Abstract
The phenols and ammonia–nitrogen compounds in semi-coking wastewater are used to react with formaldehyde to generate phenolic resin and curing agent urotropine, so as to achieve the resourceful preparation of thermosetting phenolic resin. The effects of pH value of wastewater, reaction time, reaction temperature, formaldehyde addition amount and other conditions on the quality of phenolic resin were investigated. Response surface methodology was used to optimize the preparation conditions of phenolic resin, and the effect of ammonia on the preparation of phenolic resin was explored. Moreover, solid content, carbon yield, gel time and viscosity of the prepared phenolic resin were determined. The experimental results showed that the quality of phenolic resin prepared when the pH value of wastewater was 9.5, the reaction time was 2.5 h, the reaction temperature was 140 °C, and the additive amount of formaldehyde was 1.4 mL, phenolic resin with best quality could be obtained. The solid content of phenolic resin prepared is 94.25%, the carbon yield is 40.02%, the gel time is 92 s, and the viscosity is 1.50 × 104 mPa·s. This study has solved the problems such as high chemical oxygen demand (COD), high ammonia–nitrogen, as well as difficult degradation and treatment.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Currently, environmental pollution and energy crisis are great challenges facing human being, which will also pose a serious threat to world security (Ribeiro and Nunes, 2021; Shi et al., 2021; Yu et al., 2017). Therefore, environmental governance in the process of energy development has become a major issue that must be considered to promote the high-quality development of energy revolution in China. In recent years, with the construction and development of energy and chemical industry bases, the mining and processing of coal as the primary energy industry has caused increasingly serious environmental pollution problems, which has brought great pressure and challenges to the local environmental protection work (Shi et al., 2020; Hao et al., 2018). In particular, the use of medium–low temperature pyrolysis technology to produce semi-coke, coal tar and gas is a coal utilization mode that is more suitable for the situation of China at present (Ding et al., 2021; Xu et al., 2020; Zhou et al., 2021). However, the semi-coke burned in the carbonization furnace needs to be cooled with water, and the raw gas must also be washed with water to ensure gas purification and more tar output, which results in the semi-coking wastewater with complex composition, high pollutant concentration, high chroma, high toxicity, stable properties and extremely poor biodegradability, which is a typical industrial wastewater with high pollution and toxicity (Chen et al., 2020; Li et al., 2019, 2020; Liu et al., 2017, Liu et al., 2020a, b; Ma et al., 2016; Zhang et al., 2020). There is currently no effective treatment process for such wastewater. Therefore, in view of the particularity of the semi-coking wastewater, such as the direct resource comprehensive utilization of many phenolic substances in the semi-coking wastewater and the transformation of wastewater "treatment" into "transformation" to prepare phenolic resin, it can not only protect the environment but also create economic benefits. Meanwhile, it has very important theoretical and practical significance for the current gradually warming coal quality utilization mode.
So far, although there have been a lot of literatures about the preparation of phenolic resin, most of the raw materials for preparing phenolic resin at home and abroad are single phenol and aldehyde (Foyer et al., 2016; Chaussoy et al., 2022; Lin et al., 2016; Biedermann and Grob, 2006). Xu et al. used phenol and formaldehyde as raw materials to synthesize resol resin by microwave heating under alkaline conditions, and obtained the best reaction conditions (Xu et al., 2020). Ding et al. used m-cresol, formaldehyde and boric acid as reaction raw materials to synthesize methyl phenolic resin and boron modified methyl phenolic resin by in-situ polymerization respectively (Ding et al., 2013). The structure of the resin was characterized, its mechanical properties, heat resistance and carbon yield were tested, and the influence of resin structure on its properties was studied. Zhao et al. explored the solid content, viscosity and curing time of phenolic resin, and analyzed the infrared spectrum of multi-catalytic phenolic resin (Zhao et al., 2016). Wang et al. used phenol and formaldehyde to prepare phenolic resin adhesive by twice adding alkali and once refluxing instead of once and twice alkali (Wang et al., 2017a, b). Wang et al. synthesized a novel aryl-boron-containing phenolic resin from phenol and formaldehyde in the presence of phenylboronic acid (Wang et al., 2014). The effects of catalyst dosage, phenolic-aldehyde ratio, boric acid addition and other factors on the properties of boron modified phenolic resin were investigated. The structure and thermal properties of boron modified phenolic resin were characterized by infrared spectroscopy and thermogravimetric analyzer. However, there are few reports on the preparation of phenolic resin by direct resource utilization of phenol, ammonia and other components in semi-coking wastewater.
In this paper, based on the resource utilization of semi-coking wastewater, the "treatment" of semi-coking wastewater is changed into "transformation", and the phenolic compounds and ammonia–nitrogen compounds in semi-coking wastewater are fully utilized to react with formaldehyde to generate phenolic resin and curing agent urotropine. Recycling to prepare thermosetting phenolic resin, response surface methodology is used to optimize the preparation conditions of phenolic resin. The phenolic resin was characterized and analyzed, so as to truly realize the purpose of near zero discharge of semi-coking wastewater, and solve its difficult degradation and difficult treatment.
2 Experimental Process
2.1 Water Sample Collection
The semi-coking wastewater in this paper was collected from a semi-coke plant with an annual output of 1 million tons in Shaanxi Province, China. The wastewater has no treatment, and the COD value is about 30,000 ~ 40,000 mg·L−1, the ammonia–nitrogen value is about 5000 ~ 15,000 mg·L−1. The concentration and composition of pollutants in semi-coking wastewater vary with different coal types and production processes, so it is necessary to filter the wastewater before use to remove the filter residue in the wastewater.
2.2 Sample Preparation
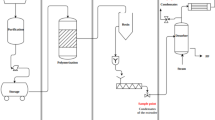
With the help of favorable conditions such as high ammonia–nitrogen catalysis, emulsification molding and waste heat at about 65 °C, the "treatment" of semi-coking wastewater is changed into "transformation". Phenolic resin and curing agent urotropine are generated by reacting phenolic and ammonia–nitrogen compounds in semi-coking wastewater with formaldehyde, and thermosetting phenolic resin is prepared by recycling. The preparation process is shown in Fig. 1.
2.3 Characterization and Analysis of Semi-Coking Wastewater Based Phenolic Resin
2.3.1 Determination of Solid Content
Three clean surface dishes were taken and put into muffle furnace. They were baked at 100 °C for about 20 min. After they were cooled to room temperature, they were weighed and recorded as m1, and then about 2.0 g of phenolic resin was weighed and recorded as m2. The weighed phenolic resin was put into the surface dish, baked at 120 °C for 2 h in muffle oven, and then cooled to room temperature, weighed and recorded as m3. The average value was determined three times. The calculation formula of solid content is as follows (EI-Shahawi et al., 2017).
2.3.2 Determination of Residual Carbon Rate
The dried crucible was weighed as w1, about 2.0 g phenolic resin was weighed as w2, the phenolic resin was placed in the crucible and placed in a muffle furnace, calcined at 800 °C for 7 min, and the total mass was weighed as w3 after cooling to room temperature. By measuring three times, the average value was recorded as the carbon yield of phenolic resin. The formula is as follows (EI-Shahawi et al., 2017).
2.3.3 Determination of Gel Time
About 1.0 g phenolic resin was placed on a heated iron plate at 150 °C, timing was started when the phenolic resin is placed on the iron plate, and the phenolic resin was continuously stirred with a glass rod until the first droplet of phenolic resin appeared, and the time at this time was the gel time of phenolic resin.
2.3.4 Determination of Viscosity
50.0g phenolic resin was dissolved in 50 mL ethanol to prepare 50% phenolic resin ethanol solution, and its viscosity was measured by NDJ-8S viscometer.
3 Results and Discussion
3.1 Effect of Preparation Conditions on the Quality of Phenolic Resin
3.1.1 Effect of pH Value of Wastewater on the Quality of Phenolic Resin
In order to explore the effect of pH value of wastewater on the quality of phenolic resin prepared, 70 mL semi-coking wastewater was taken as the treatment object, ammonia water was used to adjust the pH value of wastewater to 9.18, 9.50, 10.00, 10.50 and 11.00 respectively, 1.40 mL formaldehyde was added to react at 120 °C for 3 h, then filtered and dried to prepare a series of phenolic resins, the output of which is shown in Fig. 2(a).
Figure 2(a) is a graph showing the effect of different pH value of wastewater on the quality of phenolic resin. It can be seen from the figure that the quality of phenolic resin firstly increases and then decreases with the increase of pH value. The reason may be that with the increase of pH value of wastewater, the hydrogen on the ortho-position of phenol is more active and easier to react with formaldehyde to generate phenolic resin. When the pH value of wastewater is further increased, ammonia water ionizes ammonium ions excessively and reacts with formaldehyde to generate urotropine (hexamethylenetetramine). The generated urotropine is used as curing agent of phenolic resin, thus causing the quality of phenolic resin to decrease (Hu et al., 2019). Therefore, the amount of phenolic resin produced at pH 9.5 is the largest.
3.1.2 Effect of Reaction Time on the Quality of Phenolic Resin
Taking 70 mL semi-coking wastewater as the treatment object, a series of phenolic resins were prepared under the conditions of wastewater pH for 9.50, reaction temperature for 120 °C and addition of formaldehyde for 1.40 mL. The effect of reaction time on the quality of synthesized phenolic resin was investigated. The experimental results are shown in Fig. 2(b).
Figure 2(b) is a graph showing the effect of different reaction time on the quality of phenolic resin. It can be seen from the figure that the quality of phenolic resin shows an increasing trend with the extension of reaction time. The reasons may be that the initial reaction time is short, the reaction between formaldehyde and phenol is insufficient, addition reaction mainly occurs, polycondensation reaction rarely occurs, and the quality of phenolic resin prepared is less. With the extension of reaction time, phenol and formaldehyde can fully react under the action of ammonia, and hydroxymethyl groups also undergo polycondensation with each other, thus increasing the quality of phenolic resin. With the further increase of reaction time, the reaction of phenol gradually tends to be complete, and the quality of phenolic resin prepared tends to be stable and then increases slowly. When the reaction time is greater than 2.5 h, the quality of phenolic resin is still increasing, but the change is not obvious. Considering comprehensively, the optimal reaction time is 2.5 h.
3.1.3 Effect of Reaction Temperature on the Quality of Phenolic Resin
Reaction temperature is also one of the important factors affecting the quality of phenolic resin. In this experiment, 70 mL semi-coking wastewater was selected, the pH value was adjusted to 9.50, 1.40 mL formaldehyde was added to carry out reaction at different temperature for 2.5 h, a series of phenolic resins were obtained by filtration and drying, and the effect of reaction temperature on the quality of phenolic resin was studied. The change relationship is shown in Fig. 2(c).
Figure 2(c) shows the effect of different reaction temperature on the quality of phenolic resin. It can be seen from the figure that the quality of phenolic resin firstly increases and then decreases with the increase of reaction temperature. The reason may be that the reaction is slow and incomplete at low temperature. Ammonium ions react with phenol first to generate phenoxy anions, and phenoxy anions react with formaldehyde to generate methylol phenol. The quality of phenolic resin generated is relatively small. With the increase of temperature, addition reaction and polycondensation reaction proceed at the same time, and then polycondensation reaction occurs between methylol phenol and phenol (Bing et al., 2016; Gao et al., 2021; Liu et al., 2020a, b; Wang et al., 2017a, b), thus forming phenolic resin, and its content increases significantly. When the reaction temperature increases further, the temperature has no significant effect on the quality of phenolic resin, and because formaldehyde is volatile and cannot react with phenol, it is not conducive to the formation of phenolic resin, so the quality of phenolic resin decreases. Therefore, the best reaction temperature is 140 °C.
3.1.4 Effect of Formaldehyde Addition Amount on the Quality of Phenolic Resin
70 mL semi-coking wastewater was selected to adjust pH to 9.50 with ammonia water, and the amount of formaldehyde added was 0.80 mL, 1.00 mL, 1.20 mL, 1.40 mL, 1.60 mL, 1.80 mL, 2.00 mL and 2.20 mL, respectively. After reacting at 140 °C for 2.5 h, filtering and drying, a series of phenolic resins were prepared, and the effects of different formaldehyde additions on the quality of phenolic resins were explored.
Figure 2(d) is a graph showing the effect of different formaldehyde addition amount on the quality of phenolic resin. It can be seen from the figure that the quality of phenolic resin firstly increases, then stabilizes and then decreases with the increase of formaldehyde addition. The possible reason is that when the amount of formaldehyde is low, the ortho on phenol is inactive, and less methylol phenol is produced, and the quality of phenolic resin is less. With the increase of formaldehyde addition, formaldehyde and phenol can fully react, and the quality of phenolic resin produced gradually increases (Hu et al., 2019; Mougel et al., 2019). With increasing amount of formaldehyde furtherly, the phenol in the wastewater is continuously reduced because of the constant amount of semi-coking wastewater, which is not enough to react with formaldehyde, and the quality of phenolic resin is basically unchanged. Considering comprehensively, the optimal amount of formaldehyde added is 1.4 mL.
3.2 Preparation Mechanism of Phenolic Resin
Because there are many phenolic substances in semi-coking wastewater, after they react with the added formaldehyde, a variety of corresponding phenolic resins will produce. The total reaction equation for preparing phenolic resin is Eq. 3.
Meanwhile,the effect of ammonia in semi-coking wastewater on the preparation of phenolic resin is complex, ammonia is a weak base, which participates in the whole reaction as a catalyst in the reaction process; The ionized hydroxide ion and phenol generate phenoxy anion, the phenoxy anion and formaldehyde are added to generate methylol phenol, and the condensation of methylol phenol will generate phenolic resin; In addition, the ionized ammonium ion will also react with formaldehyde to generate hexamethylenetetramine (urotropine) (EI-Shahawi et al., 2017; Hu et al., 2019), which is a very important curing agent, resulting in the obtained phenolic resin being thermosetting phenolic resin. Ammonia in wastewater reacts with formaldehyde as follows.
3.3 Preparation Conditions Optimization of Phenolic Resin by Response Surface Methodology
3.3.1 Response Surface Design
Response surface methodology is a method that uses modeling analysis to determine various factors and their interactions, so as to achieve the purpose of optimizing experiments (Pereira et al., 2021). Through the above single factor experimental analysis results, using Design Expert 7.0 software and response surface methodology Box-Behnken Design (BBD) design, the influence of pH, reaction temperature, reaction time and other three factors and their levels on the quality of phenolic resin in the preparation process of phenolic resin was further studied. In terms of design level range, the pH value was 9.18 ~ 10, the reaction time was 1 ~ 4 h, and the amount of formaldehyde added was 1.2 ~ 1.8 mL. The experimental factors and independent variable levels are shown in Table 1.
3.3.2 Response Surface Model and Significance Test of Regression Equation
The temperature of the optimization experiment is fixed at 140 °C. The response surface methodology design experiment adopts the three-factor and three-level experiment designed in Table 1. The experimental design scheme and analysis results are shown in Table 2. The predicted value in Table 2 is the result of regression analysis of the real data obtained from the experiment by software. The relative error is the ratio of absolute error to real value, while the absolute error is the difference between the real data obtained from the experiment and the predicted data. It can be found from the table that the error between the experimental prediction value and the experimental value is less than 6%. In addition, the real experimental data are compared with the predicted data. As shown in Fig. 3, the distribution of experimental data is basically consistent with the distribution of predicted data, indicating that the established model can be applied to the prediction of optimization conditions in the preparation process of phenolic resin.
ANOVA in Design Expert 7.0 software is used to perform quadratic multinomial regression fitting on the data in Table 2. The coefficients of the regression equation and the variance analysis of the response surface model are shown in Table 3. The F value in the table is the ratio of the regression mean square deviation to the error mean square deviation, and the P value is the probability value corresponding to the F value. When the P value of model term or regression equation coefficient is less than 0.05, it has a significant impact on the model. From the table, it can be seen that the P value of the regression model is less than 0.05, indicating that the model has significant influence. The mismatch term is a reflection of the inconsistency between the experimental data and the model. The model can be used for experimental simulation when the value is greater than 0.1, while the P value in the phenolic resin preparation regression model is 0.0568, indicating that the model mismatch is not significant. In addition, A, B, C, AC, A2, B2, C2 have significant effects on the regression equation of phenolic resin preparation. The binary multi-term regression equation of phenolic resin quality is as follows.
3.3.3 Response Surface Analysis
In order to determine the best preparation conditions of phenolic resin, according to the influence of two factors (solution pH values-reaction time; solution pH values-formaldehyde addition; reaction time-formaldehyde addition) in the model equation on the quality of phenolic resin, a three-dimensional response surface diagram and its contour diagram (Figs. 4, 5 and 6) are drawn respectively. The influence results of each factor and their interaction on the quality of phenolic resin can be directly reflected through the diagram.
Figure 4 shows the interactive effect of the pH value of wastewater and reaction time on the quality of phenolic resin. It can be seen from the figure that both the pH value of wastewater and the reaction time have important influence on the quality of phenolic resin, showing a trend of increasing first and then decreasing. When the reaction time is 3.4 ~ 4 h and the pH value is 9.59 ~ 10.00, its mass shows an obvious downward trend. Through contour analysis, it can be concluded that the reaction time and the pH value of wastewater have an interactive effect on phenolic resin, and the optimal region of the pH value is between 9.38 and 9.69, and the reaction time is between 2.2 and 3.4 h.
Figure 5 shows an interactive effect of the pH value of wastewater and formaldehyde addition on phenolic resin quality. From the response surface diagram, it can be known that the quality of phenolic resin increases first and then decreases with the increase of pH value and formaldehyde addition in wastewater. The obvious downward trend appeared when the amount of formaldehyde added was 1.65 ~ 1.80 mL, and the pH value of wastewater appeared in 9.59 ~ 10.00. Through contour analysis, it can be concluded that the pH value of wastewater and the amount of formaldehyde added have obvious interactive effects on the quality of phenolic resin. The optimal range of the pH value is between 9.28 and 9.79, and the amount of formaldehyde added is between 1.40 and 1.70 mL.
Figure 6 shows that interaction of reaction time and formaldehyde addition on the quality of phenolic resin. From the response surface diagram, it can be observed that the reaction time and the amount of formaldehyde have a greater impact on the quality of phenolic resin. With the extension of the reaction time and the increase of the amount of formaldehyde, the quality of phenolic resin presents a trend of first increasing and then decreasing. The obvious decreasing trend appears in the reaction time about 3.4 ~ 4 h, and the amount of formaldehyde added appears between 1.65 ~ 1.80 mL. There is an optimal region of the reaction time is 2.5 ~ 3.4 h, and the amount of formaldehyde added is 1.40 ~ 1.70 mL. In this area, the quality of phenolic resin prepared is the highest.
3.3.4 Verification Experiment
The optimal conditions for the preparation of phenolic resin were obtained that the pH value was 9.5, the reaction time was 2.8 h, and the amount of formaldehyde added was 1.5 mL from the optimize model of Design Expert 7.0. This value can also be obtained by finding the first partial derivative of the regression equation (Eq. 5).
Under the conditions that the pH value was 9.48, the reaction time was 2.8 h, and the amount of formaldehyde added was 1.5 mL, the experiment was repeated three times. If the relative error is not more than 15%, it shows that the model has a good prediction result for the preparation conditions of phenolic resin. The experimental results are shown in Table 4.
As can be seen from Table 4, the above relative errors are not more than 15%, indicating that the model has a good prediction effect on the preparation conditions of phenolic resin.
3.4 Characterization Analysis of Semi-Coking Wastewater Based Phenolic Resin
3.4.1 Determination and Analysis of Solid Content
Three phenolic resin samples with mass of 1.9988 g, 2.0071 g and 2.0003 g were weighed respectively, and placed in muffle furnace at 120 °C for 2 h to determine the solid content of phenolic resin.
Table 5 is the solid content measurement table of phenolic resin. From the above table, the solid content of phenolic resin is 94.25%, which is larger than that of conventional phenolic resin. This may be because some ions in semi-coking wastewater play a role in strengthening the solid content of phenolic resin. Phenolic resin with large solid content has the advantages of fast drying and low transportation cost.
3.4.2 Determination and Analysis of Carbon Yield
Three phenolic resin samples with mass of 2.4156 g, 2.4327 g and 2.4927 g were taken and calcined in muffle furnace at 800 °C for 7 min, and then the carbon yield of phenolic resin was determined.
Table 6 shows the measurement data of carbon yield of phenolic resin. It can be seen that the carbon yield of semi-coking wastewater based phenolic resin at 800 °C is 40.02%, and the carbon yield of semi-coking wastewater based phenolic resin is relatively high, which well explains that the preparation of phenolic resin is thermosetting phenolic resin and is the best choice for refractory materials.
3.4.3 Determination and Analysis of Gel Time
Three phenolic resin samples with mass of 1.0002 g, 1.0008 g and 1.0006 g were taken respectively, and the gel time of phenolic resin was measured on a heated iron plate at 150 °C.
Table 7 is the measurement data of the gel time of the semi-coking wastewater based phenolic resin, From the table, the gel time of the semi-coking wastewater based phenolic resin is 92 s, The gel time of pure phenolic resin is 121 s, and the gel time of semi-coking wastewater based phenolic resin is shorter than that of pure phenolic resin, which may result from the fact that the activation energy required for synthesizing phenolic resin from semi-coking wastewater is lower than that required for synthesizing pure phenolic resin, so the gel time of semi-coking wastewater based phenolic resin is shorter.
3.4.4 Determination and Analysis of Viscosity
50 g semi-coking wastewater based phenolic resin was taken, 50 mL ethanol solution was added, stirred and shaken well, and its viscosity was measured by NDJ-8S viscometer.
Table 8 shows the measurement data of the viscosity of the semi-coking wastewater based phenolic resin. According to the table, the viscosity of the semi-coking wastewater based phenolic resin is 1.50 × 104 mPa·s, the viscosity of pure phenolic resin is 1.00 × 104 mPa·s, and the viscosity of semi-coking wastewater based phenolic resin is higher than that of pure phenolic resin, which may be due to the modification of some ions in semi-coking wastewater in the process of generating phenolic resin. The effect makes the viscosity of semi-coking wastewater based phenolic resin higher than that of pure phenolic resin.
3.4.5 SEM and FT-IR Analysis of Semi-Coking Wastewater Based Phenolic Resin
The SEM image of the prepared phenolic resin is represented in Figure S1, which is enlarged by 5000 times. Most phenolic resins are spherical shaped, which is consistent with previous literatures (Liao et al., 2021; Jeong et al., 2021). However, compared with phenolic resin prepared by pure single component, the phenolic resins prepared from semi-coking wastewater possess different sizes and aggregates into large clumps, attributing to the difference reaction speed between formaldehyde and the different phenolic substances contained in the wastewater, and resulting in the spherical structure of different sizes. FT-IR spectra of semi-coking wastewater based phenolic resin is represented in Figure S1. The characteristic peak at 1456 cm−1 assigns to CH2. The characteristic peak at 1620 cm−1 corresponds to benzene ring and the characteristic peak in 3396 cm−1 attributes to the phenolic hydroxyl group. Especially, the presence of methylene bridge functional group at around 1464 cm−1 confirms the formation of phenolic resins (Yaakob et al., 2022), indicating that phenolic resins was successfully prepared from semi-coking wastewater.
In addition, water quality characteristics before and after preparation of phenolic resin from semi-coking wastewater is shown in Table S1. Figure S3 shows the color comparison of semi-coking wastewater before and after preparation. After the preparation of phenolic resin, the color of the wastewater changes from dark black to light red. Chemical oxygen demand (COD), ammonianitrogen and total phenol are greatly decreased. The removal rate of phenols is as high as 99.2%. The toxicity of the wastewater is dramatically reduced, which is conducive to subsequent biochemical treatment.
4 Conclusion
70 mL semi-coking wastewater was used as the research object, and a certain amount of formaldehyde was added to prepare phenolic resin at a certain reaction temperature and time. Through this experiment, the following conclusions can be drawn:
(1) The effects of the pH value of wastewater, reaction time, reaction temperature, formaldehyde addition amount and other conditions on the quality of phenolic resin are investigated, and it is found that more phenolic resin could be prepared when the pH value of wastewater is 9.5, reaction time is 2.5 h, reaction temperature is 140 °C, and formaldehyde addition is 1.4 mL.
(2) The Box-Behnken Design (BBD) of response surface method was used to further study the interaction of the pH value of wastewater, reaction time and formaldehyde addition of wastewater on the quality of phenolic resin, so as to obtain the optimal conditions for the preparation of phenolic resin. The experimental results showed that the resin quality is the best when the pH value of wastewater is 9.5, the reaction time is 2.8 h and the addition amount of formaldehyde is 1.5 mL.
(3) The solid content of phenolic resin prepared from semi-coking wastewater is 94.25%, the carbon yield is 40.02%, the gel time is 92 s, and the viscosity is 1.50 × 104 mPa·s. A series of data show that the semi-coking wastewater based phenolic resin has better physical and chemical properties.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request, and all data generated and analysed during this study are included in this manuscript. Moreover, the datasets generated and analysed during the current study are not publicly available due to this technology is facing the achievement transformation but are available from the corresponding author on reasonable request.
References
Biedermann, M., & Grob, K. (2006). Phenolic resins for can coatings: I. Phenol-based resole analysed by GC–MS, GC×GC, NPLC–GC and SEC. LWT-Food Science and Technology, 39, 633–646. https://doi.org/10.1016/j.lwt.2005.04.008
Bing, L., Feng, S. H., Niasar, H. S., Zhang, Y. S., Schmidt, J., & Xu, C. (2016). Preparation and Characterization of Bark-derived Phenol Formaldehyde Foams. RSC Advances, 6(47), 40975–40981. https://doi.org/10.1039/C6RA05392K
Chaussoy, N., Brandt, D., & Gérard, J. F. (2022). Versatile method to reduce the free formaldehyde content in phenolic resins for high-temperature applications. ACS Applied Polymer Materials, 4, 4454–4463. https://doi.org/10.1021/acsapm.2c00148
Chen, B. K., Yang, S. Y., Wu, Y. Y., Suo, M. Y. Z., & Qian, Y. (2020). Intensified phenols extraction and oil removal for industrial semi-coking wastewater: A novel economic pretreatment process design. Journal of Cleaner Production, 242, 118453–118453. https://doi.org/10.1016/j.jclepro.2019.118453
Ding, J. P., Tao, Z. Q., Fan, L., & Yang, S. Y. (2013). Preparation and characterization of flame retardant epoxy resins based on phosphorus-containing biphenyl-type phenolic resin. e-Polym, 10(1), 122. https://doi.org/10.1515/epoly.2010.10.1.1372
Ding, L., Shi, X. G., Zhang, J., Wu, Y. Y., Wang, C. X., Gao, J. S., & Lan, X. Y. (2021). Modeling on the pyrolysis of particles of low-rank coal and CaO mixture. Journal of Analytical and Applied Pyrolysis, 156, 105169. https://doi.org/10.1016/j.jaap.2021.105169
El-Shahawi, M. S., Bashammakh, A. S., Alwael, H., Alsibaai, A. A., & Dowaidar, A. M. (2017). Adsorption characteristics of polycyclic aromatic hydrocarbons from non-aqueous media using activated carbon derived from phenol formaldehyde resin: kinetics and thermodynamic study. Environmental Science and Pollution Research International, 24(4228), 4240. https://doi.org/10.1007/s11356-015-4936-2
Foyer, G., Chanfi, B. H., Boutevin, B., Caillol, S., & David, G. (2016). New method for the synthesis of formaldehyde-free phenolic resins from lignin-based aldehyde precursors. European Polymer Journal, 74, 296–309. https://doi.org/10.1016/j.eurpolymj.2015.11.036
Gao, Z. W., Lang, X. L., Chen, S., & Zhao, C. (2021). Mini-Review on the Synthesis of Lignin-Based Phenolic Resin. Energy & Fuels, 35(22), 18385–18395. https://doi.org/10.1021/acs.energyfuels.1c03177
Hao, P., Ma, X. Y., Xie, J. F., Lei, F. C., Li, L. Y., Zhu, W. Q., Cheng, X., Cui, G. W., & Tang, B. (2018). Removal of toxic metal ions using chitosan coated carbon nanotube composites for supercapacitors. Science China: Chemistry, 61, 797–805. https://doi.org/10.1007/s11426-017-8215-7
Hu, Z. X., Hu, X. M., Cheng, W. M., & Lu, W. (2019). Influence of synthetic conditions on the performance of melamine-phenol-formaldehyde resin microcapsules High Perform. Polym., 31(2), 197–210. https://doi.org/10.1177/0954008318758489
Jeong, H., Kang, Y. G., Ryu, S. S., Lee, S. M., & Kim, S. (2021). Fabrication of high-strength macroporous carbons with tunable pore size by a simple powder process using phenolic resin microspheres. Ceramics International, 47, 8820–8825. https://doi.org/10.1016/j.ceramint.2020.12.002
Li, Z. Q., Jiang, L. Y., & Tang, C. J. (2019). Investigation on removing recalcitrant toxic organic polluters in coking wastewater by forward osmosis. Chinese Journal of Chemical Engineering, 28, 122–135. https://doi.org/10.1016/j.cjche.2019.07.011
Li, J. N., Wang, S. Z., Li, Y. H., Wang, L. S., Xu, T. T., Zhang, Y. S., & Jiang, Z. H. (2020). supercritical water oxidation of semi-coking wastewater: Effect of operating parameters, reaction mechanism and process enhancement. Science of the Total Environment, 710, 134396. https://doi.org/10.1016/j.scitotenv.2019.134396
Liao, Y. K., Wang, J. X., Song, X., Zhang, G., & Chen, B. Y. (2021). Low cost and large mass producible phenolic resin for water disinfection and antibacterial coating under weak visible light LED or sunlight irradiation. Applied Catalysis B Environmental, 292, 120189. https://doi.org/10.1016/j.apcatb.2021.120189
Lin, C. T., Lee, H. T., & Chen, J. K. (2016). Preparation of vanadium-modified phenolic resin/modified zirconia composites and its applied properties in cubic boron nitride (cBN) grinding wheels. Polymer Composites, 37, 3354–3364. https://doi.org/10.1002/pc.23533
Liu, Y. J., Liu, J., Zhang, A. N., & Liu, Z. (2017). Treatment effects and genotoxicity relevance of the toxic organic pollutants in semi-coking wastewater by combined treatment process. Environmental Pollution, 220, 13–19. https://doi.org/10.1016/j.envpol.2016.04.095
Liu, J., Xuan, D. P., Chai, J., Guo, D. D., Huang, Y. B., Liu, S. Q., Chew, Y. T., Li, S. R., & Zheng, Z. F. (2020a). Synthesis and Thermal Properties of Resorcinol-Furfural Thermosetting Resin. ACS Omega, 5(17), 10011–10020. https://doi.org/10.1021/acsomega.0c00365
Liu, Y., Liu, Y. J., & Liu, J. (2020b). Study on the Removal Effects and Genotoxicity Evaluation of Phenols in a Semi-Coking Wastewater Treatment Stages. Journal of Water Chemistry and Technology, 42, 297–304. https://doi.org/10.3103/s1063455x20040086
Ma, X. Y., Wang, X. C., Liu, Y. J., Gao, J., & Wang, Y. K. (2016). Variations in toxicity of semi-coking wastewater treatment processes and their toxicity prediction. Ecotoxicology and Environmental Safety, 138, 163–169. https://doi.org/10.1016/j.ecoenv.2016.09.031
Mougel, C., Garnier, T., Cassagnau, P., & Sintes-Zydowicz, N. (2019). Phenolic foams: A review of mechanical properties, fire resistance and new trends in phenol substitution. Polym., 164, 86–117. https://doi.org/10.1016/j.polymer.2018.12.050
Pereira, L. M. S., Milan, L. M., & Tapia-Blácido, D. R. (2021). Using Response Surface Methodology (RSM) to optimize 2G bioethanol production: A review. Biomass and Bioenergy, 151, 106166. https://doi.org/10.1016/j.biombioe.2021.106166
Ribeiro, J. P., & Nunes, M. I. (2021). Recent trends and developments in Fenton processes for industrial wastewater treatment - A critical review. Environmental Research, 197, 110957. https://doi.org/10.1016/j.envres.2021.110957
Shen, L. J., & Xu, W. (2002). Synthesis of bakelite under microwave radiation. Petrochemical Technology, 31, 191–193. https://doi.org/10.1007/s11670-002-0022-7
Shi, J. X., Xu, C. Y., Han, Y. X., & Han, H. J. (2020). Case study on wastewater treatment technology of coal chemical industry in China. Critical Reviews in Environment Science and Technology, 51, 1003–1044. https://doi.org/10.1080/10643389.2020.1742530
Shi, J. X., Huang, W. P., Han, H. J., & Xu, C. Y. (2021). Pollution control of wastewater from the coal chemical industry in China: Environmental management policy and technical standards. Renewable Sustainable Energy Rev., 143, 110883. https://doi.org/10.1016/j.rser.2021.110883
Wang, S. J., Jing, X. L., Wang, Y., & Si, J. J. (2014). Synthesis and characterization of phenolic resins containing aryl-boron backbone and their utilization in polymeric composites with improved thermal and mechanical properties. Polymers for Advanced Technologies, 25, 152–159. https://doi.org/10.1002/pat.3216
Wang, F., Kuai, J. T., Hu, G. Q., & Gao, Q. W. (2017a). Synthesis and properties of phenolic modified enzymatic hydrolysis lignin-epoxy resin/epoxy resin composites. Acta Materiae Com‐positae Sinica, 34(12), 2681–2688. https://doi.org/10.13801/j.cnki.fhclxb.20170401.001
Wang, Z. Q., Gao, S. S., You, J., Yu, J. L., Jiang, T., He, D. H., Gong, H., Zhang, T. T., Wei, J. J., & Guo, S. Z. (2017b). Synthesis and application of water-soluble phenol-formaldehyde resin crosslinking agent. IOP Conference Series: Earth and Environmental Science, 61(1), 012150. https://doi.org/10.1088/1755-1315/61/1/012150
Xu, K., Hu, S., Zhang, L. L., Li, H. J., Chen, Y. F., Xiong, Z., Xu, J., Jiang, L., Wang, Y., Su, S., Xu, H., & Xiang, J. (2020). Effect of temperature on Shenfu coal pyrolysis process related to its chemical structure transformation. Fuel Processing Technology, 213, 106662. https://doi.org/10.1016/j.fuproc.2020.106662
Yaakob, M. N. A. Y., Roslan, R., Salim, N., & Mustapha, S. N. H. (2022). Structural and thermal behavior of lignin-based formaldehyde-free phenolic resin. Materials Today: Proceedings, 51, 1388–1391. https://doi.org/10.1016/j.matpr.2021.11.526
Yu, D. D., Wang, H., Yang, J., Niu, Z. Q., Lu, H. T., Yang, Y., Cheng, L. W., & Guo, L. (2017). Dye Wastewater Cleanup by Graphene Composite Paper for Tailorable Supercapacitors. ACS Applied Materials & Interfaces, 9, 21298–21306. https://doi.org/10.1021/acsami.7b05318
Zhang, Y., Qin, L., Cui, Y., Liu, W. F., Liu, X. G., & Yang, Y. Z. (2020). A hydrophilic surface molecularly imprinted polymer on a spherical porous carbon support for selective phenol removal from coking wastewater. New Carbon Materials, 35, 220–231. https://doi.org/10.1016/S1872-5805(20)60486-3
Zhao, Y., Zhang, J. Z., Zhang, S. F., Gao, Q., Li, J. Z., & Zhang, W. (2016). Synthesis and Mechanism of Metal-Mediated Polymerization of Phenolic Resins. Polymers, 8, 159. https://doi.org/10.3390/polym8050159
Zhou, L. M., Wang, X. B., Chen, M. A., Liu, S., & Liu, J. J. (2021). Volatility of cadmium during pyrolysis of typical low rank coals. Journal of Fuel Chemistry and Technology, 49, 648–656. https://doi.org/10.1016/S1872-5813(21)60066-X
Acknowledgements
This work was funded in part by National Natural Science Foundation of China (22168043), Science and Technology Plan Project of Shaanxi Province of China (2024SF-YBXM-574), Science and technology project of Yulin City (2023-CXY-160), Pre-research fund of Yulin University (22GK08), Joint Fund of Clean Energy Innovation Institute of Chinese Academy of Sciences and Yulin University (YLUDNL202202).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work; there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, S., Yang, Y., Wang, Y. et al. Preparation Conditions and Property Characterizations of Semi-coking Wastewater based Phenolic Resin. Water Air Soil Pollut 235, 569 (2024). https://doi.org/10.1007/s11270-024-07367-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07367-z