Abstract
This study was aimed at studying the effect of heavy metals and arsenic on the survival and biofilm formation of some saprotrophic microorganisms: Bacillus megaterium var. phosphaticum, Bacillus mucilaginosus, Pectobacterium carotovorum, and Escherichia coli. As a source of heavy metals and arsenic, we used aqueous solutions of NaAsO2, Cd(CH3COO)2, and Pb(NO3)2 (2.5, 25, 250 mg L−1). The cultures in the liquid medium had different resistance to the toxicants under study: B. megaterium > B. mucilaginosus > P. carotovorum > E. coli. The toxicity of the tested solutions of heavy metals and arsenic for microorganisms can be arranged (decreasing toxicity) as follows: cadmium acetate > lead nitrate > sodium metaarsenite. These experiments revealed some regularities related to the mechanisms of toxic effect of As, Pb, and Cd solutions on the formation of biofilms of soil bacteria, changes in bacterial cellular forms, and their survival. Bacillus megaterium strain was the most resistant to high As concentrations and was able to influence the formation of highly structured colonies of bacterial cells with the honeycomb-like structure. The immobilization of heavy metals can be achieved due to their binding into strong compounds with the matrix substances of biofilms and polymeric organic compounds formed during the sporulation of rhizobacteria. It can increase the resistance of bacteria to high concentrations of heavy metals and arsenic. The results obtained can be of great practical importance in the development of biotechnologies related to soil bioremediation, in the field of nanotechnology, crop production, and medicine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Anthropogenic pollution of the environment with heavy metals leads to their accumulation in the soil, water, and food chain of living organisms. Plant-microbial and soil ecosystems are known to be closely interrelated (McLean et al., 2014; Miki and Doi, 2016), including the interrelations through the exchange of elements. Technogenic pollution of the environment inevitably leads to the involvement of xenobiotics in this exchange process. The role of the saprotrophic soil bacteria in this process, in particular under conditions of technogenesis, is recently being widely discussed (Chen et al., 2018; Khan et al., 2017; Mani & Kumar, 2014; Ullah et al., 2015). In this respect, the study of As and heavy metals such as Pb and Cd and their biogenic behavior on the soil bacteria is of particular relevance since the compounds of these elements are toxic, particularly as low molecular organic compounds, able to migrate in food chains and accumulate in living organisms (Kabata-Pendias, 2011). Microorganisms play a major role in the transformation of mineral components in all biosphere environments.
As shown earlier, Bacillus mucilaginosus secretes the silicase enzyme and provides silicon and other macro- and microelements from natural silicates to the rhizosphere of plants (Vaishlya et al., 2013). The rhizobacteria are effective as components of biological agriculture, able to increase soil microbiological activity and thus crop production (Sokolova et al., 2011). The effect of As and heavy metals on microorganisms can be discussed in various aspects. Microorganisms are known to reduce the concentrations of chemical elements in the environment due to the adsorption of metals on the surface of bacterial cells. It is a passive process being not dependent on metabolism of bacterial cells. Immobilization of heavy metals can occur both inside live and dead bacterial cells and in the biofilm matrix (Liu & Fang, 2002; Singh and Cameotra, 2004; Tabak et al., 2005). All the above processes rely on a number of factors: bacteria species, timing of their contact with xenobiotics, pH in the environment, metal concentration, and their speciation.
Since biofilms are the preferential growth lifestyle of microorganisms able to protect microbial cells from stressful environmental conditions, their composition, structure, and mechanisms of interrelations between microorganisms, contained in biofilms, and xenobiotics are one of the priorities in their study (Van Hullebusch et al., 2003). Biofilms are intensively studied in medicine, as they play a leading role in the growth and chronicity of infectious diseases. They can inlay permanent catheters, internal implants, contact lenses, and prostheses (Azevedo et al., 2017; Wu et al., 2019). Biofilms can be formed on various organs and tissues in bodies of humans and animals. The ability of bacteria to form biofilms is now considered as a factor of their pathogenicity as the bacterial cells contained in the biofilm are highly resistant to antibiotics and disinfectants (Borriello et al., 2004; Hall & Mah, 2017; Yuan et al., 2020). Such a resistance creates significant difficulties in the control of biofilms and presents a serious epidemiological problem in medical institutions (Aslam & Darouiche, 2011; Ielapi et al., 2020; Kim et al., 2012; Walz et al., 2010). Moreover, biofilms of some bacteria can be applicable in the following areas: in industries involving long biotransformation of toxic substances, due to the increased bacterial tolerance to xenobiotics (Nadagouda et al., 2012; Nocelli et al., 2016; Grujic et al., 2017; Al-Amshawee et al., 2020); in the biological purification of water, air, and other media by creating microbial communities of a given composition that effectively decompose harmful compounds (Hall-Stoodley et al., 2004; Abebe, 2020); in biological control of phytopathogens causing plant diseases; as well as in medicine when using medications with probiotic activity of living bacteria (Alreshidi et al., 2020; Bais et al., 2004).
Rhizosphere microorganisms form biofilms on the surface of plant roots. These microorganisms are able to stimulate plant growth, secreting a huge amount of various biologically active compounds and to protect plants from pathogenic soil microflora and from contamination by heavy metals (Castiblanco & Sundin, 2016).
Many microorganisms have the ability to resist the toxic effect of heavy metals, which is explained by their increased resistance, developed in the process of their evolution. One of the ways to counteract this was the formation of biofilms, due to polymer organic materials released by microorganisms. Possible and critical concentrations of heavy metals required for the vital activity of bacteria are very important issues which are to be studied for certain microorganisms and metals individually. To develop effective methods of metal bioaccumulation, it is required to investigate the mechanisms of this process, to isolate active bacterial strains, to select microbial communities, and to analyze the influence of external conditions on the bioaccumulation process. The solution of a number of environmental problems depends both on the ability of microorganisms to precipitate or accumulate heavy metals and on the analysis of the toxic effect of heavy metals on microorganisms. Therefore, it is important to study the patterns of biofilm formation under the influence of various chemical compounds on biofilms and the ability of bacteria to survive at different concentrations of xenobiotics.
Since the effect of heavy metals on soil microorganisms, particularly on their biofilms remains an insufficiently studied biogeochemical sector, this study was aimed at analyzing the effect of heavy metals and arsenic on the survival and biofilm formation of some saprotrophic microorganisms. Better understanding of heavy metal-biofilm interactions can be useful in managing the life of natural microbial populations and in the development of nanotechnologies and biotechnologies, related to soil remediation, crop production, and medicine.
2 Objects and Methods
In this study, we used the strains Bacillus megaterium var. phosphaticum Pl-04 (ACM (All-Russian collection of microorganisms) B-2357 D), Bacillus mucilaginosus (ACM B-1574), Pectobacterium carotovorum ssp. carotovorum ACM B-1247, and Escherichia coli XL-1 Blue (“Stratagene”, USA).
Bacillus megaterium soil microorganisms are able to convert phosphate from insoluble to a plant-accessible form; Bacillus mucilaginosus can stimulate root formation and supply silicon and other macro- and microelements from natural silicates to a plant rhizosphere (Bao et al., 2014; Stefanescu, 2015). Biological preparations used in the study are environmentally friendly, non-toxic, and safe for humans and animals plant growth stimulants (Vaishlya et al., 2013; Sokolova et al., 2011).
The causative agent of the black rot of P. carotovorum potato leads to wilting of the stems during the growing season and causes rotting of the tubers during both the growing season and storage. The sanitary-indicative microorganism E. coli can be found in the environment with fecal contamination and can signal the sanitary inadequacy of soils for human economic needs. The used plasmidless cells of E. coli strain XL1-Blue are not pathogenic.
As a source of heavy metals and arsenic, we used aqueous solutions of sodium metaarsenite NaAsO2, cadmium acetate Cd(CH3COO)2, and lead nitrate Pb(NO3)2 (2.5, 25, 250 mg L−1). Their bactericidal concentrations were determined by the disc-diffusion method. On the surface of the fish-peptone agar culture medium (FBIS “State Research Center for Applied Microbiology & Biotechnology”), sterile discs of filter paper with a diameter of 6 mm, sodden with solutions of the investigated toxicants in different concentrations, were impregnated in Petri dishes. After the lawns from the microorganisms grew, the diameter of the growth inhibition zones of the test cultures was measured.
The influence of heavy metals and arsenic on biofilm formation was assessed using spectrophotometry by comparing the sorption of the dye with biofilms in the control and experimental groups. One hundred fifty microliters of a mixture consisting of 113 μl of the suspension of the test strain of the microorganism (D = 0.2), washed with physiologic buffered saline (PBS) with 0.5% glucose (PBSG) and 37 μl of PBSG with a dissolved toxicant (10, 100, 1000 mg L−1) was pipetted in 96-well plates. Suspensions of microbial cells were cultured for 3 days at 31 °C. The optical density of the biofilms was measured on the 4th day on the plate spectrophotometer (Bio-Rad, USA) at a wavelength of 590 nm before staining and of 495 nm after it. Before staining, plankton cells were shaken out from wells with contents.
Then, 150 μl of distilled water and 20 μl of 1% alcohol solution of gentian violet were added to the wells of the plate, followed by a 45-min incubation at room temperature. The biomass of the films was assessed by measuring the amount of the dye associated with them. For this purpose, 200 μl of 96% ethanol was added to each well for extraction of the dye from the surface of the plate, and the optical density of this solution was immediately measured. The degree of film formation corresponded to the staining intensity of the well contents (Turskaya et al., 2017).
To study the effect of arsenic and heavy metals on changes in cell length and number of spores as well as on the appearance of biofilm matrix, biofilms were grown on coverslips in Petri dishes and then they were microscopized. So, defatted sterile coverslips were placed in Petri dishes filled with 20 ml of a mixture consisting of 1:3 of a toxicant solution (10, 100, 1000 mg L−1) in phosphate buffer and a suspension of the microorganisms under study (D = 0.2, PBSG), respectively. Coverslips should be completely immersed within the fluid. Instead of the toxicant solution, PBSG was added in the control experiment. Petri dishes were incubated for 3 days at 28 °C. Then, the liquid contents of the Petri dishes were drained and 5 ml of 2% glutaraldehyde in PBS was added thereto. Within 2 h, the dishes were exposed at 4 °C and then washed three times in PBSG at an interval of 15 min to remove glutaraldehyde. The biofilms were visualized on the coverslips using an Axio Observer Z1 microscope (Carl Zeiss, Germany). The number and dimensions of the cells were determined in 10 fields of view of each sample using the Axio Vision program.
To study the sorption of heavy metals and arsenic by microbial biofilms using ICP-MS inductively coupled plasma mass spectrometry, their content was determined in overbiofilm liquid. The ICP-MS with the magnetic sector ELEMENT 2 (Finnigan MAT, Germany) which has double focusing and the ability to register the signal at various resolutions was used. The determination error did not exceed 5–7%. To estimate the concentrations, calibration was carried out using the certified CLMS-1–4 solutions from SPEX (USA) with element concentrations of 0.1; 1.0; and 5.0 ng mL−1. The levelling of the matrix effect was achieved by diluting the prepared solutions of the samples for the analysis in 1000 and 5000 times. Chemical analyses were accomplished with the scientific equipment of the certified analytical center of collective use “Isotopic–geochemical investigations” at the Institute of Geochemistry, SB RAS.
2.1 Statistical Analysis
Standard deviation (SD) was calculated with a probability p < 0.05 in the analysis of chemical elements. Error control was analyzed using the Thermo SPEC spectrometer software (version 4.1). The tables show mean values and their standard deviations. Chemical analysis was accomplished using several measurements (3 analytical replicates).
Graphs of the effects of heavy metals and arsenic on bacterial survival were constructed using Microsoft Excel 2010 and STATISTICA 10 for Windows. The graphs and tables show the arithmetic means and their standard deviations for visual clarity. Significant differences from the control growth of microorganisms for the 3rd day are marked by the symbol “*”. The significance of the results obtained was determined using the non-parametric Wilcoxon test.
3 Results
3.1 Evaluation of Heavy Metals and Arsenic Effect on Microorganisms Using the Disc-Diffusion Method
Solutions of sodium metaarsenite regardless of the concentration did not affect the growth of B. megaterium, but had a bacteriostatic effect on B. mucilaginosus. In this case, a weaker growth of microorganisms was observed. The growth suppression zone in this case was 10 ± 1 and 8 ± 0.5 mm in diameter at 250 and 25 mg L−1, respectively (Fig. 1). With regard to P. carotovorum and Escherichia coli, the solutions showed a weak bactericidal effect: Small, clear zones of no growth of microorganisms with a diameter of 7 and 8 mm, respectively, were formed in all concentrations used.
Lead nitrate solutions in concentrations of 250–2.5 mg L−1 inhibited the growth of all the test cultures (Fig. 1). The growth suppression zone was 13 ± 1 mm in diameter at 250 mg L−1 in both bacilli and 10 ± 1 mm in P. carotovorum and Escherichia coli. The most toxic agent was cadmium acetate. Its solutions significantly inhibited the growth of the lawn of the all test cultures (Fig. 1). The growth suppression zone was 27 ± 1 and 12 ± 1 mm in diameter at 250 and 25 mg L−1 in B. mucilaginosus, 12 ± 1 and 6 ± 1 mm in B. megaterium, 20 ± 1 and 15 ± 1 in E. coli, and 15 ± 1 and 10 ± 1 in P. carotovorum, respectively.
Thus, cadmium acetate was found to be the most toxic solution, followed by lead nitrate and sodium metaarsenite. In terms of decreasing resistance to the action of all the selected toxicants on a dense nutrient medium, the cultures can be arranged as follows: B. megaterium > P. carotovorum > E. coli > B. mucilaginosus.
3.2 Evaluation of Heavy Metals and Arsenic Effect on the Survival of Microorganisms
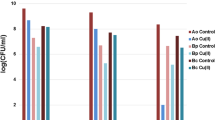
All the solutions of toxicants significantly suppressed the increase of B. mucilaginosus cells in the suspension with the exception of sodium metaarsenite, which did not influence the growth of culture in an amount of 2.5 mg L−1. The most pronounced inhibitory effect was found for the cadmium acetate solution (Fig. 2A).
Influence of cadmium acetate—2, sodium metaarsenite—3, and lead nitrate—4 (mg L−1) on the survival rate of Bacillus mucilaginosus (A), Bacillus megaterium (B), P. carotovorum (C), and E. coli (D) on the 3rd day of incubation. D0 is the initial concentration of bacteria; “*” means significant differences from the control growth of microorganisms (PBSG) on the 3rd day. 1—PBSG is physiologic buffered saline with 0.5% glucose
Solutions of cadmium acetate and lead nitrate at all the taken concentrations suppressed the increase in B. megaterium var. phosphaticum by the 3rd day of incubation as compared with the control experiment (PBSG) (Fig. 2B). Herewith, a direct concentration dependence has been found. Cadmium solutions were more toxic for microorganisms as opposed to lead nitrate ones. However, a 2.5 mg L−1 lead nitrate solution did not significantly influence Bacillus megaterium growth. As opposed, solutions of sodium metaarsenite had a significant stimulating effect on the growth of biomass in all the concentrations taken.
Solutions of lead nitrate and cadmium acetate 2.5 mg L−1 and sodium metaarsenite 2.5 and 250 mg L−1 significantly stimulated the growth of P. carotovorum by the 3rd day of incubation compared with PBSG (Fig. 2C). In comparison with the initial optical density (0 days), the biomass did not increase in the solution of cadmium acetate 250 mg L−1. Solutions of lead nitrate 25 and 250 mg L−1 effected indifferently P. carotovorum. The growth of microorganisms in the presence of cadmium acetate 25 mg L−1 did not significantly differ from the values of biomass buildup in PBSG.
Lead nitrate and sodium metaarsenite 250 mg L−1 stimulated, while cadmium acetate 25 and 250 mg L−1 suppressed the increase in E. coli biomass (Fig. 2D). Other variants of the experiment showed no significant effect.
The cultures in the liquid medium had different sensitivity to the action of the toxicants taken: B. megaterium > B. mucilaginosus > P. carotovorum > E. coli. The toxicity of the tested solutions of heavy metals and arsenic for microorganisms can be arranged (decreasing toxicity) as follows: cadmium acetate > lead nitrate > sodium metaarsenite.
3.3 Evaluation of Heavy Metals and Arsenic Effect on Biofilm Formation Activity of Microorganisms
The process of biofilm formation is known to be stimulated under unfavorable conditions. However, solutions of cadmium acetate 2.5–250 mg L−1 and lead nitrate 250 mg L−1 were so toxic that biofilm formation of B. mucilaginosus was significantly suppressed on the 3rd day of incubation (Fig. 3A). Solutions of sodium metaarsenite 250 mg L−1 did not affect the optical density of B. mucilaginosus biofilm.
Influence of cadmium acetate—2; sodium metaarsenite—3; and lead nitrate—4 (mg L−1) on biofilm formation of Bacillus mucilaginosus (A), Bacillus megaterium (B), P. carotovorum (C), and E. coli (D) on the 3rd day of incubation. “*” means significant differences from biofilm in the control variant of the experiment (PBSG) on the 3rd day; 1—PBSG is physiologic buffered saline with 0.5% glucose
Biofilm formation of B. megaterium var. phosphaticum was stimulated by sodium metaarsenite 2.5 and 250 mg L−1 and lead nitrate 2.5 and 25 mg L−1 (Fig. 3B). Cadmium acetate 2.5–250 mg L−1 and lead nitrate 250 mg L−1 significantly inhibited this process. In case of sodium metaarsenite 25 mg L−1, a decrease in the amount of biofilms was slightly less than in the control level.
Stimulation of biofilm formation of P. carotovorum was observed in all the variants of the experiment, except for cadmium acetate, 250 mg L−1 (Fig. 3C).
Cadmium acetate 2.5 mg L−1, lead nitrate, and sodium metaarsenite were found to stimulate the formation of biofilms at all the concentrations taken, while cadmium acetate 250 mg L−1 suppressed the biofilm formation of E. coli (Fig. 3D). Cadmium acetate 25 mg L−1 did not affect E. coli biofilm formation.
Thus, cadmium acetate suppressed biofilm formation in almost all the concentrations taken, and sodium metaarsenite and lead nitrate mainly stimulated this process.
3.4 Evaluation of Heavy Metals and Arsenic Effect on Cell Length and Number of Spores
Microscopy of coverslips with B. mucilaginosus and B. megaterium biofilms grown in the presence of applied toxicants showed the change in the shape of microorganism cells, the number of spores, and the appearance of the biofilm matrix fragments (Figs. 4 and 5, Table 1). Under the influence of sodium metaarsenite 25 mg L−1, the number of spores in B. mucilaginosus decreased by 5 times. Meanwhile, multicellular structures similar to biofilms were formed (Fig. 4b). Lead nitrate 25 mg L−1 and cadmium acetate 2.5 mg L−1 induced the formation of more spores (1.4 and 6.5 times higher than in the control), leading to a decrease in cell length by almost 1.5 times compared to the control (Fig. 4c and d, Table 1). Solutions of cadmium acetate 25 and 250 mg L−1 led to a complete elimination of microorganism cells.
Sodium metaarsenite 25 mg L−1 also influenced the change in the shape of the cells of Bacillus megaterium (Fig. 5b, Table 1). The cells became shorter (by 1.4 times) and wider, and were filamentous-like grouped, resembling a honeycomb shape. The number of spores increased by 8.7 times. Lead nitrate 25 mg L−1 also led to an insignificant decrease in cell length. Fragments of the biofilm matrix were observed (Fig. 5c, Table 1). The number of spores increased approximately by 2 times. In the experiment with cadmium acetate 2.5 mg L−1, the number of spores increased by 3 times compared to the control experiment; the cells grew longer (by 1.4 times) and thinner (Fig. 5d, Table 1).
Change in the Concentration of Heavy Metals in the Overbiofilm Liquid Bacillus megaterium var. phosphaticum and Bacillus mucilaginosus.
The intercellular matrix of biofilm, consisting mostly of polysaccharides and proteins, is able to bind various compounds (Van Hullebusch et al., 2003). We assumed that As, Cd, and Pb can be sorbed by the matrix, which would allow us to recommend these microorganisms for bioremediation. So, the metal content in the initial culture solution was compared at the beginning of the experiment and 3 days after (Table 2). The heavy metals and arsenic contents were found to decrease during the cultivation. Pollutants accumulated to different degrees in biofilms with different types of bacteria. Thus, it was found that at the same initial concentrations of heavy metals and arsenic in the initial cultivation medium, there is a significant decrease in the metal content in the solution over the microbial mass of the B. mucilaginosus strain (Table 2).
Although, as previous experiments showed, these bacilli were more sensitive to the toxic effect of the pollutants, they bound them better. In the variant with Pb, the reduction was 96%, whereas in the experiment with B. megaterium, it was only 56% (with the initial Pb content of 6 mg L−1). With initial Cd content of 275 mg L−1, a reduction in the cadmium content was 67% in the presence of B. mucilaginosus, while it amounted only 30% in the presence of B. megaterium. However, the bacillus strains used did not significantly alter the amount of sodium metaarsenite with As content of 150 mg L−1. Therefore, the concentrations of As, Cd, and Pb decreased in the overbiofilm liquid of B. mucilaginosus and B. megaterium, which may be stipulated by their sorption in the biofilm matrix.
4 Discussion
These experiments revealed some regularities related to the mechanisms of toxic effect of As, Pb, and Cd solutions on the formation of biofilms of B. megaterium, B. mucilaginosus, P. carotovorum, and E. coli; changes in bacterial cellular forms; and their survival. The saprotrophic bacteria under study showed different resistance to As, Pb, and Cd.
Solutions of sodium metaarsenite displayed a bacteriostatic effect on B. mucilaginosus but had little effect on the growth of B. megaterium regardless of concentration on lawns. The solutions showed a weak bactericidal effect on P. carotovorum and E. coli. In a liquid medium, solutions of sodium metaarsenite inhibited the growth of B. mucilaginosus cell suspension (except of 2.5 mg L−1) and stimulated the accumulation of biomass in all other bacterial strains (Fig. 2). Arsenic solutions did not affect the biofilm-forming process of the B. mucilaginosus strain. They stimulated this process of B. megaterium, P. carotovorum, and E. coli at a maximum As concentration in the solution (Fig. 3). Microscopy of coverslips with B. megaterium grown in the presence of As solution revealed its specific behavior: The cells became shorter and wider and were grouped into separate chains, independent colonies, similar to honeycomb-like structures (Fig. 5). In the presence of As solution, B. mucilaginosus cells were spiral-shaped concentrated (Fig. 4).
With initial As content of 28 mg L−1, in the presence of B. mucilaginosus and B. megaterium, As concentration in the overbiofilm liquid decreased by 42%, while its concentration did not change in the presence of both cultures at the maximum As initial concentration (Table 1), which can be explained by both high migration mobility of the element and specific features of biofilm matrix. Soil bacteria can play a significant role to stimulate arsenic migration (Fitz & Wenzel, 2002). At the same time, there is a growing body of evidence that soil bacteria can accumulate As in less toxic biomethylated forms (Кabata-Pendias 2011). Since arsenic is widely distributed in nature and is chemically similar to phosphorus, a biophile element, many microorganisms have the ability to use arsenic in metabolic processes, changing its valence. As+5 can enter cells via phosphate transporters and interfere in oxidative phosphorylation by replacing phosphate. Some bacteria use As+3 as an electron donor. Arsenic oxidation suggests aerobic respiration. In such a transformation, more toxic As+3 transforms into less toxic As+5 (Lieutaud et al., 2010; Perelomov and Chulin, 2014).
In terms of decreasing resistance to the action of heavy metals and arsenic on a dense nutrient medium, the cultures can be arranged as follows: B. megaterium > P. carotovorum > E. coli > B. mucilaginosus. The same cultures on a liquid medium showed a different sensitivity to the action of toxicants: B. megaterium > B. mucilaginosus > P. carotovorum > E. coli. These two methods indicate that the Bacillus megaterium var. phosphaticum strain had a higher resistance to high concentrations of arsenic. This strain was also able to influence the translocation of phosphorus compounds into plants. Thus, there is a special interaction of As with the Bacillus megaterium var. phosphaticum strain. Currently, there are discussions that some bacteria in critical situations can use arsenic instead of phosphorus for their vital activity, since these elements are similar in their chemical properties (Oremland et al., 2009).
As was shown earlier (Belogolova et al., 2019), B. megaterium var. phosphaticum is able to initiate As accumulation in the rhizosphere. This strain has a significant effect on As migration, its mobilization, and immobilization in the rhizosphere due to arsenic leaching from mineral and difficult-to-destroy compounds and its accumulation in the rhizosphere soil and plant roots. The authors (Belogolova et al., 2015) describe a combined effect of Bacillus megaterium, Bacillus mucilaginosus, and Azotobacter on immobilization of high As contents in rhizosphere soil. A complex effect of bacteria can enhance the processes of biosorption of xenobiotics in the biofilm matrix (Nocelli et al., 2016). The study of the biogeochemical activity of arsenic in the rhizosphere can also be of great practical importance, due to the possibility to use strains of living soil bacteria Bacillus megaterium var. phosphaticum, Bacillus mucilaginosus, and Azotobacter for soil remediation.
Solutions of lead nitrate in our experiments inhibited the growth of the lawns of all test cultures, irrespective of the concentration. In a liquid medium, lead nitrate solutions inhibited the growth of B. mucilaginosus and B. megaterium (Fig. 2). Solutions of lead nitrate 250 mg L−1 inhibited the biofilm-forming ability of B. mucilaginosus and B. megaterium. Solutions of lead nitrate enhanced the biofilm-forming ability of P. carotovorum and E. coli in almost all the concentrations taken (Fig. 3). Microscopy of coverslips with B. megaterium biofilms grown in the presence of lead solution displays an insignificant decrease in cell length and the appearance of the biofilm matrix fragments (Fig. 5c).
In the presence of B. mucilaginosus and B. megaterium, Pb concentration in the overbiofilm liquid significantly decreased, in particular of B. mucilaginosus (Table 2). It means that this strain initiated lead fixation in the biomass. However, earlier experiments showed that this strain was more sensitive to lead toxic effect, but it bound lead better.
It is known that microorganisms are able to adsorb trace elements on the surface of cells and accumulate them inside the cells due to biological sorption (Tabak et al., 2005). Lead can also be accumulated in the biofilm matrix (Koechler et al., 2015). As opposed to As, lead has lesser migration mobility in natural environments and is able to form strong bonds with organic matter (Kabata-Pendias, 2011). For instance, B. mucilaginosus displayed higher spore-forming ability. Spore formation leads to the accumulation of polymeric organic substances. These compounds could initiate lead sorption, therefore leading to significant decrease in its concentration in the solution compared to the biomass of B. mucilaginosus (Table 2).
Microorganisms are known to adsorb heavy metals at a high rate. Dead cells are also capable of accumulating heavy metals (Das et al., 2012; Wang & Chen, 2009). The early researches showed the ability of organic lead compounds to transfer into more mobile forms in the rhizosphere soil inoculated with Azotobacter, B. megaterium, and B. mucilaginosus and to initiate its immobilization in chelate forms in rhizosphere and plant roots (Belogolova et al., 2020). These bacteria intensively affect lead mobilization and immobilization in the soil–plant system and suppress its entry into the upper parts of plants. This is also illustrated by the example of Rhodobacter sphaeroides (Li et al., 2016).
Solutions of cadmium acetate inhibited the growth of all test cultures on dense and liquid nutrient media and the biofilm-forming process, as cadmium is the most toxic element amongst heavy metals. It possesses high genotoxicity, as it readily accumulates inside the cells of living organisms. Cadmium is also known to inhibit the DNA-mediated transformation in microorganisms (Kabata-Pendias, 2011).
We studied the effect of different cadmium concentrations on the survival rate of microorganisms. The results show that when cadmium concentration decreased, microbial growth increased, while its higher concentrations suppressed the microbial growth (Fig. 2). These data are consistent with the results of the disc-diffusion method. Microscopy of coverslips with B. mucilaginosus and B. megaterium biofilms grown in the presence of Cd salts displayed an adaptive strategy of microorganism survival. Solution of cadmium acetate 2.5 mg L−1 stimulated spore-forming ability of B. mucilaginosus that resulted in shortening of cell length and appearance of biofilm matrix fragments compared with the control experiment. The same solution stimulated the spore-forming process of B. megaterium and led to elongation and thinning of cells (Fig. 5d, Table 1). This may also suggest the influence of genotoxicity of cadmium, leading to reshaping bacteria cells. B. mucilaginosus was shown to have a high spore-forming ability that may suppress the growth and inhibit biofilm formation under the effect of cadmium, which demonstrated a higher toxicity. Like in the case with Pb, cadmium concentrations significantly decreased in the overbiofilm liquid of B. mucilaginosus. It means that spore-forming processes inhibited cadmium entry into the overbiofilm solutions and initiate its immobilization that can occur under the effect of polymeric organic substances, produced from the spore-forming process.
Some authors report that extracellular polymeric organic substances may be more important in the biosorption of heavy metals than the surface of bacterial cells (Koechler et al., 2015; Van Hullebusch et al., 2003; Zakaria & Ahmad, 2020). Therefore, both bacterial cells and extracellular polymeric organic substances, formed in the biofilm matrix and in the spore formation process, are able to initiate immobilization of heavy metals.
5 Conclusion
All in all, the experiments established specific features related to mechanisms of toxic effect of As, Pb, and Cd salt solutions on biofilm formation of B. megaterium, B. mucilaginosus, P. carotovorum, and E. coli; changes in bacterial cellular forms; and their survival. The bacteria under study demonstrated different resistance to As, Pb, and Cd. The Cd solutions showed the maximum toxicity to the studied bacteria and suppressed the biofilm formation almost at all the taken concentrations while As and Pb solutions stimulated this process.
The immobilization of heavy metals can be achieved due to their binding into strong compounds with the matrix substances of biofilms and polymeric organic compounds formed during the sporulation of bacteria. The strain Bacillus megaterium was found to be the most resistant to high As concentrations and was able to influence the formation of highly structured colonies of bacterial cells with honeycomb-like structures.
The study of heavy metal-biofilm interactions can be useful in managing the life of natural microbial populations in the development of nanotechnologies and biotechnologies, related to soil bioremediation, crop production, and medicine.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abebe, G. M. (2020). The role of bacterial biofilm in antibiotic resistance and food contamination. International Journal of Microbiology. https://doi.org/10.1155/2020/1705814
Al-Amshawee, S. K., Yunus, M. Y., Azoddein, A. A. (2020). A review study of biofilm bacteria and microalgae bioremediation for palm oil mill effluent: Possible approach. IOP Conference Series: Materials Science and Engineering, https://doi.org/10.1088/1757-899X/736/2/022034.
Alreshidi, M. M., Sulieman, A. M. E., Veettil, V. N., & Snoussi, M. (2020). Antagonistic, biofilm-forming rhizospheric Pseudomonas spp. isolated from Hail province. Advancements in Life Sciences, 7(3), 170–176.
Aslam, S., & Darouiche, R. O. (2011). Role of antibiofilm-antimicrobial agents in controlling device-related infections. International Journal of Artificial Organs. https://doi.org/10.5301/ijao.5000024
Azevedo, A. S., Almeida, C., Melo, L. F., & Azevedo, N. F. (2017). Impact of polymicrobial biofilms in catheter-associated urinary tract infections. Critical Reviews in Microbiology. https://doi.org/10.1080/1040841X.2016.1240656
Bais, H. P., Fall, R., & Vivanco, J. M. (2004). Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiology. https://doi.org/10.1104/pp.103.028712
Bao, H., Jiang, L., Cai, W., Chen, C., Shen, M., He, Z., Chen, Z., Zhou, W. (2014). Biosorption mechanism of Bacillus mucilaginosus BHX-71 for heavy metal in industrial wastewater by FTIR. Reviews in Environmental Science and Bio/Technology, 434–437.
Belogolova, G. A., Gordeeva, O. N., Sokolova, M. G., Pastukhov, M. V., Poletaeva, V. I., Vaishlya, O. B. (2019). Rhizobacteria effect on arsenic migration and translocation of biogenic elements in plants. KnE Life Sciences, https://doi.org/10.18502/kls.v4i14.5684.
Belogolova, G. A., Sokolova, M. G., Gordeeva, O. N., & Vaishlya, O. B. (2015). Speciation of arsenic and its accumulation by plants from rhizosphere soils under the influence of Azotobacter and Bacillus bacteria. Journal of Geochemical Exploration. https://doi.org/10.1016/j.gexplo.2014.11.017
Belogolova, G., Gordeeva, O., Sokolova, M., Pastukhov, M., Vaishlya, O., Poletaeva, V., & Belozerova, O. (2020). Transformation of lead compounds in the soilplant system under the influence of Bacillus and Azotobacter rhizobacteria. Chemistry and Ecology. https://doi.org/10.1080/02757540.2020.1723557
Borriello, G., Werner, E., Roe, F., Kim, A. M., Ehrlich, G. D., & Stewart, P. S. (2004). Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrobial Agents and Chemotherapy. https://doi.org/10.1128/AAC.48.7.2659-2664.2004
Castiblanco, L. F., & Sundin, G. W. (2016). New insights on molecular regulation of biofilm formation in plant-associated bacteria. Journal of Integrative Plant Biology. https://doi.org/10.1111/jipb.12428
Chen, Y. M., Ding, Q. B., Chao, Y. Q., Wei, X. G., Wang, S. Z., & Qiu, R. L. (2018). Structural development and assembly patterns of the root-associated microbiomes during phytoremediation. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2018.07.095
Das, N., Basak, L. V. G., Salam, J. A., & Abigail, M. E. A. (2012). Application of biofilms on remediation of pollutants - An overview. Journal of Microbiology and Biotechnology Research, 2, 783–790.
Fitz, W. J., & Wenzel, W. W. (2002). Arsenic transformation in the soil–rhizosphere–plant system: Fundamentals and potential application to phytoremediation. Journal of Biotechnology. https://doi.org/10.1016/S0168-1656(02)00218-3
Grujic, S., Vasic, S., Comic, L., Ostojic, A., & Radojevic, I. (2017). Heavy metal tolerance and removal potential in mixed-species biofilm. Water Science and Technology. https://doi.org/10.2166/wst.2017.248
Hall-Stoodley, L., Costerton, J. W., & Stoodley, P. (2004). Bacterial biofilms: From the natural environment to infectious diseases. Nature Reviews Microbiology. https://doi.org/10.1038/nrmicro821
Hall, C. W., & Mah, T. F. (2017). Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiology Reviews. https://doi.org/10.1093/femsre/fux010
Ielapi, N., Nicoletti, E., Lore, C., Guasticchi, G., Avenoso, T., Barbetta, A., Franciscis, S., Andreucci, M., Sapienza, P., & Serra, R. (2020). The role of biofilm in central venous catheter related bloodstream infections: Evidence-based nursing and review of the literature. Reviews on Recent Clinical Trials. https://doi.org/10.2174/1574887114666191018144739
Kabata-Pendias, A. (2011). Trace elements in soil and plants. CRC Press Taylor and Francis Group LLC.
Khan, W. U., Ahmad, S. R., Yasin, N. A., Ali, A., Ahmad, A. (2017). Effect of Pseudomonas fluorescens RB4 and Bacillus subtilis 189 on the phytoremediation potential of Catharanthus roseus (L.) in Cu and Pb-contaminated soils. International Journal of Phytoremediation, https://doi.org/10.1080/15226514.2016.1254154.
Kim, H. W., Ha, U. S., Woo, J. C., Kim, S. J., Yoon, B. I., Lee, S. J., & Cho, Y. H. (2012). Preventive effect of selenium on chronic bacterial prostatitis. Journal of Infection and Chemotherapy. https://doi.org/10.1007/s10156-012-0406-7
Koechler, S., Farasin, J., Cleiss-Arnold, J., & Arsene-Ploetze, F. (2015). Toxic metal resistance in biofilms: Diversity of microbial responses and their evolution. Research in Microbiology. https://doi.org/10.1016/j.resmic.2015.03.008
Li, X., Peng, W., Jia, Y., Lu, L., & Fan, W. (2016). Bioremediation of lead contaminated soil with Rhodobacter sphaeroides. Chemosphere. https://doi.org/10.1016/j.chemosphere.2016.04.098
Lieutaud, A., van Lis, R., Duval, S., Capowiez, L., Muller, D., Lebrun, R. (2010). Arsenite oxidase from Ralstonia sp. 22: Characterization of the enzyme and its interaction with soluble cytochromes. Journal of Biological Chemistry, https://doi.org/10.1074/jbc.M110.113761.
Liu, H., & Fang, H. H. P. (2002). Characterization of electrostatic binding sites of extracellular polymers by linear programming analysis of titration data. Biotechnology and Bioengineering. https://doi.org/10.1002/bit.10432
Mani, D., & Kumar, C. (2014). Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. International Journal of Environmental Science and Technology. https://doi.org/10.1007/s13762-013-0299-8
McLean, K. L., Dodd, S. L., Minchin, R. F., Ohkura, M., Bienkowski, D., & Stewart, A. (2014). Non-target impacts of the biocontrol agent Trichoderma atroviride on plant health and soil microbial communities in two native ecosystems in New Zealand. Australasian Plant Pathology. https://doi.org/10.1007/s13313-013-0229-8
Miki, T., & Doi, H. (2016). Leaf phenological shifts and plant-microbe-soil interactions can determine forest productivity and nutrient cycling under climate change in an ecosystem model. Ecological Research. https://doi.org/10.1007/s11284-016-1333-3
Nadagouda, M. N., Bennett-Stamper, C., White, C., & Lytle, D. (2012). Multifunctional silver coated E-33/iron oxide water filters: Inhibition of biofilm growth and arsenic removal. RSC Advances. https://doi.org/10.1039/C2RA01306A
Nocelli, N., Bogino, P. C., Banchio, E., & Giordano, W. (2016). Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of rhizobia. Materials. https://doi.org/10.3390/ma9060418
Oremland, R. S., Chad, W. S., Wolfe-Simon, F., & Stolz, J. F. (2009). Arsenic in the evolution of earth and extraterrestrial ecosystems. Geomicrobiology. https://doi.org/10.1080/01490450903102525
Perelomov, L. V., & Chulin, A. N. (2014). Molecular mechanisms of interaction of microelements with microorganisms in the environment. Direct biological transformation of microelement compounds. Biology Bulletin Reviews, 4, 285–299. https://doi.org/10.1134/S2079086414040070)
Singh, P., & Cameotra, S. S. (2004). Enhancement of metal bioremediation by use of microbial surfactants. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2004.04.155
Sokolova, M. G., Akimova, G. P., & Vaishlya, O. B. (2011). Effect of phytohormones synthesized by rhyzosphere bacteria on plants. Applied Biochemistry and Microbiology. https://doi.org/10.1134/S0003683811030148
Stefanescu, I. A. (2015). Bioaccumulation of heavy metals by Bacillus megaterium from phosphogypsum waste. Scientific Study and Research: Chemistry and Chemical Engineering, Biotechnology, Food Industry, 16(1), 93–97.
Tabak, H. H., Lens, P., van Hullebusch, E. D., Dejonghe, W. (2005). Developments in bioremediation of soils and sediments polluted with metals and radionuclides – 1. Microbial processes and mechanisms affecting bioremediation of metal contamination and influencing metal toxicity and transport. Reviews in Environmental Science and Bio/Technology, https://doi.org/10.1007/s11157-005-2169-4.
Turskaya, A. L., & Ul’danova A. A., Stepanov A. V., Bukin, Y. S., Verkhoturov, V. V., Gaida, B. K., Markova, Y. A. . (2017). Formation of Pectobacterium carotovorum biofilms depending of the carbon source. Microbiology. https://doi.org/10.1134/S0026261717010143
Ullah, A., Heng, S., Munis, M. F. H., Fahad, S., & Yang, X. (2015). Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environmental and Experimental Botany. https://doi.org/10.1016/j.envexpbot.2015.05.001
Vaishlya, O. B., Amyago, D. M., & Guseva, N. V. (2013). Role of Bacillus mucilaginosus at silicon biogeochemical cycle in a system soil – plant. Mineralogical Magazine. https://doi.org/10.1180/minmag.2013.077.5.22
Van Hullebusch, E. D., Zandvoort, M. H., & Lens, P. N. L. (2003). Metal immobilisation by biofilms: Mechanisms and analytical tools. Reviews in Environmental Science and Biotechnology. https://doi.org/10.1023/B:RESB.0000022995.48330.55
Walz, J. M., Avelar, R. L., Longtine, K. J., Carter, K. L., Mermel, L. A., & Heard, S. O. (2010). Anti-infective external coating of central venous catheters: A randomized, noninferiority trial comparing 5-fluorouracil with chlorhexidine/silver sulfadiazine in preventing catheter colonization. Critical Care Medicine. https://doi.org/10.1097/CCM.0b013e3181f265ba
Wang, J. L., & Chen, C. (2009). Biosorbents for heavy metals removal and their future: A review. Biotechnology Advances. https://doi.org/10.1016/j.biotechadv.2008.11.002
Wu, Y. K., Cheng, N. C., & Cheng, C. M. (2019). Biofilms in chronic wounds: Pathogenesis and diagnosis. Trends in Biotechnology. https://doi.org/10.1016/j.tibtech.2018.10.011
Yuan, L., Sadiq, F. A., Wang, N., Yang, Z., & He, G. (2020). Recent advances in understanding the control of disinfectant-resistant biofilms by hurdle technology in the food industry. Critical Reviews in Food Science and Nutrition. https://doi.org/10.1080/10408398.2020.1809345
Zakaria, Z. A., & Ahmad, W. A. (2020). Cr(VI) removal using the combination of the Cr(VI)-resistant and Cr(VI)-reducing biofilm and the alum-polyacrylamide. Water, Air & Soil Pollution,. https://doi.org/10.1007/s11270-020-04860-z
Acknowledgements
Bacterial strains were provided by the Associate Professor of Tomsk State University Ph.D. Vaishlya O.B. for testing and research on the basis of the Siberian Institute of Plant Physiology and Biochemistry, SB RAS. The authors are grateful to Ph.D. Vaishlya O.B. We are also grateful to Khomutova M. Yu. for editing the English version of the text.
Funding
This study was funded by the governmental assignment in terms of Project № 0284–2021-0003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bybin, V.A., Belogolova, G.A., Markova, Y.A. et al. Influence of Heavy Metals and Arsenic on Survival and Biofilm Formation of Some Saprotrophic Soil Microorganisms. Water Air Soil Pollut 232, 343 (2021). https://doi.org/10.1007/s11270-021-05288-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05288-9