Abstract
We aimed to assess the quality of a midsize river that receives agricultural and urban wastewater. Nuclear abnormalities (NA), comet assays of blood and gills, and gill histopathology were evaluated in fish Astyanax aff. paranae during the summer and winter 2011 at three sites in Paraná State, Brazil: (1) a biological reserve (Rebio—reference area); (2) an agricultural site; (3) a downstream site that accumulates agricultural and urban effluents. We found the highest effects of pollutants in fish at the downstream site during the summer. The agricultural site showed an intermediate damage rate, and fish at Rebio generally had the least damage, with the exception of NA. Despite conflicting results from the biomarkers used, we observed an increase in damage associated with the accumulation of pollutants. Pesticides are probable xenobiotics in the agricultural area. Additionally, metals and substances such as pharmaceuticals and ammonia may be present at the downstream site.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Industrial development and intense urbanization along rivers introduces a large volume of different substances to waterways, including thousands of xenobiotic organic and inorganic chemical compounds (Van der Oost et al. 2003). The discharge of these pollutants from industrial, domestic, and agricultural sources negatively affects aquatic biodiversity as well as the health of humans that utilize the water source (Amorim 2003).
Many animal species can be used as bioindicators to assess the effects of xenobiotics. Fish are relatively sensitive and are widely used as bioindicators of the impact of xenobiotics on aquatic environments across several response levels (Van der Oost et al. 2003). Fishes of the genus Astyanax Baird and Girard (1854) are important components of the trophic food chain in South American rivers, and they comprise a considerable portion of the diet of larger fishes (Prioli et al. 2002). However, stocks of some species of this genus have been severely endangered by the introduction of large predators (Agostinho et al. 2007) and by anthropogenic contamination. These fish species have many properties that make them useful for the assessment of bioindicators: they are omnivorous, can be easily captured, have a convenient size for experiments, and do well under laboratory conditions (Carrasco-Letelier et al. 2006). Therefore, these organisms have been used in many recent studies, including bioassays and environmental biomonitoring (Alberto et al. 2005; Carrasco-Letelier et al. 2006; Rossi et al. 2011).
Field studies comparing impacted and unimpacted areas permit the evaluation of the health of fish in their own environment, although it is not always possible to precisely determine the causal agent of organismal change. Nonetheless, some biomarkers, such as the piscine micronucleus test, comet assay, and the histopathological index, applied to specific organs can provide a good estimate of the general and long-term quality of the environment (Martinez and Souza 2002).
Studies of environmental contamination using biomarkers are currently recommended to be complementary to environmental monitoring. According to Zhou et al. (2008), these studies have obvious advantages over conventional chemical analyses of aquatic environments; they can: (1) detect sublethal effects on organisms; (2) present integrative responses to complex mixtures of pollutants; and (3) be used for predictive analyses of pollutants, even at concentrations undetectable by analytical methods.
Biomarker analyses are important in field studies because they are easy to perform and represent low-cost tools for characterizing the effects of pollution in situ. They provide integrative data regarding the health of biota and the risks of exposure to human populations, and they have recently been used in numerous biomonitoring studies (Abdel-Moneim et al. 2012; Brito et al. 2012; Azevedo et al. 2013).
The growth of urban, industrial, and agricultural activities around water sources associated with frequently inadequate or insufficient water treatment has increased the levels of pollutants in water bodies. In the last decades, more and more xenobiotics have harmed the physical, chemical, and biological processes of ecosystems, resulting in serious disturbances. Thus, biomonitoring programs have become relevant, principally because the effects of agricultural and urban pollution on fishes can be evaluated individually or cumulatively. The results of these biomonitoring studies can be subside to conservation programs and regulatory laws (Brito et al. 2012).
Thus, our study aimed to assess, during the summer and winter of 2011, the water quality at two sites along the Campo River, which receives different types of pollutants, using the fish Astyanax aff. paranae as a bioindicator. Our hypothesis was that the damage rate in biomarkers in A. aff. paranae would increase because of the cumulative effects of pollutants and that this would become more intense during the summer.
Materials and Methods
The study area is located in two low-order tributaries of the Upper Paraná River Basin, southern Brazil. The fish species, A. aff. paranae, is restricted to these tributaries, principally in the areas closest to the headwaters (Garutti and Britski 2000). This species is a small characiform fish that undertakes short reproductive migrations (Suzuki et al. 2004), although it usually remains in a specific area. It has a short life cycle and omnivorous habits (Barbieri 1992).
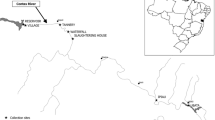
The sampling sites are shown in Fig. 1. MP is located in the Campo River, upstream of Campo Mourão, Paraná, South Brazil. It is surrounded only by agricultural areas and does not cross any urban or industrial zones. This site probably receives only agricultural effluents. JP is located in the same river, downstream of the city, and is characterized by the discharge of different urban effluents, including effluents from a municipal sewage treatment plant and industrial waste. Flocculent materials, including non-natural forms, were evident at this site, in violation of the Brazilian law established by Resolution number 357 (Brasil 2005). At this site, it is necessary to note the accumulation of agricultural and urban pollutants. Furthermore, an artificial dam located between these two sites prohibits the migration of fish. Campo Mourão has a population of 90,000 people, and its economy is predominantly based on agriculture, which occupies approximately 84 % of the municipal area of the city. Levels of crop production, particularly of soy and corn, are very high due to the low relief and deep soil, which have allowed the development of mechanized agriculture in the region. These favorable conditions for intensive cultivation have led to environmental conservation being neglected, and inadequate conservation practices that favor soil erosion are still common in the region (Mizote 2008). Moreover, agricultural activity is very important in this region as it hosts the largest agricultural cooperative in Brazil and the third largest in the world.
The third site (RE) is located in the Concordia Stream in the Biological Reserve of Perobas (Rebio), in the city of Tuneiras do Oeste. A Biological Reserve is a category of conserved area stated in Brazilian law No. 9985 (National System of Conservation Units—SNUC, Brasil 2000), which aims for the integral preservation of biota and other attributes existing in their natural limits. In this area, the only human interference permitted is for procedures to recover modified ecosystems and for management activities needed to restore and preserve the natural balance, biodiversity, and natural ecological processes (Brasil 2000). This site is characterized by the lowest level of anthropogenic impact.
The climate of the three sampling sites, according to the Köppen climate classification, is moist mesothermal subtropical (Cfa), with warm summers and infrequent frosts. Summer is often the wettest season, without a defined dry season. The mean maximum temperature in the warm months is approximately 22°C, falling below 18°C in winter, with a mean annual temperature of approximately 20°C. Annual rainfall at these sites varies between 1,300 and 1,600 mm, and relative humidity is 75 %. The winds are predominantly from the northeast quadrant, but there is the possibility of frost in winter months when they blow from the south or southwest (Maack 1981). Both the Concordia Stream and the Campo River are situated on the third plateau of Paraná, at an altitude of approximately 600 m (Paraná 2005; Silva and Filho 2011).
The regions of Rebio and Campo Mourão are characterized by contact between seasonal semi-deciduous submontane forest containing perobas (Aspidosperma spp.), cedar (Cedrela fissilis), and palmettos (Euterpe edulis), and the Araucaria Forest, dominated by Pinheiro-do-Paraná (Araucaria angustifolia) (Castella and Britez 2004). The cerrado, which is characterized by aluminum-rich soils, can be found in both areas. Unfortunately, this phytogeographical component in Campo Mourão has been reduced to a few fragments, many of them defaced by human action and biological contamination by invasive species, e.g., Leucaena leucocephala (Guerreiro and Parolin 2008).
We sampled approximately 20 fishes (A. aff. paranae) at each site during the winter (July 2011) and summer (November 2011). All the procedures in this study were carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals (following the Canadian Council on Animal Care 2003).
Fishes were transported to the laboratory, anesthetized with benzocaine, and euthanized by an excess of anesthesia. The procedures described by Heddle (1973) and Schmid (1975), as modified by Ferraro et al. (2004), were used to perform the piscine micronuclei (MN) and nuclear alteration test (NA). Blood samples were collected with a heparinized insulin syringe and smeared on clean microscope slides. Cells were fixed with ethanol PA and stained with 10 % Giemsa for 10 min. One thousand cells from each fish were analyzed using blind scoring under 1,000× magnification. Only cells with intact cellular and nuclear membranes were scored. Round or ovoid-shaped, non-refractory particles with color and structure similar to chromatin and a diameter 1/3rd that of the main nucleus, and clearly detached from it, were interpreted as MN. At the same time, consistent variations from the normally smooth, elliptical shape of the erythrocyte nucleus were recorded. Following the description by Carrasco et al. (1990), the nuclear morphological alterations were classified as blebbed, lobed, vacuolated, binucleated, or notched nuclei. All of these features were considered “nuclear alterations” (NA) and were grouped together with MN for statistical analysis.
Comet assays were performed with blood and gill samples (Speit and Hartmann 1999, with modifications by Ferraro et al. 2004). Gill and blood cells were homogenized (Potter-type homogenizer at 1,500 rpm). A 15-μL sample was taken from each replicate and mixed with 120 μL of low-melting point agarose (0.5 %). The suspension was spread onto slides previously coated with a normal agarose layer. The resultant gel electrophoresis “comets” were scored using a Leica® epifluorescence microscope. One hundred nucleoids from each fish were analyzed (Kobayashi et al. 1995) using a visual classification system based on the migration of DNA fragments, defined as class 0 (no visible damage), class 1 (little damage), class 2 (medium damage), class 3 (extensive damage), and class 4 (maximally damaged). The comet score was calculated by multiplying the number of nuclei in a class by the class number.
Gill samples were preserved in Alfac fixative solution for 12 h (85 mL of 80 % ethanol; 10 mL of 40 % formaldehyde; and 5 mL of glacial acetic acid per 100 mL of solution), dehydrated in a graded series of ethanol baths, and embedded in Paraplast-Plus resin (Sigma®). Sections (5 µm thick) were stained with hematoxylin/eosin and observed under a Leica® photomicroscope. Morphological lesions were graded according to the injury index described by Bernet et al. (1999), wherein observations of injury to the gills were classified under three severity factors (minimal, moderate, and marked pathological importance) and an index was obtained. The following lesions were quantified: total fusion, hyperplasia, aneurism, epithelium displacement, necrosis, and epithelial atrophy.
The use of all of the above cited biomarkers can be justified because, according to Moore and Simpson (1992), biomarkers are best used as selected batteries of tests rather than individually. In field studies like ours, a large number of pollutants are present in complex mixtures, and they are responsible for multiple effects on the many organisms present (Bolognesi and Hayashi 2011). Therefore, the evaluation of responses to mixtures of pollutants requires a set of complementary biomarkers (Flammarion et al. 2002). For this reason, the investigated endpoints were selected in order to examine several negative biological impacts such as cytotoxicity, mutagenicity, DNA damage, biochemical alterations, and histopathology (Van der Oost et al. 2003). Thus, comparative analysis of the results obtained at different biological levels may lead to substantial improvement in the knowledge of integrated fish responses to toxin exposure.
All data were first tested for assumptions of normality and homoscedasticity (Kolmogorov–Smirnov and Levene’s test, respectively). These assumptions were satisfied; thus, analysis of variance (ANOVA) was used. We used a two-way ANOVA model, and, in the presence of an interaction, the factors “season” and “sampling site” were analyzed together. In the absence of interaction, the factors were analyzed individually. Group comparisons were performed using Turkey’s test. The level of significance adopted was 0.05 (Quinn and Keough 2002).
Results and Discussion
We observed considerable variations in the normal elliptical nuclear shape of erythrocytes in the analyzed fishes, such as MN and other nuclear abnormalities (NA). The different types of abnormalities observed in nuclei are presented in Table 1. In general, the frequencies of NAs were observed in the following rank order: notched > lobed > binucleated > MN > vacuolated > blebbed. MN were found exclusively during the winter in Rebio and the agricultural site. They were found in low frequency, which is common in fishes because their small chromosomes are difficult to visualize (Carrasco et al. 1990). Probably, the contaminants that caused the high frequency of other nuclear morphological alterations in Rebio during the summer, such as notched and lobed NA, did not cause alterations in the MN test. Unlike other NA, it is known that MN originate from the displacement of a whole chromosome or an acentric chromosome lost in a malsegregation (Schmid 1975). Thus, MNs are only detected if there is a complete cell cycle and the contamination occurred before anaphase in mitosis (Fenech 2007). Alterations in nuclear morphology can be attributed to damage to lamina, a cytoskeletal protein responsible for maintaining the shape of the nucleus (Alberts et al. 2002). Probably, contaminants that reach this endpoint are most common in Rebio (summer), where contaminants cause breaks in chromosomes or centromeres.
Notched nuclei, the most frequent NA found in this study, have a well-defined slit of uniform width extending an appreciable depth into the nucleus. Aneuploidy is due to the failure of tubulin aggregation to form the spindle and cytokinesis under the aneugenic action of toxicants; it can result in the formation of notched nuclei and binucleated cells (Fernandes et al. 2007). Binucleation is an indicator of abnormal cell division due to the blocking of cytokinesis. This abnormal cell division is considered to result in a genetic imbalance in the cells, potentially leading to carcinogenesis (Çavas et al. 2005). Ateeq et al. (2002), while elaborating the sequence of cellular degradation under the impact of toxicants, suggested that toxicants cause hypoxic conditions, which reduce ATP, leading to abnormally shaped erythrocytes. Further, toxicants interrupt the lipid solubility of membranes of erythrocytes, resulting in vacuolated cells, and ultimately lead to apoptosis.
A lobed nucleus has evaginations larger than those of a blebbed nucleus. Lobed nuclei are generally rarer than MN in normal cells, but can be found in up to 20 % of cells treated with genotoxic substances such as antineoplastic agents (Çavas and Ergene-Gözükara 2005a). There are two hypotheses regarding the formation of lobed and blebbed nuclei: (1) they result from problems in the segregation of tangled and attached chromosomes; and (2) they are caused by gene amplification via the breakage-fusion-bridge cycle during the elimination of amplified DNA from the nucleus (Çavas and Ergene-Gözükara 2005a). Further, Von Sonntag hypothesized that these two abnormalities arise due to the damage caused to the genetic material by free radicals produced under oxidative stress caused by toxicants (Von Sonntag 1987).
Several experimental studies have demonstrated that NA other than MN are sensitive and informative biomarkers (Pacheco and Santos 2002; Ateeq et al. 2002; Çavas and Ergene-Gözükara 2005a). Consistent with the results from our study, Çavas and Ergene-Gözükara (2005a) found the following rank order of NA frequency: binucleated = notched > lobed > blebbed. In this study, the frequencies of MN and other NA in fish captured from areas polluted by different types of industrial effluent and aromatic hydrocarbons were significantly higher than those in fish from the reference area.
Some studies have attempted to associate different NAs in fish with local or specific pollutants. For example, Çavas and Ergene-Gözükara (2005b) found a significant induction of lobed and blebbed nuclei in fishes exposed to petroleum. Fernandez et al. (2011) observed significant differences in notched and blebbed nuclei comparing between fish from a preserved site and a region under the influence of urban and harbor activities and diverse discharge sources in Brazil. These results are consistent with those of our study, since we also found notches to be the most common NA. Azevedo et al. (2012) compared a Brazilian estuary that was relatively unaffected by human activity to three regions impacted by various anthropogenic activities. They also observed no significant differences in MN frequency.
Nevertheless, it is difficult to associate different types of NA with a specific source of pollution (Carrasco et al. 1990). However, in recent years, increasing attention has been paid to NA other than MN, and it was demonstrated that this biomarker may serve as an index of genotoxic and cytotoxic damage (Pacheco and Santos 2002; Çavas and Ergene-Gözükara 2005b; Frenzilli et al. 2009; Rybakovas et al. 2009).
A statistically significant interaction was observed between the factors “season” and “site” (p < 0.001) in NA data. Although Rebio is considered to be a reference area, since it is located in a protected area designated as a biological reserve, high NA frequency was found in the summer in this region (Fig. 2a). This fish species has a short migration period during warm seasons (Suzuki et al. 2000), and the specimens may have migrated from agricultural areas outside of Rebio to the head of the river within the reserve, explaining the result. Thus, we observed a high value for NA in Rebio during the summer, probably due to a specific contaminant present in the buffer zone of Rebio in this season, which affects only this endpoint (genotoxic and cytotoxic damage). In comparison with other biomarkers, only NA exhibited a high value at this site, showing the specificity of the responsible pollutant. The buffer zone of Rebio is defined as 500 m on a horizontal projection from the edge of the reserve, and aims to minimize the negative environmental impacts of Rebio (Instituto Chico Mendes 2012). In this zone, agricultural activities are allowed, artificial grassland and sugarcane are cultivated, and pesticides are sprayed (Instituto Chico Mendes 2012). Sugarcane is an uncommon crop in Campo Mourão, and this can explain why similar damage was not observed in the Campo River, which is also affected by pesticides. Fishes exposed to pesticides used to treat sugarcane crops can suffer a high level of cytotoxic and mutagenic damage as determined by the NA test, particularly during the rainy season (summer) (Silva et al. 2014). A study by Armas et al. (2005) examined the pesticides used in a sugarcane crop in Brazil and found that the most used were glyphosate (19.9 %), atrazine (14.5 %), ametrine (14.4 %), 2,4-D (10.6 %), metribuzin (9.4 %), diuron (7.8 %), and acetochlor (7.3 %). Sharma and Vig (2012) showed that atrazine and diuron caused chromosomal aberrations and MN, which increased proportionally with increasing concentrations of these pesticides. Çavas (2011) also demonstrated the induction of MN in a fish exposed to atrazine. Xie et al. (2004) found that an acetochlor-based pesticide could increase the frequencies of MN and nuclear anomalies, and had significant mutagenic activities on erythrocyte nuclei in fish. For 2,4-D, a time- and dose-dependent response of the MN frequency formation was demonstrated in fish (Farah et al. 2003).
Biomarkers in Astyanax aff. paranae sampled in Rebio and upstream and downstream of Campo Mourão city during winter and summer 2011. a Mean nuclear alteration frequency (‰) considering micronucleus and other morphological nuclear abnormalities – ANOVA F (2,100) = 96.012, p = 0.0000. b Blood comet assay score – ANOVA F (2, 97) = 8.1486, p = 00054. c Bernet’s index calculated for gill histopathology – ANOVA F (2, 101) = 3.8408, p = 0.02468. d Gill comet assay score – ANOVA F (2,105) = 9.6371, p = 0.00014. Different letters (a, b, c, **) indicate significant differences in Turkey test, p < 0.05. C.I. confidence interval
Despite the high NA value recorded in summer, the results derived from other biomarkers support the use of Rebio as a reference site, making it more probable that these NA results were affected by an indeterminate specific pollutant. In field studies like ours, an assessment of several biomarkers, comparing the results obtained at different biological levels may lead to a substantial improvement in the knowledge of integrated fish responses to toxin exposure.
By excluding the NA result for Rebio in the summer, the other results showed a general trend in the formation of abnormalities, which corroborates the comet assay data (Fig. 2a, b); the lowest damage index was observed in Rebio (winter), the middle index in the agricultural area, and the highest was detected downstream, near the sewage treatment plant.
We also found that the histopathological damages in gills tended to increase with pollutant accumulation, particularly in the summer. The lowest Bernet’s Index was found in Rebio in the winter, and the highest mean was at the downstream site in the summer (Fig. 2c). The results from Rebio/winter and downstream/summer were significantly different from each other and from other sites/seasons. These samples represented a group with an intermediate damage rate, without statistically significant differences among them.
The histological alterations observed in gills may reflect long-term exposure (Bernet et al. 1999). The main histological alterations found in our study, independent of season or site, were lamellar fusion and hyperplasia, which affect gas exchange, and epithelial displacement, aneurisms, and lamellar hypertrophy (Fig. 3). Most of these histopathological alterations can be interpreted as non-specific responses to stress, and they are generally described in fishes exposed to a broad spectrum of pollutants, including metals, hydrocarbons, organic contaminants, and other substances (Mallatt 1985). Pereira et al. (2013) demonstrated that the proliferation of the lamellar epithelium and consequent lamellar fusion increased concomitant with a decrease in water quality, and that this was very severe in areas with particularly poor ecological conditions. These histological changes, promoting cell proliferation, are defense mechanisms that enlarge the diffusion distance between the water and blood (Pereira et al. 2013) and have already been described as responses to specific contaminants by other authors. For example, Arellano et al. (1999) found that fishes exposed to copper showed lifting and swelling of the lamellar epithelium. Similar results were found in fishes exposed to nickel (Pane et al. 2004) and to naphthenic acids, ions, and polycyclic aromatic hydrocarbons (PAHs) (Nero et al. 2006). Jiraungkoorskul et al. (2003) observed cell proliferation, lamellar cell hyperplasia, lamellar fusion, epithelial lifting, and aneurysm in gill filaments after sub-chronic exposure to glyphosate. These are potential pollutants in agricultural areas and downstream sites in our study.
Gill biomarkers seem to corroborate the order of the study areas in the amount of fish exposure to pollutants. In the comet assay for gills, the lowest damage rate was found in Rebio in the summer (Fig. 2d). This rate was significantly lower than that at the sites in the Campo River during this season, in which no significantly different results were found. In fishes collected during the winter, an intermediate amount of damage was observed in the agricultural area, but there was no significant difference between damage in upstream fishes and fish from Rebio. Comparison between seasons revealed that considering the NA and histopathology results, the damage rate was higher in the summer than in the winter.
The detection of DNA fragmentation using the comet assay is used as a biomarker of recent exposure (Wirzinger et al. 2007). Differences in the response between gill cells and blood cells in the comet assay may be due to tissue-specific physiochemical activities, concerning either activation/detoxification mechanisms or the repair of different types of strand breaks (Ali et al. 2009). Furthermore, the number of alkali-labile sites in DNA could vary among different tissues, and different cell types can have considerably different levels of DNA single-strand breaks due to variation in excision repair activity, metabolic activity, anti-oxidant concentrations, and other factors (Lee and Steinert 2003).
In general, the agricultural site upstream of the city had an intermediate level of damage. Despite no effects of the influence of urban pollution, this site is affected by more diffuse forms of pollution because it is surrounded by agricultural areas and receives effluents from crop farms bordering the river. According to data from the Department of Agriculture and Supply of Paraná State (SEAB/PR) (Paraná 2010), the region of this study receives an input of 10 tons of pesticides each year, principally herbicides (59 %), insecticides (18 %), fungicides (9 %), adjuvants (7 %), and other chemicals (7 %). Among the most popular herbicides in the region used in soy and corn crops (the major crops) are glyphosate (>30 % of the total), triazine, sulfonylurea, and imidazolinone. Organophosphorous and pyrethroid insecticides, benzimidazole, and triazole-based fungicides are among the most applied in Campo Mourão. In the same department, most of these pesticides are ranked as class II (very dangerous) or III (dangerous). Currently, glyphosate-based herbicides are the most used around the world to control weeds (Çavas and Könen 2007), but their genotoxic, mutagenic, and histopathological effects on fishes have been reported by Çavas and Könen (2007); Marc et al. (2004); Jiraungkoorskul et al. (2003), and many others. The study by Çavas (2011) demonstrated the genotoxic potential of triazine on fishes, showing significant increases in the frequencies of MN and DNA strand breaks in erythrocytes of Carassius auratus following exposure. This pesticide also causes histopathological damage (Mela et al. 2013). The organophosphorous insecticides are known to cause histopathological damage in the liver and gills (Fanta et al. 2003) and to inhibit acetylcholinesterase activity (Silva et al. 1993; Bálint et al. 1995).
Our hypothesis that the negative effects would increase because of the cumulative effects of pollutants was supported in the downstream area by most of the biomarkers studied. Pesticides are most likely discharged into agricultural areas, and the addition of sewage treatment plant and industrial effluents can contribute to the effects observed in the downstream site. The cumulative effects of pesticides and sewage effluent are understudied, but it is known that their mixture in aquatic environments can have a synergistic effect on organisms (Rouimi et al. 2012; Gust et al. 2013).
Our downstream site was located in the area of effluent discharge from the municipal sewage treatment plant, and this may have contributed to the highest effect on fishes. This has been supported by the harmful effects of sewage treatment plant effluents on fishes noted in previous studies. Talapatra and Banerjee (2007) concluded that fishes cultivated in sewage-fed fish farms develop high rates of NA and necrotic and apoptotic cells. Bucher and Hofer (1993) exposed fishes to dilutions of biologically treated domestic wastewater, and noted histopathological alterations in the liver and kidneys, but not in the gills. Narain et al. (1990) observed remarkable histological gill alterations in fishes exposed to sewage pollution stress, with hyperplasia as the most frequent alteration. Similar results were found in our study.
Most sewage treatment plants perform only primary and secondary treatment, which removes settleable solids and organic matter (p and n) without the removal of specific pollutants, generally toxic or non-biodegradable compounds (Von Sperling 1996). Furthermore, the situation is concerning because heavy metals, hormones, plasticizers, and surfactants can remain in the effluents, which may cause endocrine disorders in fishes (Silva de Assis et al. 2013). The mixture of heavy metal ions in water could have toxic effects on fish to different extents, such as affecting their breathing, immunity, enzyme activity, and embryonic development, as well as causing DNA damage by physicochemical, physiological, and detoxification processes (Zhang et al. 2008). The main mechanism of DNA damage caused by heavy metal ions is the induction of a large number of free radicals. These free radicals break DNA double chains. If the broken DNA strands are not repaired rapidly, the functioning of DNA is impaired, resulting in genotoxicity (Zhang et al. 2008).
In summary, the lowest alteration index was recorded in Rebio during the winter, followed by the agricultural site with an intermediate rate and the highest index in the downstream site, downstream of Campo Mourão city. This highest alteration index probably occurred due to the cumulative effects of pollutants from agricultural, industrial, and urban sources, as well as from the municipal sewage treatment plant discharges at this site, and therefore it requires more effective monitoring. We recommend further studies with other biomarkers to corroborate the effects of sewage and pesticides on fishes and other organisms.
References
Abdel-Moneim AM, Al-Kahtani MA, Elmenshawy OM (2012) Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments, Saudi Arabia. Chemosphere 88:1028–1035. doi:10.1016/j.chemosphere.2012.04.001
Agostinho AA, Gomes LC, Pelicice FM (2007) Ecologia e manejo de recursos pesqueiros em reservatórios do Brasil, 1st edn. EDUEM, Maringá
Alberto A, Camargo AFM, Verani JR et al (2005) Health variables and gill morphology in the tropical fish Astyanax fasciatus from a sewage-contaminated river. Ecotoxicol Environ Saf 61:247–255. doi:10.1016/j.ecoenv.2004.08.009
Alberts B, Johnson A, Lewis J et al (2002) Molecular biology of the cell, 4th edn. Garland, New York
Ali D, Nagpure NS, Kumar S et al (2009) Assessment of genotoxic and mutagenic effects of chlorpyrifos in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem Toxicol 47:650–656. doi:10.1016/j.fct.2008.12.021
Amorim LCA (2003) Os biomarcadores e sua aplicação na avaliação da exposição aos agentes químicos ambientais. Rev Bras Epidemiol 6:158–170
Arellano JM, Storch V, Sarasquete C (1999) Histological Changes and Copper Accumulation in Liver and Gills of the Senegales Sole, Solea senegalensis. Ecotoxicol Environ Saf 72:62–72
Armas E, Monteiro R, Amâncio A et al (2005) Uso de Agrotóxicos em Cana-de-Açúcar na Bacia do Rio Corumbataí e o Risco de Poluição Hídrica. Quim Nova 28:975–982
Ateeq B, Abul farah M, Niamat Ali M, Ahmad W (2002) Induction of micronuclei and erythrocyte alterations in the catfish Clarias batrachus by 2,4-dichlorophenoxyacetic acid and butachlor. Mutat Res 518:135–144
Azevedo JDS, Braga EDS, de Ribeiro CAO (2012) Nuclear abnormalities in erythrocytes and morphometic index in the Catfish Cathorops spixii (Ariidae) from different sites on the southeastern Brazilian coast. Brazilian J Oceanogr 60:323–330
Azevedo JS, Braga ES, Silva de Assis HC, Ribeiro CAO (2013) Biochemical changes in the liver and gill of Cathorops spixii collected seasonally in two Brazilian estuaries under varying in fl uences of anthropogenic activities. Ecotoxicol Environ Saf 96:220–230. doi:10.1016/j.ecoenv.2013.06.021
Bálint T, Szegletes T, Szegletesb Z et al (1995) Biochemical and subcellular changes in carp exposed to the organophosphorus methidathion and the pyrethroid deltamethrin. Aquat Toxicol 33:279–295
Barbieri GM (1992) Biologia de Astyanax scabripinnis paranae (Characiformes, Characidade) do ribeirão do Fazzari. São Carlos. Estado de São Paulo. II. Aspectos quantitativos da reprodução. Rev Bras Biol 52:589–596
Bernet D, Schmidt H, Meier W, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34
Bolognesi C, Hayashi M (2011) Micronucleus assay in aquatic animals. Mutagenesis 26:205–213. doi:10.1093/mutage/geq073
Brasil (2000) Law n. 9985, June 18th 2000. SNUC. 18
Brasil (2005) Resolução do Conselho Nacional do Meio Ambiente no. 357, de 17 de março de 2005. 1–23
Bucher F, Hofer R (1993) The effects of treated domestic sewage on three organs (Gills, Kidney, Liver) of Brown trout (Salmo trutta). Water Res 27:255–261
Carrasco K, Tilbury K, Myers M (1990) Assessment of the piscine micronucleus test as in situ biological indicator of chemical contaminant effects. Can J Fish Aquat Sci 47:2123–2136
Carrasco-Letelier L, Eguren G, de Mello FT, Groves PA (2006) Preliminary field study of hepatic porphyrin profiles of Astyanax fasciatus (Teleostei, Characiformes) to define anthropogenic pollution. Chemosphere 62:1245–1252. doi:10.1016/j.chemosphere.2005.07.005
Castella PR, Britez RM (2004) A floresta com araucária no Paraná: conservação e diagnóstico dos remanescentes florestais. 236
Çavas T (2011) In vivo genotoxicity evaluation of atrazine and atrazine-based herbicide on fish Carassius auratus using the micronucleus test and the comet assay. Food Chem Toxicol 49:1431–1435. doi:10.1016/j.fct.2011.03.038
Çavas T, Ergene-Gözükara S (2005a) Micronucleus test in fish cells: a bioassay for in situ monitoring of genotoxic pollution in the marine environment. Environ Mol Mutagen 46:64–70. doi:10.1002/em.20130
Çavas T, Ergene-Gözükara S (2005b) Induction of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aquat Toxicol 74:264–271. doi:10.1016/j.aquatox.2005.06.001
Çavas T, Könen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 22:263–268. doi:10.1093/mutage/gem012
Çavas T, Garanko NN, Arkhipchuk VV (2005) Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food Chem Toxicol 43:569–574. doi:10.1016/j.fct.2004.12.014
de Andrade Brito I, Freire CA, Yamamoto FY et al (2012) Monitoring water quality in reservoirs for human supply through multi-biomarker evaluation in tropical fish. J Environ Monit JEM 14:615–625. doi:10.1039/c2em10461j
Fanta E, Rios FSA, Romão S et al (2003) Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicol Environ Saf 54:119–130
Farah MA, Ateeq B, Ali MN, Ahmad W (2003) Evaluation of genotoxicity of PCP and 2,4-D by micronucleus test in freshwater fish Channa punctatus. Ecotoxicol Environ Saf 54:25–29. doi:10.1016/S0147-6513(02)00037-4
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104. doi:10.1038/nprot.2007.77
Fernandes TCC, Mazzeo DEC, Marin-Morales MA (2007) Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pestic Biochem Physiol 88:252–259. doi:10.1016/j.pestbp.2006.12.003
Fernandez WS, Dias JF, Ribeiro CAO, Azevedo JDS (2011) Liver damages and nuclear abnormalities in erythrocytes of Atherinella brasiliensis (Actynopterigii, Atherinopsidade) from two beaches in southeast of Brazil. Braz J Med Biol Res 59:163–169
Ferraro MVM, Fenocchio AS, Mantovani MS et al (2004) Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests. Genet Mol Biol 27:103–107
Flammarion P, Devaux A, Nehls S et al (2002) Multibiomarker responses in fish from the Moselle river (France). Ecotoxicol Environ Saf 51:145–153. doi:10.1006/eesa.2001.2134
Frenzilli G, Nigro M, Lyons BP (2009) The Comet assay for the evaluation of genotoxic impact in aquatic environments. Mutat Res 681:80–92. doi:10.1016/j.mrrev.2008.03.001
Garutti V, Britski HA (2000) Descrição de uma espécie nova de Astyanax (Teleostei: Characidae) da bacia do alto rio Paraná e considerações sobre as demais espécies do gênero na bacia. Comun do Mus Ciências e Tecnol da PUCRS- Série Zoológica 14:65–88
Guerreiro RL, Parolin M (2008) Espécies Nativas de Cerrado na Cidade de Campo Mourão: potencial para Recuperação? I Simpósio de Estudos Urbanos: desenvolvimento regional e dinâmica ambiental p 16
Gust M, Fortier M, Garric J et al (2013) Effects of short-term exposure to environmentally relevant concentrations of different pharmaceutical mixtures on the immune response of the pond snail Lymnaea stagnalis. Sci Total Environ 445–446:210–218. doi:10.1016/j.scitotenv.2012.12.057
Heddle JA (1973) A rapid in vivo test for chromosomal damage. Mutat Res 18:187–190
Instituto Chico Mendes I (2012) Plano de Manejo da Reserva Biológica das Perobas. 199
Jiraungkoorskul W, Upatham ES, Kruatrachue M et al (2003) Biochemical and histopathological effects of glyphosate herbicide on Nile Tilapia (Oreochromis niloticus). Environ Toxicol 4:260–267. doi:10.1002/tox.10123
Kobayashi H, Sugiyama C, Morikawa Y et al (1995) A comparison between manual microscopic analysis and computerized image analysis in the single cell gel electrophoresis. Mamm Mutagen Study Gr Commun 3:103–115
Lee RF, Steinert S (2003) Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat Res Mutat Res 544:43–64. doi:10.1016/S1383-5742(03)00017-6
Maack R (1981) Geografia física do Estado do Paraná, 2nd ed. 450p
Mallatt J (1985) Fish Gills structural changes inducted by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci 42:630–648
Marc J, Mulner-Lorillon O, Bellé R (2004) Glyphosate-based pesticides affect cell cycle regulation. Biol Cell 96:245–249. doi:10.1016/j.biolcel.2003.11.010
Martinez BR, Souza MM (2002) Acute effects of nitrite on ion regulation in two neotropical fish species. Comp Biochem Physiol Part A 133:151–160
Mela M, Guiloski IC, Doria HB et al (2013) Effects of the herbicide atrazine in neotropical cat fish (Rhamdia quelen). Ecotoxicol Environ Saf 93:13–21. doi:10.1016/j.ecoenv.2013.03.026
Mizote LTM (2008) Agenda 21 Local de Campo Mourão: do projeto ao processo. 238
Moore MN, Simpson MC (1992) Molecular and celular pathology in environmental impact assessment. Aquat Toxicol 22:313–322
Narain AS, Srivastava AK, Singh BB (1990) Gill Lesions in the Perch, Anabas testudineus, subjected to sewage toxicity. Bull Environ Contam Toxicol 45:235–242
Nero V, Farwell A, Lister A et al (2006) Gill and liver histopathological changes in yellow perch (Perca flavescens) and goldfish (Carassius auratus) exposed to oil sands process-affected water. Ecotoxicol Environ Saf 63:365–377. doi:10.1016/j.ecoenv.2005.04.014
Pacheco M, Santos MA (2002) Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol Environ Saf 53:331–347
Pane EF, Haque A, Wood CM (2004) Mechanistic analysis of acute, Ni-induced respiratory toxicity in the rainbow trout (Oncorhynchus mykiss): an exclusively branchial phenomenon. Aquat Toxicol 69:11–24. doi:10.1016/j.aquatox.2004.04.009
Paraná (2005) Plano de Manejo do Parque Estadual do Lago Azul. Encarte II: 1–45. Available in: http://www.iap.pr.gov.br/arquivos/File/Plano_de_Manejo/Parque_Estadual_Lago_Azul/4_PELA_ENCARTE_II.pdf. Accessed 20 jan 2014
Paraná (2010) Capítulo 1- Dimensão Ambiental. In: IPARDES (ed) Indicadores Ambient. por bacias hidrográficas do Estado do Paraná. Instituto Paranaense de Desenvolvimento Econômico e Social, Curitiba, p 223
Pereira S, Pinto AL, Cortes R et al (2013) Gill histopathological and oxidative stress evaluation in native fish captured in Portuguese northwestern rivers. Ecotoxicol Environ Saf 90:157–166. doi:10.1016/j.ecoenv.2012.12.023
Prioli SMAP, Prioli AJ, Júlio HF Jr et al (2002) Identification of Astyanax altiparanae (Teleostei, Characidae) in the Iguaçu River, Brazil, based on mitochondrial DNA and RAPD markers. Genet Mol Biol 430:421–430
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. 537p
Rossi SC, Piancini LDS, Ribeiro CAO et al (2011) Sublethal effects of waterborne herbicides in tropical freshwater fish. Bull Environ Contam Toxicol 87:603–607. doi:10.1007/s00128-011-0397-6
Rouimi P, Zucchini-Pascal N, Dupont G et al (2012) Impacts of low doses of pesticide mixtures on liver cell defence systems. Toxicol In Vitro 26:718–726. doi:10.1016/j.tiv.2012.03.015
Rybakovas A, Barsiene J, Lang T (2009) Environmental genotoxicity and cytotoxicity in the offshore zones of the Baltic and the North Seas. Mar Environ Res 68:246–256. doi:10.1016/j.marenvres.2009.06.014
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Sharma S, Vig AP (2012) Genotoxicity of Atrazine, Avenoxan, Diuron and Quizalofop-P-ethyl Herbicides using the Allium cepa Root Chromosomal Aberration Assay. Terr Aquat Environ Toxicol 6:90–95
Silva JRR, Filho HO (2011) Dípteros ectoparasitas (Insecta, Diptera) em morcegos (Chiroptera, Mammalia) na Reserva Biológica das Perobas Paraná, Brasil. Iheringia, Sér Zool 101:220–224
Silva de Assis HC, Simmons DBD, Zamora JM et al (2013) Estrogen-like effects in male gold fish Co-exposed to fluoxetine and. Environ Sci Technol 47:5372–5382
Silva HC, Medina HSG, Fanta E, Bacilat M (1993) Sub-Lethal effect of the Organophosphate Folidol 600 (Methyl Parathion) on Callichthys callichthys (Pisces: Teleostei). Comp Biochem Physiol 105C:197–201
Silva MD, Rossi SC, Ghisi NDC et al (2014) Using multibiomarker approach as a tool to improve the management plan for a Private Reserve of Natural Heritage (RPPN). Bull Environ Contam Toxicol 92:602–608. doi:10.1007/s00128-014-1230-9
Speit G, Hartmann A (1999) The comet assay (single-cell gel test), a sensitive genotoxicity test for the detection of DNA damage and repair. In: Henderson DS (ed) Methods mol. biol. DNA repair protoc—eukaryot syst, 113th edn. Human Press, Totowa, pp 203–211
Suzuki H, Agostinho AA, Winemiller KO (2000) Relationship between oocyte morphology and reproductive strategy in loricariid catfishes of the Paraná River, Brazil. J Fish Biol 57:791–807. doi:10.1006/jfbi.2000.1352
Suzuki HI, Vazzoler AEAM, Marques EE, et al. (2004) Reproductive ecology of the fish assemblage. Up. Parana River its floodplain Phys. Asp. Ecol. Conserv
Talapatra SN, Banerjee SK (2007) Detection of micronucleus and abnormal nucleus in erythrocytes from the gill and kidney of Labeo bata cultivated in sewage-fed fish farms. Food Chem Toxicol 45:210–215. doi:10.1016/j.fct.2006.07.022
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Von Sonntag C (1987) The chemical basis of radiation biology. 515
Von Sperling M (1996) Princípios básicos do tratamento de esgotos—Princípios do tratamento biológico de águas residuárias, 2nd ed. 211
Wirzinger G, Weltje L, Gercken J, Sordyl H (2007) Genotoxic damage in field-collected three-spined sticklebacks (Gasterosteus aculeatus L.): a suitable biomonitoring tool? Mutat Res 628:19–30. doi:10.1016/j.mrgentox.2006.11.011
Xie Z, Cai Y, Chen G et al (2004) Induction of micronuclei and nuclear anomalies in erythrocytes of Misgurnus anguillicaudatus by herbicide acetochlor. Fish Sci 23:17–19
Zhang Y, Wang Y, Yu R et al (2008) Effects of heavy metals Cd2 +, Pb2 + and Zn2 + on DNA damage of loach Misgurnus anguillicaudatus. Front Biol China 3:50–54. doi:10.1007/s11515-008-0012-3
Zhou Q, Zhang J, Fu J et al (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150. doi:10.1016/j.aca.2007.11.018
Acknowledgments
The authors acknowledge the logistical support from the Chico Mendes Institute for Biodiversity Conservation of the Reserve of Perobas; to Nupélia (Limnology, Ichthyology and Aquiculture Research Center/UEM); to financial support from the Araucaria Foundation and SETI (Parana State Agency for Science and Technology) and to CAPES (PROEX - Brazilian Agency for Science and Technology). We greatly thank Jeffrey D. Muehlbauer for English language revisions. Voucher specimens of collected individuals were stored at the Ichthyological Collection of the Limnology, Ichthyology and Aquiculture Research Center: the JP and MP fishes NUP 13381 and Field: NCG2011103101 and the Rebio fishes NUP13382 and Field NCG2011071701.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghisi, N.C., de Oliveira, E.C., Fávaro, L.F. et al. In Situ Assessment of a Neotropical Fish to Evaluate Pollution in a River Receiving Agricultural and Urban Wastewater. Bull Environ Contam Toxicol 93, 699–709 (2014). https://doi.org/10.1007/s00128-014-1403-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1403-6