(3), 247–264.
Destro, A. L. F., Silva, S. B., Gregorio, K. P., de Oliveira, J. M., Lozi, A. A., Zuanon, J. A. S., Salaro, A. L., da Matta, S. L. P., Gonçalves, R. V., & Freitas, M. B. (2021). Effects of subchronic exposure to environmentally relevant concentrations of the herbicide atrazine in the Neotropical fish Astyanax altiparanae. Ecotoxicology and Environmental Safety, 208, 111601. https://doi.org/10.1016/j.ecoenv.2020.111601
Diao, Z.-H., Zhang, W.-X., Liang, J.-Y., Huang, S.-T., Dong, F.-X., Yan, L., Qian, W., & Chu, W. (2021). Removal of herbicide atrazine by a novel biochar based iron composite coupling with peroxymonosulfate process from soil: Synergistic effect and mechanism. Chemical Engineering Journal, 409, 127684.
Dikdim, J. M. D., Gong, Y., Noumi, G. B., Sieliechi, J. M., Zhao, X., Ma, N., Yang, M., & Tchatchueng, J. B. (2019). Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation. Chemosphere, 217, 833–842. https://doi.org/10.1016/j.chemosphere.2018.10.177
Dong, J., Wang, L., Ma, F., Yang, J., Qi, S., & Zhao, T. (2016). The effect of Funnelliformis mosseae inoculation on the phytoremediation of atrazine by the aquatic plant Canna indica L. var. flava Roxb. RSC advances, 6(27), 22538–22549. https://doi.org/10.1039/C5RA23583A
Donnelly, P., Entry, J., & Crawford, D. (1993). Degradation of atrazine and 2, 4-dichlorophenoxyacetic acid by mycorrhizal fungi at three nitrogen concentrations in vitro. Applied and Environmental Microbiology, 59(8), 2642–2647.
Duttagupta, S., Mukherjee, A., Bhattacharya, A., & Bhattacharya, J. (2020). Wide exposure of persistent organic pollutants (PoPs) in natural waters and sediments of the densely populated Western Bengal basin. India. Science of the Total Environment, 717, 137187. https://doi.org/10.1016/j.scitotenv.2020.137187
Eissa, F., Al-Sisi, M., & Ghanem, K. (2021). Occurrence, human health, and ecotoxicological risk assessment of pesticides in surface waters of the River Nile’s Rosetta Branch. Egypt. Environmental Science and Pollution Research, 28(39), 55511–55525. https://doi.org/10.1007/s11356-021-14911-5
Elias, D., & Bernot, M. J. (2014). Effects of atrazine, metolachlor, carbaryl and chlorothalonil on benthic microbes and their nutrient dynamics. PLoS ONE, 9(10), e109190. https://doi.org/10.1371/journal.pone.0109190
Esparza-Naranjo, S. B., da Silva, G. F., Duque-Castaño, D. C., Araújo, W. L., Peres, C. K., Boroski, M., & Bonugli-Santos, R. C. (2021). Potential for the biodegradation of atrazine using leaf litter fungi from a subtropical protection area. Current Microbiology, 78(1), 358–368.
Fan, X., & Song, F. (2014a). Bioremediation of atrazine: Recent advances and promises. Journal of Soils and Sediments, 14(10), 1727–1737.
Fan, X., & Song, F. (2014b). Bioremediation of atrazine: Recent advances and promises. Journal of Soils and Sediments, 14, 1727–1737.
Fan, Y., Ji, Y., Zheng, G., Lu, J., Kong, D., Yin, X., & Zhou, Q. (2017). Degradation of atrazine in heterogeneous Co3O4 activated peroxymonosulfate oxidation process: Kinetics, mechanisms, and reaction pathways. Chemical Engineering Journal, 330, 831–839. https://doi.org/10.1016/j.cej.2017.08.020
Fan, X., Chang, W., Sui, X., Liu, Y., Song, G., Song, F., & Feng, F. (2020). Changes in rhizobacterial community mediating atrazine dissipation by arbuscular mycorrhiza. Chemosphere, 256, 127046.
Fareed, A., Hussain, A., Nawaz, M., Imran, M., Ali, Z., & Haq, S. U. (2021). The impact of prolonged use and oxidative degradation of Atrazine by Fenton and photo-Fenton processes. Environmental Technology & Innovation, 24, 101840. https://doi.org/10.1016/j.eti.2021.101840
Fernández, L. A., Valverde, C., & Gómez, M. A. (2013). Isolation and characterization of atrazine-degrading Arthrobacter sp. strains from Argentine agricultural soils. Annals of microbiology, 63(1), 207–214. https://doi.org/10.1007/s13213-012-0463-2
Fikarová, J., Kříženecká, S., Elznicová, J., Faměra, M., Lelková, T., Matkovič, J., & Matys Grygar, T. (2018). Spatial distribution of organic pollutants (PAHs and polar pesticides) in the floodplain of the Ohře (Eger) River, Czech Republic. Journal of Soils and Sediments, 18(1), 259–275. https://doi.org/10.1007/s11368-017-1807-0
Fisch, K., Brockmeyer, B., Gerwinski, W., Schulz-Bull, D. E., & Theobald, N. (2021). Seasonal variability, long-term distribution (2001–2014), and risk assessment of polar organic micropollutants in the Baltic Sea. Environmental Science and Pollution Research, 28(29), 39296–39309. https://doi.org/10.1007/s11356-021-13254-5
Furtado, R. X. d. S., Azevedo, E. B., & Motheo, A. d. J. (2019). Electrochemical degradation of aqueous alachlor and atrazine: products identification, lipophilicity, and ecotoxicity. Eclética Química Journal, 44(SI), 12–25.
Galíndez-Nájera, S. P., Ramos-Monroy, O., Ruiz-Ordaz, N., Salmerón-Alcocer, A., Juárez-Ramírez, C., Ahuatzi-Chacón, D., Curiel-Quesada, E., & Galíndez-Mayer, J. (2011). Simultaneous degradation of atrazine and simazine by a binary culture of Stenotrophomonas maltophilia and Arthrobacter sp. in a two-stage biofilm reactor. Journal of Chemical Technology & Biotechnology, 86(4), 554–561. https://doi.org/10.1002/jctb.2550
Gao, J., Song, P., Wang, G., Wang, J., Zhu, L., & Wang, J. (2018). Responses of atrazine degradation and native bacterial community in soil to Arthrobacter sp. strain HB-5. Ecotoxicology and Environmental Safety, 159, 317–323.
Geng, Y., Ma, J., Jia, R., Xue, L.-Q., Tao, C.-J., Li, C.-J., Ma, X.-D., & Lin, Y. (2013). Impact of long-term atrazine use on groundwater safety in Jilin Province China. J Integr Agric, 12, 305–313.
Geret, F., Burgeot, T., Haure, J., Gagnaire, B., Renault, T., Communal, P.-Y., & Samain, J.-F. (2013). Effects of low-dose exposure to pesticide mixture on physiological responses of the pacific oyster. Crassostrea Gigas. Environmental Toxicology, 28(12), 689–699. https://doi.org/10.1002/tox.20764
Glaze, W. H., Kang, J.-W., & Chapin, D. H. (1987). The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. https://doi.org/10.1080/01919518708552148
Góngora-Echeverría, V. R., Martin-Laurent, F., Quintal-Franco, C., Lorenzo-Flores, A., Giácoman-Vallejos, G., & Ponce-Caballero, C. (2019). Dissipation and adsorption of 2, 4-D, atrazine, diazinon, and glyphosate in an agricultural soil from Yucatan State, Mexico. Water, Air, & Soil Pollution, 230, 1–15.
Gustafson, D. I. (1989). Groundwater ubiquity score: A simple method for assessing pesticide leachability. Environmental Toxicology and Chemistry: An International Journal, 8(4), 339–357.
Hanson, M. L., Solomon, K. R., Van Der Kraak, G. J., & Brian, R. A. (2019). Effects of atrazine on fish, amphibians, and reptiles: Update of the analysis based on quantitative weight of evidence. Critical Reviews in Toxicology, 49(8), 670–709. https://doi.org/10.1080/10408444.2019.1701985
He, Y., Wang, L., Chen, Z., Huang, X., Wang, X., Zhang, X., & Wen, X. (2022). Novel catalytic ceramic membranes anchored with MnMe oxide and their catalytic ozonation performance towards atrazine degradation. Journal of Membrane Science, 648, 120362. https://doi.org/10.1016/j.memsci.2022.120362
Heemann, T. P., Arantes, S., Andrade, E., Viana, D., & Sella, H. (2018). Phytoremediation capacity of forest species to herbicides in two types of soils. Floresta e Ambiente, 25. https://doi.org/10.1590/2179-8087.046517
Herrero-Hernández, E., Rodríguez-Cruz, M. S., Pose-Juan, E., Sánchez-González, S., Andrades, M. S., & Sánchez-Martín, M. J. (2017). Seasonal distribution of herbicide and insecticide residues in the water resources of the vineyard region of La Rioja (Spain). Science of the Total Environment, 609, 161–171. https://doi.org/10.1016/j.scitotenv.2017.07.113
Hertfordshire, U. (2017). PPDB: Pesticide Properties DataBase. University of Hertfordshire.
Hong, J., Boussetta, N., Enderlin, G., Merlier, F., & Grimi, N. (2022). Degradation of residual herbicide atrazine in agri-food and washing water. Foods, 11(16), 2416.
Huang, X., He, J., Yan, X., Hong, Q., Chen, K., He, Q., Zhang, L., Liu, X., Chuang, S., & Li, S. (2017). Microbial catabolism of chemical herbicides: Microbial resources, metabolic pathways and catabolic genes. Pesticide Biochemistry and Physiology, 143, 272–297. https://doi.org/10.1016/j.pestbp.2016.11.010
Hvězdová, M., Kosubová, P., Košíková, M., Scherr, K. E., Šimek, Z., Brodský, L., Šudoma, M., Škulcová, L., Sáňka, M., & Svobodová, M. (2018). Currently and recently used pesticides in Central European arable soils. Science of the Total Environment, 613, 361–370.
Ibrahim, S., Lateef, M. A., Khalifa, H., & Monem, A. A. (2013). Phytoremediation of atrazine-contaminated soil using Zea mays (maize). Annals of Agricultural Sciences, 58(1), 69–75. https://doi.org/10.1016/j.aoas.2013.01.010
Illatou, O. E. F. M., Spinelli, S., Avezac, M., Bertrand, M., Gonzalez, C., & Vinches, M. (2023). Occurrences, distribution and risk assessment of polar pesticides in Niger River valley and its tributary the Mekrou River (Niger Republic). Environmental Science and Pollution Research, 30(8), 20804–20820.
Jablonowski, N. D., Schäffer, A., & Burauel, P. (2011). Still present after all these years: Persistence plus potential toxicity raise questions about the use of atrazine. Environmental Science and Pollution Research, 18(2), 328–331.
Jakinala, P., Lingampally, N., Kyama, A., & Hameeda, B. (2019). Enhancement of atrazine biodegradation by marine isolate Bacillus velezensis MHNK1 in presence of surfactin lipopeptide. Ecotoxicology and Environmental Safety, 182, 109372. https://doi.org/10.1016/j.ecoenv.2019.109372
Jian, Y., Yunting, X., Xianghong, T., Rong, Z., & Zhanqiang, B. (2021). Atrazine and its metabolites (ATZs) in source water, finished water, and tap water from drinking water treatment plants and its human risk assessment in Zhoukou City, China. Human and Ecological Risk Assessment: An International Journal, 27(7), 1926–1938.
Jiang, Z., Zhang, X., Wang, Z., Cao, B., Deng, S., Bi, M., & Zhang, Y. (2019). Enhanced biodegradation of atrazine by Arthrobacter sp. DNS10 during co-culture with a phosphorus solubilizing bacteria: Enterobacter sp. P1. Ecotoxicology and Environmental Safety, 172, 159–166. https://doi.org/10.1016/j.ecoenv.2019.01.070
Jiang, Z.-Y., Ma, Y.-K., Ke, Q.-F., Chu, L.-F., Guo, C.-X., & Guo, Y.-P. (2021). Hydrothermal deposition of CoFe2O4 nanoparticles on activated carbon fibers promotes atrazine removal via physical adsorption and photo-Fenton degradation. Journal of Environmental Chemical Engineering, 9(5), 105940. https://doi.org/10.1016/j.jece.2021.105940
Jiang, Q., Jiang, S., Li, H., Zhang, R., Jiang, Z., & Zhang, Y. (2022). A stable biochar supported S-nZVI to activate persulfate for effective dichlorination of atrazine. Chemical Engineering Journal, 431, 133937. https://doi.org/10.1016/j.cej.2021.133937
Jin, X., Yu, X., Zhu, G., Zheng, Z., Feng, F., & Zhang, Z. (2016). Conditions optimizing and application of laccase-mediator system (LMS) for the laccase-catalyzed pesticide degradation. Scientific Reports, 6(1), 1–7.
Juhel, G., Bayen, S., Goh, C., Lee, W. K., & Kelly, B. C. (2017). Use of a suite of biomarkers to assess the effects of carbamazepine, bisphenol A, atrazine, and their mixtures on green mussels. Perna Viridis. Environmental Toxicology and Chemistry, 36(2), 429–441. https://doi.org/10.1002/etc.3556
Kennelly, J. P., van der Veen, J. N., Nelson, R. C., Leonard, K.-A., Havinga, R., Buteau, J., Kuipers, F., & Jacobs, R. L. (2018). Intestinal de novo phosphatidylcholine synthesis is required for dietary lipid absorption and metabolic homeostasis. Journal of Lipid Research, 59(9), 1695–1708.
Khandarkhaeva, M., Batoeva, A., Aseev, D., Sizykh, M., & Tsydenova, O. (2017). Oxidation of atrazine in aqueous media by solar-enhanced Fenton-like process involving persulfate and ferrous ion. Ecotoxicology and Environmental Safety, 137, 35–41. https://doi.org/10.1016/j.ecoenv.2016.11.013
Khatoon, H., & Rai, J. (2020). Optimization studies on biodegradation of atrazine by Bacillus badius ABP6 strain using response surface methodology. Biotechnology Reports, 26, e00459. https://doi.org/10.1016/j.btre.2020.e00459
Krishnasamy, L., Krishna, K., & Subpiramaniyam, S. (2022). Photocatalytic degradation of atrazine in aqueous solution using La-doped ZnO/PAN nanofibers. Environmental Science and Pollution Research, 1–10. https://doi.org/10.1007/s11356-022-19665-2
Lee, K.-H., Bartsch, H., Nair, J., Yoo, D.-H., Hong, Y.-C., Cho, S.-H., & Kang, D. (2006). Effect of short-term fasting on urinary excretion of primary lipid peroxidation products and on markers of oxidative DNA damage in healthy women. Carcinogenesis, 27(7), 1398–1403.
Lerro, C. C., Beane Freeman, L. E., Portengen, L., Kang, D., Lee, K., Blair, A., Lynch, C. F., Bakke, B., De Roos, A. J., & Vermeulen, R. C. (2017). A longitudinal study of atrazine and 2, 4-D exposure and oxidative stress markers among iowa corn farmers. Environmental and Molecular Mutagenesis, 58(1), 30–38. https://doi.org/10.1002/em.22069
Li, C., Gao, N., Wang, L., & Shen, Y. (2012). Hydrogen peroxide-assisted low pressure UV photodegradation of atrazine in aqueous solution. International Journal of Environmental Studies, 69(4), 625–634. https://doi.org/10.1080/00207233.2012.674780
Li, J., Hu, J., Xu, W., Ling, M., & Yao, J. (2014). Hydrolysis reaction mechanism in atrazine metabolism and prediction of its metabolites’ toxicities. Journal of Agricultural and Food Chemistry, 62(21), 4852–4863.
Li, H., Qu, M., Lu, X., Chen, Z., Guan, S., Du, H., & Zhu, D. (2019a). Evaluation of the potential of Potamogeton crispus and Myriophyllum spicatum on phytoremediation of atrazine. International Journal of Environmental Analytical Chemistry, 99(3), 243–257. https://doi.org/10.1080/03067319.2019.1588967
Li, J., Wan, Y., Li, Y., Yao, G., & Lai, B. (2019b). Surface Fe (III)/Fe (II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Applied Catalysis b: Environmental, 256, 117782.
Li, P., Li, X., Yao, L., Wu, Y., & Li, B. (2020). Soybean isoflavones prevent atrazine-induced neurodegenerative damage by inducing autophagy. Ecotoxicology and Environmental Safety, 190, 110065. https://doi.org/10.1016/j.ecoenv.2019.110065
Li, B., Jiang, Y., Wang, T., He, X., Ma, L., Li, B., & Li, Y. (2021a). Effect of atrazine on accumulation of iron via the iron transport proteins in the midbrain of SD rats. Science of the Total Environment, 780, 146666. https://doi.org/10.1016/j.scitotenv.2021.146666
Li, P., Yao, L.-Y., Jiang, Y.-J., Wang, D.-D., Wang, T., Wu, Y.-P., Li, B.-X., & Li, X.-T. (2021b). Soybean isoflavones protect SH-SY5Y neurons from atrazine-induced toxicity by activating mitophagy through stimulation of the BEX2/BNIP3/NIX pathway. Ecotoxicology and Environmental Safety, 227, 112886.
Li, D., Ali, J., Shahzad, A., Gendy, E. A., Nie, H., Jiang, W., Xiao, H., Chen, Z., & Wang, S. (2022). Persulfate coupled with Cu2+/LDH-MoS4: A novel process for the efficient atrazine abatement, mechanism and degradation pathway. Chemical Engineering Journal, 436, 134933. https://doi.org/10.1016/j.cej.2022.134933
Lin, C., Lerch, R., Garrett, H., & George, M. (2008). Bioremediation of atrazine-contaminated soil by forage grasses: Transformation, uptake, and detoxification. Journal of Environmental Quality, 37(1), 196–206.
Lin, Z., Roede, J. R., He, C., Jones, D. P., & Filipov, N. M. (2014). Short-term oral atrazine exposure alters the plasma metabolome of male C57BL/6 mice and disrupts α-linolenate, tryptophan, tyrosine and other major metabolic pathways. Toxicology, 326, 130–141. https://doi.org/10.1016/j.tox.2014.11.001
Liu, Z., Wang, Y., Zhu, Z., Yang, E., Feng, X., Fu, Z., & Jin, Y. (2016). Atrazine and its main metabolites alter the locomotor activity of larval zebrafish (Danio rerio). Chemosphere, 148, 163–170.
Liu, J., Hua, R., Lv, P., Tang, J., Wang, Y., Cao, H., Wu, X., & Li, Q. X. (2017). Novel hydrolytic de-methylthiolation of the s-triazine herbicide prometryn by Leucobacter sp. JW-1. Science of the Total Environment, 579, 115–123. https://doi.org/10.1016/j.scitotenv.2016.11.006
Liu, Y., Wang, S., Shi, L., Lu, W., & Li, P. (2020). Enhanced degradation of atrazine by microbubble ozonation. Environmental Science: Water Research & Technology, 6(6), 1681–1687. https://doi.org/10.1039/D0EW00227E
Liu, B., Zeng, Q., Chen, H., Liao, J., Bai, Y., Han, Q., Qiao, N., Wang, S., Mehmood, K., & Hussain, R. (2021). The hepatotoxicity of altrazine exposure in mice involves the intestinal microbiota. Chemosphere, 272, 129572. https://doi.org/10.1016/j.chemosphere.2021.129572
Liu, D., Liu, Y., He, H., Liu, J., Yang, X., Zhang, L., Tang, Y., & Zhu, H. (2023). Functional Bimetal/Carbon Composites Co/Zr@ AC for Pesticide Atrazine Removal from Water. Molecules, 28(5), 2071.
Liu, Y., Ji, X., Yang, J., Tang, W., Zhu, Y., Wang, Y., Zhang, Y., Zhang, Y., Duan, J., & Li, W. (2022). Degradation of the typical herbicide atrazine by UV/persulfate: Kinetics and mechanisms. Environmental Science and Pollution Research, 1–14. https://doi.org/10.1007/s11356-022-18717-x
Lopes, R. D. O., Pereira, P. M., Pereira, A. R. B., Fernandes, K. V., Carvalho, J. F., França, A. D. S. D., Valente, R. H., da Silva, M., & Ferreira-Leitão, V. S. (2020). Atrazine, desethylatrazine (DEA) and desisopropylatrazine (DIA) degradation by Pleurotus ostreatus INCQS 40310. Biocatalysis and Biotransformation, 38(6), 415–430.
Lopes-Lima, M., Sousa, R., Geist, J., Aldridge, D. C., Araujo, R., Bergengren, J., Bespalaya, Y., Bódis, E., Burlakova, L., & Van Damme, D. (2017). Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biological Reviews, 92(1), 572–607. https://doi.org/10.1111/brv.12244
Loureiro, D. B., Lario, L. D., Herrero, M. S., Salvatierra, L. M., Novo, L. A., & Pérez, L. M. (2023). Potential of Salvinia biloba Raddi for removing atrazine and carbendazim from aquatic environments. Environmental Science and Pollution Research, 30(8), 22089–22099.
Lu, Y.-S., Yang, S.-L., Gou, C.-L., Wang, X.-L., Wen, X., He, X.-R., Guo, X.-X., Xu, Y.-Y., Yu, J., & Qiu, J. (2022). Integrated metabolomics and transcriptomics analysis reveals new biomarkers and mechanistic insights on atrazine exposures in MCF-7 cells. Ecotoxicology and Environmental Safety, 232, 113244. https://doi.org/10.1016/j.ecoenv.2022.113244
Luo, M., Zhou, D.-D., Shang, A., Gan, R.-Y., & Li, H.-B. (2021). Influences of food contaminants and additives on gut microbiota as well as protective effects of dietary bioactive compounds. Trends in Food Science & Technology, 113, 180–192. https://doi.org/10.1016/j.tifs.2021.05.006
Lutze, H. V., Bircher, S., Rapp, I., Kerlin, N., Bakkour, R., Geisler, M., von Sonntag, C., & Schmidt, T. C. (2015). Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter. Environmental Science & Technology, 49(3), 1673–1680. https://doi.org/10.1021/es503496u
Ma, L., Chen, S., Yuan, J., Yang, P., Liu, Y., & Stewart, K. (2017). Rapid biodegradation of atrazine by Ensifer sp. strain and its degradation genes. International Biodeterioration & Biodegradation, 116, 133–140. https://doi.org/10.1016/j.ibiod.2016.10.022
Madariaga-Navarrete, A., Rodríguez-Pastrana, B. R., Villagómez-Ibarra, J. R., Acevedo-Sandoval, O. A., Perry, G., & Islas-Pelcastre, M. (2017). Bioremediation model for atrazine contaminated agricultural soils using phytoremediation (using Phaseolus vulgaris L) and a locally adapted microbial consortium. Journal of Environmental Science and Health, Part B, 52(6), 367–375. https://doi.org/10.1080/03601234.2017.1292092
Mahlalela, L. C., Casado, C., Marugan, J., Septien, S., Ndlovu, T., & Dlamini, L. N. (2020). Photocatalytic degradation of atrazine in aqueous solution using hyperbranched polyethyleneimine templated morphologies of BiVO4 fused with Bi2O3. Journal of Environmental Chemical Engineering, 8(5), 104215. https://doi.org/10.1016/j.jece.2020.104215
Mai, C., Theobald, N., Lammel, G., & Hühnerfuss, H. (2013). Spatial, seasonal and vertical distributions of currently-used pesticides in the marine boundary layer of the North Sea. Atmospheric Environment, 75, 92–102.
Maqbool, Z., Hussain, S., Imran, M., Mahmood, F., Shahzad, T., Ahmed, Z., Azeem, F., & Muzammil, S. (2016). Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: A critical review. Environmental Science and Pollution Research, 23(17), 16904–16925.
McDaniel, M. A., Maier, S. F., & Einstein, G. O. (2002). “Brain-specific” nutrients: A memory cure? Psychological Science in the Public Interest, 3(1), 12–38.
McKnight, A. M., Gannon, T. W., & Yelverton, F. (2022). Phytoremediation potential of three terrestrial plant species for removal of atrazine, azoxystrobin, and imidacloprid. International Journal of Phytoremediation, 24(2), 187–195.
Metcalfe, C. D., Helm, P., Paterson, G., Kaltenecker, G., Murray, C., Nowierski, M., & Sultana, T. (2019). Pesticides related to land use in watersheds of the Great Lakes basin. Science of the Total Environment, 648, 681–692. https://doi.org/10.1016/j.scitotenv.2018.08.169
Mili, C., Kalita, S., & Roy, S. (2022). Microbes as a potential bioremediation tool for atrazine-contaminated soil: A review. Journal of Applied Biology and Biotechnology, 11(1), 8–15.
Moeini, Z., Azhdarpoor, A., Yousefinejad, S., & Hashemi, H. (2019). Removal of atrazine from water using titanium dioxide encapsulated in salicylaldehydeNH2MIL-101 (Cr): Adsorption or oxidation mechanism. Journal of Cleaner Production, 224, 238–245. https://doi.org/10.1016/j.jclepro.2019.03.236
Montiel-León, J. M., Munoz, G., Duy, S. V., Do, D. T., Vaudreuil, M.-A., Goeury, K., Guillemette, F., Amyot, M., & Sauvé, S. (2019). Widespread occurrence and spatial distribution of glyphosate, atrazine, and neonicotinoids pesticides in the St. Lawrence and Tributary Rivers. Environmental Pollution, 250, 29–39. https://doi.org/10.1016/j.envpol.2019.03.125
Moselhy, W., Nabil, T., Abdel-Halim, B., & Helmy, N. (2016). Effect of atrazine and glyphosate on the reproductive system of female rats: histological and immunohistochemical studies. Assiut Veterinary Medical Journal, 62(148), 101–111. https://doi.org/10.21608/AVMJ.2016.169224
Muniz, J. F., McCauley, L., Scherer, J., Lasarev, M., Koshy, M., Kow, Y., Nazar-Stewart, V., & Kisby, G. (2008). Biomarkers of oxidative stress and DNA damage in agricultural workers: A pilot study. Toxicology and Applied Pharmacology, 227(1), 97–107.
Navarra, W., Sacco, O., Daniel, C., Venditto, V., Vaiano, V., Vignati, D. A. L., Bojic, C., Libralato, G., Lofrano, G., & Carotenuto, M. (2022). Photocatalytic degradation of atrazine by an N-doped TiO2/polymer composite: Catalytic efficiency and toxicity evaluation. Journal of Environmental Chemical Engineering, 10(4), 108167. https://doi.org/10.1016/j.jece.2022.108167
Navarro, S., Vela, N., Giménez, M. J., & Navarro, G. (2004). Persistence of four s-triazine herbicides in river, sea and groundwater samples exposed to sunlight and darkness under laboratory conditions. Science of the Total Environment, 329(1–3), 87–97.
Ngigi, A. N., Getenga, Z. M., Boga, H. I., & Ndalut, P. K. (2012). Biodegradation of s-triazine herbicide atrazine by Enterobacter cloacae and Burkholderia cepacia sp from long-term treated sugarcane-cultivated soils in Kenya. Journal of Environmental Science and Health, Part B, 47(8), 769–778. https://doi.org/10.1080/03601234.2012.676364
Nowell, L. H., Moran, P. W., Schmidt, T. S., Norman, J. E., Nakagaki, N., Shoda, M. E., Mahler, B. J., Van Metre, P. C., Stone, W. W., & Sandstrom, M. W. (2018). Complex mixtures of dissolved pesticides show potential aquatic toxicity in a synoptic study of Midwestern US streams. Science of the Total Environment, 613, 1469–1488.
Organization, W. H. (2004). Guidelines for drinking-water quality (Vol. 1). World Health Organization.
Ouyang, W., Zhang, Y., Lin, C., Wang, A., Tysklind, M., & Wang, B. (2021). Metabolic process and spatial partition dynamics of Atrazine in an estuary-to-bay system, Jiaozhou bay. Journal of Hazardous Materials, 414, 125530. https://doi.org/10.1016/j.jhazmat.2021.125530
Palma, P., Köck-Schulmeyer, M., Alvarenga, P., Ledo, L., Barbosa, I., De Alda, M. L., & Barceló, D. (2014). Risk assessment of pesticides detected in surface water of the Alqueva reservoir (Guadiana basin, southern of Portugal). Science of the Total Environment, 488, 208–219. https://doi.org/10.1016/j.scitotenv.2014.04.088
Papadopoulos, N., & Zalidis, G. (2019). The use of Typha Latifolia L. in constructed wetland microcosms for the remediation of herbicide Terbuthylazine. Environmental Processes, 6(4), 985–1003. https://doi.org/10.1007/s40710-019-00398-3
Pereira, P., Brunetti, I., Castro, K., Chiarotti, L., Santos, B., Moraes, J., & Cruz, C. (2019). Acute toxicity of herbicides and sensibility of aquatic plant Wolffia brasiliensis as a bioindicator organism. Planta Daninha, 37. https://doi.org/10.1590/S0100-83582019370100092
Picard, J.-C., Munoz, G., Duy, S. V., & Sauvé, S. (2021). Longitudinal and vertical variations of waterborne emerging contaminants in the St. Lawrence Estuary and Gulf during winter conditions. Science of The Total Environment, 777, 146073. https://doi.org/10.1016/j.scitotenv.2021.146073
Plaza, J., Arencibia, A., & López-Muñoz, M. J. (2021). Evaluation of nZVI for the degradation of atrazine in heterogeneous Fenton-like systems at circumneutral pH. Journal of Environmental Chemical Engineering, 9(6), 106641. https://doi.org/10.1016/j.jece.2021.106641
Poonia, K., Hasija, V., Singh, P., Khan, A. A. P., Thakur, S., Thakur, V. K., Mukherjee, S., Ahamad, T., & Raizada, S. A. (2022). Photocatalytic degradation aspects of atrazine in water: Enhancement strategies and mechanistic insights. Journal of Cleaner Production, 367, 133087.
Props, A. J., Richards, H. J., Hooser, S. B., Burcham, G. N., & Wilson-Frank, C. R. (2021). Atrazine intoxication in cattle, confirmed by gas chromatography–mass spectrometry. Journal of Veterinary Diagnostic Investigation, 33(6), 1163–1167.
Rehan, M., Kluge, M., Fränzle, S., Kellner, H., Ullrich, R., & Hofrichter, M. (2014). Degradation of atrazine by Frankia alni ACN14a: Gene regulation, dealkylation, and dechlorination. Applied Microbiology and Biotechnology, 98(13), 6125–6135. https://doi.org/10.1007/s00253-014-5665-z
Ren, Z., Ren, B., Pan, H., Li, S., Ren, B., Xu, S., Chon, T.-S., Wang, W., & Chen, B. (2019). Is circadian rhythm a good indicator in the environmental assessment? The toxic effects of contaminants in trace level on the behavior responses of goldfish (Carassius auratus). Ecological Indicators, 105, 700–708. https://doi.org/10.1016/j.ecolind.2018.08.058
van Rensburg, G. J., Wepener, V., Horn, S., & Greenfield, R. (2022a). Oxidative stress in the freshwater shrimp Caridina africana following exposure to atrazine. Bulletin of Environmental Contamination and Toxicology, 1–7. https://doi.org/10.1007/s00128-022-03526-2
Rimayi, C., Odusanya, D., Weiss, J. M., de Boer, J., & Chimuka, L. (2018). Seasonal variation of chloro-s-triazines in the Hartbeespoort Dam catchment, South Africa. Science of the Total Environment, 613, 472–482.
Rodríguez-González, N., Uzal-Varela, R., González-Castro, M., Muniategui-Lorenzo, S., & Beceiro-González, E. (2017). Reliable methods for determination of triazine herbicides and their degradation products in seawater and marine sediments using liquid chromatography-tandem mass spectrometry. Environmental Science and Pollution Research, 24(8), 7764–7775.
Rohr, J. R., & McCoy, K. A. (2010). A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environmental Health Perspectives, 118(1), 20–32. https://doi.org/10.1289/ehp.0901164
Rostami, S., Jafari, S., Moeini, Z., Jaskulak, M., Keshtgar, L., Badeenezhad, A., Azhdarpoor, A., Rostami, M., Zorena, K., & Dehghani, M. (2021). Current methods and technologies for degradation of atrazine in contaminated soil and water: A review. Environmental Technology & Innovation, 24, 102019.
Roy, D., Neogi, S., & De, S. (2022). Visible light assisted activation of peroxymonosulfate by bimetallic MOF based heterojunction MIL-53 (Fe/Co)/CeO2 for atrazine degradation: Pivotal roles of dual redox cycle for reactive species generation. Chemical Engineering Journal, 430, 133069. https://doi.org/10.1016/j.cej.2021.133069
Sacco, O., Vaiano, V., Han, C., Sannino, D., & Dionysiou, D. D. (2015). Photocatalytic removal of atrazine using N-doped TiO2 supported on phosphors. Applied Catalysis b: Environmental, 164, 462–474. https://doi.org/10.1016/j.apcatb.2014.09.062
Sadeghnia, H., Shahba, S., Ebrahimzadeh-Bideskan, A., Mohammadi, S., Malvandi, A. M., & Mohammadipour, A. (2021). Atrazine neural and reproductive toxicity. Toxin Reviews, 1–14. https://doi.org/10.1080/15569543.2021.1966637
Salazar-Ledesma, M., Prado, B., Zamora, O., & Siebe, C. (2018). Mobility of atrazine in soils of a wastewater irrigated maize field. Agriculture, Ecosystems & Environment, 255, 73–83.
Samsudin, E. M., Abd Hamid, S. B., Juan, J. C., Basirun, W. J., Kandjani, A. E., & Bhargava, S. K. (2015). Controlled nitrogen insertion in titanium dioxide for optimal photocatalytic degradation of atrazine. RSC Advances, 5(55), 44041–44052. https://doi.org/10.1039/C5RA00890E
Sánchez, O. F., Lin, L., Bryan, C. J., Xie, J., Freeman, J. L., & Yuan, C. (2020). Profiling epigenetic changes in human cell line induced by atrazine exposure. Environmental Pollution, 258, 113712. https://doi.org/10.1016/j.envpol.2019.113712
Satsuma, K. (2010). Mineralization of s-triazine herbicides by a newly isolated Nocardioides species strain DN36. Applied Microbiology and Biotechnology, 86(5), 1585–1592. https://doi.org/10.1007/s00253-010-2460-3
Scherr, K. E., Bielská, L., Kosubová, P., Dinisova, P., Hvězdová, M., Šimek, Z., & Hofman, J. (2017). Occurrence of Chlorotriazine herbicides and their transformation products in arable soils. Environmental Pollution, 222, 283–293.
Shahid, M., Zaidi, A., Ehtram, A., & Khan, M. S. (2019). In vitro investigation to explore the toxicity of different groups of pesticides for an agronomically important rhizosphere isolate Azotobacter vinelandii. Pesticide Biochemistry and Physiology, 157, 33–44. https://doi.org/10.1016/j.pestbp.2019.03.006
Shan, W., Hu, W., Wen, Y., Ding, X., Ma, X., Yan, W., & Xia, Y. (2021). Evaluation of atrazine neurodevelopment toxicity in vitro-application of hESC-based neural differentiation model. Reproductive Toxicology, 103, 149–158. https://doi.org/10.1016/j.reprotox.2021.06.009
Sharma, K., Dutta, V., Sharma, S., Raizada, P., Hosseini-Bandegharaei, A., Thakur, P., & Singh, P. (2019). Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. Journal of Industrial and Engineering Chemistry, 78, 1–20.
Shaw, J., Moore, M., Readman, J., Mou, Z., Langston, W., Lowe, D., Frickers, P., Al-Moosawi, L., Pascoe, C., & Beesley, A. (2019). Oxidative stress, lysosomal damage and dysfunctional autophagy in molluscan hepatopancreas (digestive gland) induced by chemical contaminants. Marine Environmental Research, 152, 104825.
Shen, T., Wang, X., Xu, P., Yang, C., Li, J., Wang, P., & Zhang, G. (2022). Effect of dielectric barrier discharge plasma on persulfate activation for rapid degradation of atrazine: Optimization, mechanism and energy consumption. Environmental Research, 212, 113287.
Silveyra, G. R., Silveyra, P., Vatnick, I., Medesani, D. A., & Rodríguez, E. M. (2018). Effects of atrazine on vitellogenesis, steroid levels and lipid peroxidation, in female red swamp crayfish Procambarus clarkii. Aquatic Toxicology, 197, 136–142. https://doi.org/10.1016/j.aquatox.2018.02.017
Silveyra, G. R., Canosa, I. S., Rodriguez, E. M., & Medesani, D. A. (2017). Effects of atrazine on ovarian growth, in the estuarine crab Neohelice granulata. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 192, 1–6.
Singh, S., Kumar, V., Chauhan, A., Datta, S., Wani, A. B., Singh, N., & Singh, J. (2018). Toxicity, degradation and analysis of the herbicide atrazine. Environmental Chemistry Letters, 16, 211–237.
Singh, S., Rawat, M., Malyan, S. K., Singh, R., Tyagi, V. K., Singh, K., Kashyap, S., Kumar, S., Sharma, M., & Panday, B. (2023). Global distribution of pesticides in freshwater resources and their remediation approaches. Environmental Research, 225, 115605.
Slaby, S., Marin, M., Marchand, G., & Lemiere, S. (2019). Exposures to chemical contaminants: What can we learn from reproduction and development endpoints in the amphibian toxicology literature? Environmental Pollution, 248, 478–495. https://doi.org/10.1016/j.envpol.2019.02.014
Sobahi, T. R., & Amin, M. (2021). Photocatalytic oxidation of atrazine using BaTiO3-MWCNT nanocomposites under visible light. Ceramics International, 47(10), 14366–14374.
Sogos, V., Caria, P., Porcedda, C., Mostallino, R., Piras, F., Miliano, C., De Luca, M. A., & Castelli, M. P. (2021). Human neuronal cell lines as an in vitro toxicological tool for the evaluation of novel psychoactive substances. International Journal of Molecular Sciences, 22(13), 6785. https://doi.org/10.3390/ijms22136785
Song, F., Li, J., Fan, X., Zhang, Q., Chang, W., Yang, F., & Geng, G. (2016). Transcriptome analysis of Glomus mosseae/Medicago sativa mycorrhiza on atrazine stress. Scientific Reports, 6(1), 20245.
Sousa, J. C., Barbosa, M. O., Ribeiro, A. R., Ratola, N., Pereira, M. F., & Silva, A. M. (2020). Distribution of micropollutants in estuarine and sea water along the Portuguese coast. Marine Pollution Bulletin, 154, 111120. https://doi.org/10.1016/j.marpolbul.2020.111120
Srivastava, A., Suyal, A., & Srivastava, P. C. (2017). Persistence behavior of penoxsulam herbicide in two different soils. Bulletin of Environmental Contamination and Toxicology, 99(4), 470–474. https://doi.org/10.1007/s00128-017-2171-x
Stipičević, S., Galzina, N., Udiković-Kolić, N., Jurina, T., Mendaš, G., Dvoršćak, M., Petrić, I., Barić, K., & Drevenkar, V. (2015). Distribution of terbuthylazine and atrazine residues in crop-cultivated soil: The effect of herbicide application rate on herbicide persistence. Geoderma, 259, 300–309. https://doi.org/10.1016/j.geoderma.2015.06.018
Stradtman, S. C., & Freeman, J. L. (2021). Mechanisms of neurotoxicity associated with exposure to the herbicide atrazine. Toxics, 9(9), 207.
Su, L., Chen, X., Wang, H., Wang, Y., & Lu, Z. (2022). Oxygen vacancies promoted heterogeneous catalytic ozonation of atrazine by defective 4A zeolite. Journal of Cleaner Production, 336, 130376. https://doi.org/10.1016/j.jclepro.2022.130376
Sun, X., Liu, F., Shan, R., & Fan, Y. (2019). Spatiotemporal distributions of Cu, Zn, metribuzin, atrazine, and their transformation products in the surface water of a small plain stream in eastern China. Environmental Monitoring and Assessment, 191(7), 1–13. https://doi.org/10.1007/s10661-019-7556-3
Sun, C., Xu, Y., Hu, N., Ma, J., Sun, S., Cao, W., Klobučar, G., Hu, C., & Zhao, Y. (2020). To evaluate the toxicity of atrazine on the freshwater microalgae Chlorella sp. using sensitive indices indicated by photosynthetic parameters. Chemosphere, 244, 125514. https://doi.org/10.1016/j.chemosphere.2019.125514
Surmeier, D. J., Obeso, J. A., & Halliday, G. M. (2017). Selective neuronal vulnerability in Parkinson disease. Nature Reviews Neuroscience, 18(2), 101–113. https://doi.org/10.1038/nrn.2016.178
Tai, J. K. A. C., Horzmann, K. A., Franco, J., Jannasch, A. S., Cooper, B. R., & Freeman, J. L. (2021). Developmental atrazine exposure in zebrafish produces the same major metabolites as mammals along with altered behavioral outcomes. Neurotoxicology and Teratology, 85, 106971. https://doi.org/10.1016/j.ntt.2021.106971
Tian, S.-Q., Qi, J.-Y., Wang, Y.-P., Liu, Y.-L., Wang, L., & Ma, J. (2021). Heterogeneous catalytic ozonation of atrazine with Mn-loaded and Fe-loaded biochar. Water Research, 193, 116860. https://doi.org/10.1016/j.watres.2021.116860
Triassi, M., Montuori, P., Provvisiero, D. P., De Rosa, E., Di Duca, F., Sarnacchiaro, P., & Díez, S. (2022). Occurrence and spatial-temporal distribution of atrazine and its metabolites in the aquatic environment of the Volturno River estuary, southern Italy. Science of the Total Environment, 803, 149972. https://doi.org/10.1016/j.scitotenv.2021.149972
Trousil, S., Lee, P., Pinato, D. J., Ellis, J. K., Dina, R., Aboagye, E. O., Keun, H. C., & Sharma, R. (2014). Alterations of choline phospholipid metabolism in endometrial cancer are caused by choline kinase alpha overexpression and a hyperactivated deacylation pathway. Cancer Research, 74(23), 6867–6877.
Tuğaç, H. M., Oba, O. A., & Aydinlik, N. P. (2023). Removal of atrazine from aqueous solutions using activated carbon from novel hackberry seeds: kinetics and equilibrium studies. Chemical Engineering Communications, 211(1), 1–12.
Tulcan, R. X. S., Ouyang, W., Gu, X., Lin, C., Tysklind, M., & Wang, B. (2021). Typical herbicide residues, trophic transfer, bioconcentration, and health risk of marine organisms. Environment International, 152, 106500.
van Rensburg, G. J., Wepener, V., Horn, S., & Greenfield, R. (2022b). Oxidative stress in the freshwater shrimp Caridina africana following exposure to atrazine. Bulletin of Environmental Contamination and Toxicology, 109(3), 443–449.
Vizioli, B. D. C., da Silva, G. S., de Medeiros, J. F., & Montagner, C. C. (2023). Atrazine and its degradation products in drinking water source and supply: Risk assessment for environmental and human health in Campinas. Brazil. Chemosphere, 336, 139289.
Vonberg, D., Vanderborght, J., Cremer, N., Pütz, T., Herbst, M., & Vereecken, H. (2014). 20 years of long-term atrazine monitoring in a shallow aquifer in western Germany. Water Research, 50, 294–306. https://doi.org/10.1016/j.watres.2013.10.032
Vryzas, Z., Papadakis, E. N., Oriakli, K., Moysiadis, T. P., & Papadopoulou-Mourkidou, E. (2012). Biotransformation of atrazine and metolachlor within soil profile and changes in microbial communities. Chemosphere, 89(11), 1330–1338.
Wang, D., Xu, H., Ma, J., Lu, X., Qi, J., & Song, S. (2018). Strong promoted catalytic ozonation of atrazine at low temperature using tourmaline as catalyst: Influencing factors, reaction mechanisms and pathways. Chemical Engineering Journal, 354, 113–125. https://doi.org/10.1016/j.cej.2018.07.032
Wang, P., Liu, X., Qiu, W., Wang, F., Jiang, H., Chen, M., Zhang, W., & Ma, J. (2020). Catalytic degradation of micropollutant by peroxymonosulfate activation through Fe (III)/Fe (II) cycle confined in the nanoscale interlayer of Fe (III)-saturated montmorillonite. Water Research, 182, 116030.
Wang, Q., Zhang, A., Li, P., Héroux, P., Zhang, H., Yu, X., & Liu, Y. (2021a). Degradation of aqueous atrazine using persulfate activated by electrochemical plasma coupling with microbubbles: Removal mechanisms and potential applications. Journal of Hazardous Materials, 403, 124087. https://doi.org/10.1016/j.jhazmat.2020.124087
Wang, Z., Ouyang, W., Tysklind, M., Lin, C., & Wang, B. (2021b). Seasonal variations in atrazine degradation in a typical semienclosed bay of the northwest Pacific ocean. Environmental Pollution, 283, 117072. https://doi.org/10.1016/j.envpol.2021.117072
Wang, P., Cao, J., Mao, L., Zhu, L., Zhang, Y., Zhang, L., Jiang, H., Zheng, Y., & Liu, X. (2022). Effect of H3PO4-modified biochar on the fate of atrazine and remediation of bacterial community in atrazine-contaminated soil. Science of the Total Environment, 851, 158278.
Weber, G., Christmann, N., Thiery, A.-C., Martens, D., & Kubiniok, J. (2018). Pesticides in agricultural headwater streams in southwestern Germany and effects on macroinvertebrate populations. Science of the Total Environment, 619, 638–648.
Wilkinson, A. D., Collier, C. J., Flores, F., & Negri, A. P. (2015). Acute and additive toxicity of ten photosystem-II herbicides to seagrass. Scientific Reports, 5(1), 1–11. https://doi.org/10.1038/srep17443
Wood, R. J., Mitrovic, S. M., Lim, R. P., & Kefford, B. J. (2017). Chronic effects of atrazine exposure and recovery in freshwater benthic diatoms from two communities with different pollution histories. Aquatic Toxicology, 189, 200–208. https://doi.org/10.1016/j.aquatox.2017.06.013
Wu, S., He, H., Li, X., Yang, C., Zeng, G., Wu, B., He, S., & Lu, L. (2018). Insights into atrazine degradation by persulfate activation using composite of nanoscale zero-valent iron and graphene: Performances and mechanisms. Chemical Engineering Journal, 341, 126–136. https://doi.org/10.1016/j.cej.2018.01.136
Xie, H., Wang, X., Chen, J., Li, X., Jia, G., Zou, Y., Zhang, Y., & Cui, Y. (2019). Occurrence, distribution and ecological risks of antibiotics and pesticides in coastal waters around Liaodong Peninsula, China. Science of the Total Environment, 656, 946–951.
Xu, L., Zang, H., Zhang, Q., Chen, Y., Wei, Y., Yan, J., & Zhao, Y. (2013). Photocatalytic degradation of atrazine by H3PW12O40/Ag–TiO2: Kinetics, mechanism and degradation pathways. Chemical Engineering Journal, 232, 174–182. https://doi.org/10.1016/j.cej.2013.07.095
Xue, L., Yingnan, L., Xiaoyan, Z., & CHENG, S., Xianjie, C., & Weisong, Y. (2021a). Fabrication porous carbon nitride for photocatalytic degradation of atrazine: Influencing parameters and mechanism. Environmental Chemistry, 40(12), 3927–3935. https://doi.org/10.7524/j.issn.0254-6108.2020072302
Xue, Y., Zhang, Z.-M., Zhang, R.-R., Li, Y.-Q., Sun, A.-L., Shi, X.-Z., Chen, J., & Song, S. (2021b). Aquaculture-derived distribution, partitioning, migration, and transformation of atrazine and its metabolites in seawater, sediment, and organisms from a typical semi-closed mariculture bay. Environmental Pollution, 271, 116362.
Yang, W., & Wu, T. (2022). Evaluation of plasmon-enhanced catalytic ozonation for the abatement of micropollutants in environmental matrices. Water Research, 211, 118072. https://doi.org/10.1016/j.watres.2022.118072
Yang, L., & Zhang, Y. (2020). Effects of atrazine and its two major derivatives on the photosynthetic physiology and carbon sequestration potential of a marine diatom. Ecotoxicology and Environmental Safety, 205, 111359. https://doi.org/10.1016/j.ecoenv.2020.111359
Yang, X., Wei, H., Zhu, C., & Geng, B. (2018). Biodegradation of atrazine by the novel Citricoccus sp. strain TT3. Ecotoxicology and Environmental Safety, 147, 144–150. https://doi.org/10.1016/j.ecoenv.2017.08.046
Yang, L., Li, H., Zhang, Y., & Jiao, N. (2019). Environmental risk assessment of triazine herbicides in the Bohai Sea and the Yellow Sea and their toxicity to phytoplankton at environmental concentrations. Environment International, 133, 105175.
Yang, N., Liu, Y., Zhu, J., Wang, Z., & Li, J. (2020). Study on the efficacy and mechanism of Fe-TiO2 visible heterogeneous Fenton catalytic degradation of atrazine. Chemosphere, 252, 126333. https://doi.org/10.1016/j.chemosphere.2020.126333
Yang, C., Lim, W., & Song, G. (2021). Reproductive toxicity due to herbicide exposure in freshwater organisms. Comparative Biochemistry and Physiology Part c: Toxicology & Pharmacology, 248, 109103. https://doi.org/10.1016/j.cbpc.2021.109103
Ye, J., Zhang, J., Gao, J., Li, H., Liang, D., & Liu, R. (2016). Isolation and characterization of atrazine-degrading strain Shewanella sp. YJY4 from cornfield soil. Letters in Applied Microbiology, 63(1), 45–52. https://doi.org/10.1111/lam.12584
Yin, J., Hong, X., Ma, L., Liu, R., & Bu, Y. (2020). Non-targeted metabolomic profiling of atrazine in Caenorhabditis elegans using UHPLC-QE Orbitrap/MS. Ecotoxicology and Environmental Safety, 206, 111170.
Yu, H., Liu, Y., Shu, X., Fang, H., Sun, X., Pan, Y., & Ma, L. (2020). Equilibrium, kinetic and thermodynamic studies on the adsorption of atrazine in soils of the water fluctuation zone in the Three-Gorges Reservoir. Environmental Sciences Europe, 32(1), 1–10.
Yue, L., Ge, C., Feng, D., Yu, H., Deng, H., & Fu, B. (2017). Adsorption–desorption behavior of atrazine on agricultural soils in China. Journal of Environmental Sciences, 57, 180–189. https://doi.org/10.1016/j.jes.2016.11.002
Zaluski, A. B., Wiprich, M. T., De Almeida, L. F., De Azevedo, A. P., Bonan, C. D., & Vianna, M. R. (2022). Atrazine and Diuron Effects on Survival, Embryo Development, and Behavior in Larvae and Adult Zebrafish. Frontiers in Pharmacology, 13. https://doi.org/10.3389/fphar.2022.841826
Želježić, D., Žunec, S., Bjeliš, M., Benković, V., Mladinić, M., Lovaković Tariba, B., Pavičić, I., Marjanović Čermak, A. M., Kašuba, V., & Milić, M. (2018). Effects of the chloro-s-triazine herbicide terbuthylazine on DNA integrity in human and mouse cells. Environmental Science and Pollution Research, 25, 19065–19081.
Zhang, Y., Cao, B., Jiang, Z., Dong, X., Hu, M., & Wang, Z. (2012). Metabolic ability and individual characteristics of an atrazine-degrading consortium DNC5. Journal of Hazardous Materials, 237, 376–381.
Zhang, C., Yang, Z., Zhang, C., & Sun, Y. (2013). Kinetics of photocatalytic degradation of atrazine on molecularly imprinted titania film. Asia-Pacific Journal of Chemical Engineering, 8(3), 318–322. https://doi.org/10.1002/apj.1662
Zhang, Y., Du, Y., Liu, D., & Bian, W. (2014). The role of dissolved oxygen in the Ta (O) N-driven visible Fenton-like degradation of atrazine. Journal of Environmental Chemical Engineering, 2(3), 1691–1698.
Zhang, C., Qin, L., Dou, D.-C., Li, X.-N., Ge, J., & Li, J.-L. (2018). Atrazine induced oxidative stress and mitochondrial dysfunction in quail (Coturnix C. coturnix) kidney via modulating Nrf2 signaling pathway. Chemosphere, 212, 974–982. https://doi.org/10.1016/j.chemosphere.2018.08.138
Zhang, J., Wu, X., Zhang, X., Pan, H., Shearer, J. E., Zhang, H., & Sun, F. (2021a). Zn2+-dependent enhancement of Atrazine biodegradation by Klebsiella variicola FH-1. Journal of Hazardous Materials, 411, 125112. https://doi.org/10.1016/j.jhazmat.2021.125112
Zhang, Y., Yang, C., Zheng, Z., Cao, B., You, F., Liu, Y., & Jiang, Z. (2021b). Mechanism for various phytotoxicity of atrazine in soils to soybean: Insights from soil sorption abilities and dissolved organic matter properties. Journal of Environmental Management, 297, 113220.
Zhang, Z., Fu, Q., Xiao, C., Ding, M., Liang, D., Li, H., & Liu, R. (2022). Impact of Paenarthrobacter ureafaciens ZF1 on the soil enzyme activity and microbial community during the bioremediation of atrazine-contaminated soils. BMC Microbiology, 22(1), 1–12.
Zhang, F., Sun, S., Rong, Y., Mao, L., Yang, S., Qian, L., Li, R., & Zheng, Y. (2023). Enhanced phytoremediation of atrazine-contaminated soil by vetiver (Chrysopogon zizanioides L.) and associated bacteria. Environmental Science and Pollution Research, 30(15), 44415–44429.
Zhang, J., Liang, S., Wang, X., Lu, Z., Sun, P., Zhang, H., & Sun, F. (2019). Biodegradation of atrazine by the novel Klebsiella variicola strain FH-1. BioMed Research International, 2019. https://doi.org/10.1155/2019/4756579
Zhao, X., Bai, S., Li, C., Yang, J., & Ma, F. (2019). Bioaugmentation of atrazine removal in constructed wetland: Performance, microbial dynamics, and environmental impacts. Bioresource Technology, 289, 121618. https://doi.org/10.1016/j.biortech.2019.121618
Zheng, L., Zhang, Y., Yan, Z., Zhang, J., Li, L., Zhu, Y., Zhang, Y., Zheng, X., Wu, J., & Liu, Z. (2017). Derivation of predicted no-effect concentration and ecological risk for atrazine better based on reproductive fitness. Ecotoxicology and Environmental Safety, 142, 464–470. https://doi.org/10.1016/j.ecoenv.2017.04.006
Zhu, L., Jiang, C., Panthi, S., Allard, S. M., Sapkota, A. R., & Sapkota, A. (2021). Impact of high precipitation and temperature events on the distribution of emerging contaminants in surface water in the Mid-Atlantic, United States. Science of the Total Environment, 755, 142552. https://doi.org/10.1016/j.scitotenv.2020.142552
Zhu, J., Zhao, Y., Li, X., & Fu, L. (2022). Characteristics of two terbutylazine-degrading bacteria and the construction of a live bacterial agent for effective degradation of terbutylazine in soil. Anais da Academia Brasileira de Ciências, 94. https://doi.org/10.1590/0001-3765202220200658
Acknowledgements
The authors thank the VIT, Vellore, Tamilnadu, India, for supporting this work
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, Mrudula Pulimi and Amitava Mukherjee; Literature survey and data analysis, Garima Gajendra.; Mrudula Pulimi,; writing— original draft preparation, Garima Gajendra.; writing—review and editing, Garima Gajendra, Mrudula Pulimi, Chandrasekaran Natarajan.; visualization,, Mrudula Pulimi.; supervision, Mrudula Pulimi. Chandrasekaran Natarajan;, project administration, Chandrasekaran Natarajan and Mrudula Pulimi. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors have no competing interests to declare that are relevant to content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gajendra, G., Pulimi, M., Natarajan, C. et al. Occurrence, Toxicodynamics, and Mechanistic Insights for Atrazine Degradation in the Environment. Water Air Soil Pollut 235, 649 (2024). https://doi.org/10.1007/s11270-024-07439-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07439-0