Abstract
Climate change is expected to affect tree leaf phenology by extending the length of the growing season (LGS), which will affect the productivity and nutrient cycling of forests. Interactions between plants and microbes will mediate the ecosystem processes further through microbe-mediated plant–soil feedback (PSF). To investigate the possible consequences of interactions between the extension of the growing season (GS) and PSF under various conditions, we developed a simple theoretical model (LGS-PSF model). The LGS-PSF model predicts that microbe-mediated PSF will intensify the negative effects of increasing temperature on the size of soil carbon stock when compared with simulations without the PSF effect. The combined effects of increasing temperature and PSF on the size of soil carbon stock occurs through enhanced activity of individual microbes and increased microbial population size. More importantly, the model also demonstrated that a longer GS mitigates this negative effect on carbon accumulation in soil, not through increased net primary production, but through intensified competition for nutrients between plants and microbes, thus suppressing microbial population growth. Our model suggested that the interactive effects of the LGS and PSF on carbon and nitrogen dynamics in forests should be incorporated into larger scale quantitative models for better forecasting of future forest functions under climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The recent increase in global air temperatures caused by greenhouse gas emissions has increased scientific focus on the basic responses of organisms to changes in temperature because those responses relate to both function and distribution, as well as ecosystem functions and services (Saxe et al. 2001; Sage and Kubien 2007; Chuine 2010). Because temperature is a major factor controlling plant productivity (Chuine and Beaubien 2001; Saxe et al. 2001), the predicted warming by 2100 of 2–6 °C compared with present temperatures in northern temperate forest regions (Christensen et al. 2007) could have substantial effects on forest productivity.
One effect of increasing air temperature caused by recent climate change is the induction of changes in the phenological timings of organisms (Fitter and Fitter 2002; Root et al. 2003; Gordo and Sanz 2005; Menzel et al. 2006; Doi and Katano 2008). Recent global warming has affected the phenological events of a variety of plant species (Menzel and Fabian 1999; IPCC 2007; Miller-Rushing et al. 2007; Gordo and Sanz 2009a, b), accelerating the timing of spring phenological events (e.g., leaf budburst and flowering), as well as delaying autumnal events (e.g., leaf coloring and fall; Menzel et al. 2006; Doi and Katano 2008; Doi et al. 2010). Consequently, these types of leaf phenological shifts are extending the length of the growing season (LGS, the days between leaf budburst and leaf fall) for various plant species (Menzel and Fabian 1999; Matsumoto et al. 2003; Gordo and Sanz 2009a, 2009b; Doi 2012). The LGS of plants has engendered substantial interest because it may exert substantial control on the ecosystem functions of forest and grassland ecosystems (White et al. 1999; Churkina et al. 2005; Hu et al. 2010). Furthermore, the recent documentation of extensions of the LGS has led to speculation that spring and autumn warming may enhance carbon (C) sequestration and extend the period of net C uptake in the future (Churkina et al. 2005). Thus, understanding changes to the LGS and the consequent leaf phenological response to climate change is important for producing better predictions of future ecosystem functions especially for deciduous forests in temperate regions. Individual plants, as well as entire forests, affect soil conditions through leaf fall and nutrient uptake via the root. In turn, these changes in soil conditions alter plant productivity through positive or negative feedback, a process termed plant–soil feedback (PSF; Van der Putten et al. 1993; Bever 1994; Ehrenfeld et al. 2005; Kulmatiski et al. 2008). Soil microbes have recently been recognized as a major driver of PSF because of changes in microbial activity and species composition that have been documented in response to changes in plant types and leaf quality, which in turn affect the plant performance (Miki et al. 2010; Miki 2012). Additionally, increasing air temperature also alters the soil conditions of forests by inducing changes in microbial activity. The majority of previous ecosystem models predicted that increasing air temperature stimulates microbial decomposition, and therefore, will decrease C stock, while increasing soil respiration (Allison et al. 2010). However, changes in the growing period will alter the relationship between plants and soil microbes, which can be competitive with respect to uptake of limited mineral nutrients, or mutualistic via nutrient recycling, and thus may cause unexpected changes to the decomposition rate of soil organic matter (SOM). Recent approaches to predicting soil conditions have only considered the direct effects of soil temperature (Allison et al. 2010). However, the microbe-mediated PSF and changes in the LGS have not been considered in predictions related to productivity and ecosystem functions/services of forests under future climate-change scenarios.
To estimate future forest productivity and functions under climate change, we propose that both the shift in the LGS in plants and interactions with microbes should be integrated into predictive methods. To date, however, predictions of forest productivity in response to CO2 and air-temperature increases have been performed with little regard to these effects (Cramer et al. 2001). Here we address this insufficiency through the introduction of a simple mechanistic model that accounts for both shifts in the LGS and PSF effects on plant productivity and nutrient cycling in a forest ecosystem in response to increasing temperature. Using the name length of the growing season-explicit plant–soil feedback model, (LGS-PSF model) we employed a set of ordinary differential equations, consists of C and nitrogen (N) compartments for growth and storage parts of plant standing biomass, soil microbial biomass, and soil organic matter, and soil inorganic N pool. Plants supply organic substrates for soil microbes as litter production, and soil microbes remineralize litter into inorganic N. They also compete for inorganic N, the strength of which depends on the C/N demand of soil microbes. The magnitude and seasonal variations in these processes are affected directly by air-temperature warming and indirectly by shifted plant leaf phenologies. Although the LGS-PSL model is not intended for quantitative forecasting or projection, the model provides new insights into how the interactive effects of a shift in the LGS and population dynamics of soil microbes can potentially determine the plant–soil co-development process in a temperate forest. In particular, its qualitative predictions demonstrate that longer GS enhances the size of C stock in soil, not through increased net primary production (NPP) and litter production, but through intensified competition for nutrients by soil microbes, thus suppressing microbial decomposition.
Methods
Model formulation
General framework of the LGS-PSF model
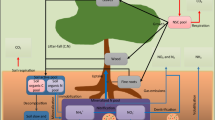
The variables used in the model plant–microbe–soil system consists of (1) C and N accumulated during growth by individual plants (C G and N G , respectively), (2) those stored in plants that are available for reallocation (C R and N R , respectively), (3) microbial C and N (C M and N M ), (4) soil organic C and N (SOC; C S , and SON; N S ), and (5) mineral N in soil (N I ; Fig. 1a). All of these variables have values per unit area, but not per unit individual of plants (see Table S1). The target ecosystem is a deciduous forest in a temperate region and the spatial scale of the model is relatively small enough so that any effects on spatial heterogeneity in plant stands and soil environment on forest dynamics can be neglected. Therefore, the LGS-PSF model uses a set of ordinary differential equations and is categorized as a process-based stand model (Medlyn et al. 2011). In the model, we defined six phases of the year along with plant and microbe phenology (Fig. 1b, Phase 1–6). Our model parameterizes the six major processes involved in ecological cycling of C and N in plant–microbe–soil systems as follows. (1) Atmospheric deposition supplies mineral N at a constant rate and a specific rate of loss occurs constantly by leaching throughout the year. (2) Photosynthesis only occurs in the plant canopy during the GS (Phase 3 in Fig. 1b) and its rate changes seasonally with the availability of light and mineral N in soil. (3) Plant respiration in growth and storage tissues occurs throughout the year but is affected by daily temperature. (4) The supply of organic matter to soil through litter fall occurs through constant herbivory (in Phase 3), and by the death and decay of plant matter, especially by leaves falling during autumn (for F L days in Phase 5). Organic C is also supplied by root excretion in the GS (in Phase 3). (5) A part of the photosynthetic product (C G , N G ) is reallocated before autumn litter fall (occurring for S L days in Phase 4) into the plant’s storage tissues (C R , N R ); this is then reused for foliation in the following year (occurring for S RG days in Phase 2). (6) Microbial decomposition of soil organic matter, microbial growth, and net mineralization (or immobilization) of N occur throughout the year but are also temperature dependent.
a Flow diagram of the length of the growing season-explicit plant–soil feedback model. The plant effects consist of four parts, C and N accumulated during growth by individual plants (C G and N G , respectively), and those stored in plants that are available for reallocation (C R and N R , respectively). There are two microbial components (C M and N M ), two soil components, organic C and N (C S , and N S , respectively), and mineral N in soil (N I ). b Phenology of the plant. Phase 1 (and Phase 6) correspond to winter, when the carbon and nitrogen biomass accumulated in plant growth tissues is zero (C G = N G = 0). Phase 2 is a short period in early spring (S RG = 5 days), during which translocation from storage (C S and N S ) to the growth tissues of the plant occurs. Phase 3 corresponds to the growing season, which extends from spring to the end of summer. During this period, photosynthesis, continuous litter fall by herbivory (0.001/day of plant growth tissues), and carbon excretion from the root (10 % of daily photosynthesis) occur. Phase 4 is a short period in early autumn (S L = 14 days), during which translocation from plant growth tissues to storage occurs prior to the litter fall period (Phase 5: F L = 14 days)
Core processes: photosynthesis and microbial decomposition
This section provides additional details regarding the formulations for core processes in our model (Processes 2 and 6). ESM 1 describes detailed formulations for other processes. For Process 2, we use a simple formula for total canopy photosynthesis [GPP (µg C m−2 s−1)] that is a function of canopy N (assumed to be half of the N G ), canopy C (assumed to be half of the C G ) converted to LAI (leaf area index, L), and absorbed photosynthetically active radiation (I a ) as follows (Franklin 2007):
with
and
In Eq. 1, h, ϕ, and θ represent day length, quantum efficiency, and a curvature parameter of leaf light response, respectively. Equation 2 represents the light extinction by self-shading with coefficient (k) from the radiation level above the canopy (I 0). Equation 3 represents the relationship between canopy C and the LAI (Poorter and Remkes 1990). Table S1 shows the definitions, units, and default values of all parameters. To focus on the effects of climate change on phonological shifts, the direct effects of elevated CO2 and temperature on GPP are not considered in our model.
For Process 6, we consider the activities of microbial decomposers limited by C or N, as determined by the availability of organic C and N, as well as mineral N, following the standard framework that assumes a fixed C:N ratio of microbial biomass (C:N)M (Manzoni and Porporato 2007, 2009). We assume that the decomposition rate of SOM itself is not limited by the availability of mineral N (C overflow hypothesis, Manzoni and Porporato 2007); thus, the decomposition rate of SOC (DECC) and that of SON (DECN) is given as:
respectively, where the decomposition coefficient k L depends on the belowground temperature T B . The amount of C and N assimilated by microbes depends on the potential assimilation rate of C \({\text{pASIM}}_{\text{C}} = e_{M} \cdot {\text{DEC}}_{\text{C}} \;(e_{M} \; < \; 1.0)\) and that of N \({\text{pASIM}}_{\text{N}} = e_{M} \cdot {\text{DEC}}_{\text{N}} + k_{I} \cdot C_{M} \cdot N_{I}\) where k I represents the maximum affinity for mineral nutrient uptake in soil. Note that the fraction (1 − e M ) of SOC and SON is respired as CO2 and released as mineral N (gross mineralization), respectively. When \({\text{pASIM}}_{\text{C}} > ( {\text{C:N)}}_{\text{M}} \cdot {\text{pASIM}}_{\text{N}}\), the excess \({\text{C}} (= {\text{pASIM}}_{\text{C}} - ( {\text{C:N)}}_{\text{M}} \cdot {\text{pASIM}}_{\text{N}}\)) is respired as CO2 and realized assimilation of C is \(( {\text{C:N)}}_{\text{M}} \cdot {\text{pASIM}}_{\text{N}}\) to maintain the fixed C:N ratio of microbes. When this condition is not met, C availability limits microbial growth, and the uptake of mineral N from the soil \((={\text{pASIM}}_{\text{C}} / ( {\text{C:N)}}_{\text{M}} - e_{M} \cdot {\text{DEC}}_{\text{N}} )\) is less than the capacity \(k_{I} \cdot C_{M} \cdot N_{I}\). Note that the uptake rate is likely to be positive because the C:N ratio of SOM (=[SOC]/[SON]) is much larger than that of microbes.
Seasonality in day length, temperature, and timing of phonological shifts in plants
In this section, we describe major formulas that are necessary to simulate the seasonal dynamics of the plant–microbe–soil model system. First, the day length (h) at latitude L (45°N) at the day of the year (J) is approximated by the CBM day length model (Forsythe et al. 1995):
where \(\beta (J) = \sin^{ - 1} \left[ {0.39795\cos \alpha (J)} \right]\) and \(\alpha (J) = 0.2163108 + 2\tan^{ - 1} \left[ {0.9671396\tan \left( {0.00860 \cdot \left( {J - 186} \right)} \right)} \right]\).
Second, the daily aboveground temperature (T A ) is approximated by a sine curve:
Third, the daily belowground temperature (T B : soil temperature) is determined by the aboveground temperature and LAI (Zheng et al. 1993), which is given by:
Fourth, we incorporate the linkage between the annual average aboveground temperature and the plant phonological timing into the model system. More specifically, we assume that the first day of foliation [(D B + 1)th day of year] occurs earlier with increased annual average temperature. It is given by:
where s represents the sensitivity coefficient [\(0 \le s \le 10\) (day °C-1)]. We assume that the first day of foliation occurs in mid-April at 18 °C average temperature with regard to foliation phenology of Japanese template trees from 1953 to 2005 (Doi and Katano 2008). Also, we assume that the timing of leaf fall in autumn is not affected by temperature, given that LGS becomes longer with elevated annual average temperature with referred to Menzel et al. (2006).
Temperature-dependent processes
We assume microbial activity (k L : see Eq. 4) and mass-specific respiration of plants (r P ) can be expressed using the Arrhenius equation. That is, k L and \(r_{P} \propto \exp \left( { - E_{a} /RT} \right)\) where E a is the activation energy (assumed as 50 kJ mol−1), R is the gas constant (8.3124 J K−1 mol−1), and T (T B or T A for microbial activity or plant respiration, respectively) is the temperature in degrees Kelvin. With these parameters, the Q10 value for microbial activity and plant respiration, which is defined as the factor by which the reaction rate increases with a 10 °C rise in temperature, is about 2.0 between 0 and 30 °C (Davidson and Janssens 2006). Although GPP was assumed to be independent from temperature, plant respiration caused NPP to be temperature dependent.
Settings for simulations
We chose climate-specific parameters (e.g. phenology) from published data on temperate forests and temperature-specific physiological parameters from review articles and modeling studies (Table S1). We first simulated the dynamics of a plant–microbe–soil system for 1000 years, using a fourth order Runge–Kutta method with a fixed interval of 0.01 day with default parameter settings (e.g., the average annual aboveground temperature was set at 18 °C). Although the time interval (0.01 day) for the integration of equations was less than 1 day, we did not consider variations of temperature within a day (see Eqs. 6, 7). The 1000-year simulation period is sufficient for the model system to achieve a stable seasonal cycle. Following the 1000-year simulation, these parameters were again set to the initial conditions of the model for further simulations to examine parameter sensitivity by running the model again for 1000 years and the responses of the model system to climate change by running the model for 100 years). We assumed that the annual average temperature increased linearly from 18 °C, with a 0–6 °C increase per 100 years. To model responses to climate change, we prepared two distinct scenarios for understanding the role of plant–microbe interactions with changing plant phonological timing in C and N cycling. In the microbe-explicit scenario with microbe-mediated PSF (Scenario 1), microbial activity dynamically changes with changing climatic conditions via altered microbial biomass and their C and N demands, as well as with the direct effects of increasing temperature, which follows the Arrhenius equation. In the microbe-implicit scenario without microbe-mediated PSF (Scenario 2), microbial activities are not dynamic. In Scenario 2, prior to the simulated climate change, we assume that daily changes in the mass-specific decomposition rate of SOC and mass-specific net mineralization from SON follow an identical pattern of seasonality as in Scenario 1, such that the C and N cycling in Scenario 2 are almost identical to that of Scenario 1. However, following climate change, daily mass-specific rates are affected only by an increase in temperature, following the Arrhenius equation. The comparison of these two scenarios elucidates the role of population dynamics of microbes and interactions with plants in C and N cycling.
Results
Our model reproduces realistic stock distribution and fluxes of C and N at an annual scale in temperate forests; the simulated values range from 21 (N biomass of growth part of plants) to 144 % (LAI) of published data (Fig. 2, see also Table S2). The sensitivity analysis indicates that the microbial parameters [C:N ratio (CNRatio_M), specific decomposition rate (Decomp_293), growth efficiency based on N (GrowthEffN_M), and mortality (Mortality_M)] have larger effects on ecosystem processes than plant-related parameters [specific uptake rate of mineral N (UPtakeN_P), daily litter production (frac_Litter0), C excretion from root (frac_rootC), and reallocation efficiency based on C (ReAlloC) and N (ReallocN); Fig. 3]. If we focus on soil standing stocks of C and N [(SOC@end Ph4), (SOC@end Ph5), (SON@end Ph4), and (SON@end Ph5)] only, daily litter production, C:N ratio of microbes, and microbial growth efficiency based on N are the most important parameters. Interestingly, the sensitivity of SOC and SON to changes in N deposition and leaching was smaller than that of other variables (e.g., LAI, representing the standing stock of aboveground plant biomass). Figure S1 shows a more detailed sensitivity analysis of all parameter values simultaneously.
Default model output and comparison with observed values from Harvard forest (42.5°N, 72°W, the data in ESM2). Values inside boxes are seasonal minimums and maximums of standing stock (g m−2) and values with arrows represent fluxes (g m−2 y−1). Asterisk indicates the data are from the other forest. (−) means no data are available. Table S1 shows parameter values and details
Summary matrix of sensitivity analysis. The sensitivity of each variable predicted by the model (rows) to each parameter (columns) was calculated by focusing on the value of each variable generated using the default parameter value (v 100) and a 50 % increase and decrease in the default parameter value (v 150, and v 50, respectively). Degree of sensitivity is categorized into four classes based on the value of |v 150/v 100 − 1| + |v 50/v 100 − 1|. Table S1 summarizes the definitions of abbreviations
We then elucidated the effects of increased temperature on biomass accumulation (Fig. 4) and elemental fluxes (Fig. 5) by focusing on the results that did not show change in plant phenology (i.e., sensitivity coefficient s = 0). Our model prediction is straightforward in that LAI increases with increased temperature (Fig. 4a, e), whereas standing stocks of SOC and SON decrease (Fig. 4b, c, f, g), regardless of whether or not the scenario included microbial population dynamics. The degree of enhanced decomposition that may result in a decreased accumulation of SOC and SON (indicated by annual net mineralization rate; Fig. 5a, e) is much larger than that of the increase in NPP (Fig. 5b, f) and litter production (Fig. 5c, d, g, h), which may result in increased accumulation of SOC and SON. This is why the net effect of increased temperature on soil organic matter is negative, resulting in the reduction of SOC and SON standing stocks (Fig. 4b, c, f, g). The difference between microbe-explicit and microbe-implicit scenarios is that the reduction of SOC and SON is much larger in the microbe-explicit scenario (Fig. 4). This probably occurs because microbe-mediated PSF enhances the process of decomposition, not only through the direct positive effects of increased temperature on decomposition per individual microbe [k L (T B ) in Eq. 4], but also by the increasing size of microbial populations (Fig. 4d).
Responses of standing stocks to temperature increase and extension of the growing season. A simulated temperature increase per 100 years of 0–6 °C, with an interval of 0.5 °C. The variation in the extended growth period of plants is generated by changing the sensitivity of the budding date (parameter s in Eq. 8) from 0 to 10 (days °C-1). a–d Microbe-explicit scenario. e–h Microbe-implicit scenario. C carbon, LAI leaf area index, SOC soil organic carbon, SON soil organic nitrogen
Responses of annual C and N fluxes to temperature increases and extension of the growing season. Settings for simulations are identical to those described for Fig. 4. Litter C+ root C represents the total organic carbon flux from plants to soil via litter fall and C root excretion. C carbon, N nitrogen, NPP net primary production
Next, we elucidated the effect of extension of the GS caused by increased temperature by focusing on the results of simulations with a fixed level of increased temperature (Figs. 4, 5). In the microbe-explicit scenario, LAI at the end of GS (LAI@end Ph3) and annual NPP are smaller, with higher sensitivity of the budding day, despite the longer growth period (Figs. 4a, 5b). In contrast, the microbe-implicit scenario predicts that LAI and NPP increase, with higher sensitivity of plant phenology and a longer growth period (Figs. 4e, 5f). The same trends are observed in annual production of litter C and N (Fig. 5c, d vs. g, h); litter production decreases with a longer growth period in the microbe-explicit scenario, but increases in the microbe-implicit scenario.
These contrasting results can be understood by comparing simulations with or without a plant phenological shift. In the microbe-explicit scenario in which a phenological shift occurs with increased temperatures, the plants start to grow earlier (Fig. 6a) and consume more mineral N than in the simulation without a phenological shift. The phenological shift in the first scenario therefore leads to lower availability of mineral N (Fig. 6b) and then a lower growth rate of microbes (Fig. 6c), which results in a lower decomposition rate of SOC (Fig. 6d). In contrast, in the microbe-implicit scenario, an earlier commencement of plant growth (Fig. 6e) and lower availability of mineral N (Fig. 6f) do not severely affect the decomposition rate of SOC (Fig. 6g).
Comparison of temporal dynamics of plant, soil, and microbes between microbe-explicit and microbe-implicit scenarios. Temporal dynamics at the 100th year after changes in temperature are shown, with a linear 4 °C increase over 100 years. Solid and dashed lines represent results without consideration of shifts in plant phenology (s = 0) and those with the shifts (s = 10), respectively. The specific growth rate of microbes and specific decomposition rate of soil organic carbon (SOC) are defined as per microbial biomass growth rate and per SOC biomass decomposition rate, respectively. a–d Microbe-explicit scenario. e–g Microbe-implicit scenario. LAI leaf area index
Finally, for the comparison with other models (see “Discussion”), we evaluated the responses of the model ecosystem under a typical scenario with +3.0 °C/100 year and a 10 day extension of the GS/°C (Fig. 7).
Annual NPP, SOC, and microbial biomass changes among the different scenarios (with/without extension of the growing season and microbes) under +3.0 °C/100 years and a 10 day extension of the growing season/°C. ±M mean microbe-implicit (−) and -explicit (+) scenario. LGS length of the growing season, NPP net primary production, SOC soil organic carbon
Discussion
Overview: effects of microbial population dynamics and interactions with LGS shift
Our results from the model showed that a longer GS resulted in an increase in NPP and tree litter production without microbe-mediated PSF, but also resulted in decreased NPP and litter production with microbe-mediated PSF. Regarding the shift in the LGS and PSF effects on forest productivity and material cycling, we found that the both the shift in the LGS and PSF strongly affected forest dynamics. An extension of the LGS altered forest productivity and material cycling, as well as the plant–soil feedback.
Considering the microbial population explicitly in the model resulted in a reduction of soil organic matter by warming, which was larger than in the scenario with a fixed microbial population size (i.e., microbe-implicit scenario; Fig. 4). However, the effects of microbial population dynamics on C and N cycling become much more profound when PSF and interactions with shifts in the LGS are considered. The shift in the LGS to a longer growing season is generally believed to buffer the negative effects of warming on C sequestration in soil via enhancing NPP and litter production (Piao et al. 2007). Our simulation also showed SOC and SON are larger with higher sensitivity of plant phenology to increased temperature (Fig. 4b, c vs. f, g), regardless of whether or not the scenario included microbial population dynamics. However, this mechanism is effective only when interactions with microbe-mediated PSF are neglected (i.e., scenario without microbial population dynamics) where the longer growth period enhances NPP (Fig. 5f). In contrast, we found a new potential mechanism for this type of a buffering effect of the shift in the LGS; earlier plant growth leads to intensified competition between plants and microbes for N (Fig. 6b), resulting in a suppression of microbial population size (Fig. 4d) and thus the litter decomposition rate (Fig. 6d), resulting in a larger accumulation of SOM. Although evidence is lacking in temperate forests, not a few grassland studies in colder climate regions demonstrate seasonal shifts exist in the competitive outcome between plants and microbes for N (Xu et al. 2011; Kuzyakov and Xu 2013) and in the seasonal partitioning of N between microbes and plants (Bardgett et al. 2005). These lines of evidence indicate the importance of seasonality in interactions between plants and microbes (Bardgett et al. 2005) and imply that the proposed effects of an extended GS on nutrient cycling through seasonally dynamic plant–microbe interactions should be considered in these types of alpine or tundra ecosystems.
Important factors related to C and N cycling
From the sensitivity analyses, we can determine which factors are more important in the control leaf phenological effects on plant and soil functions. The parameters related to microbial activity, such as specific decomposition rate, growth efficiency for N, and mortality, have larger effects on ecosystem outcomes (e.g., annual values of NPP and SON). These analyses clearly gives us ideas on how to improve the model, implying the importance of the incorporation of (1) variable (non-homeostatic) C:N for microbial demand leading to variable decomposition rates and growth efficiency for N and (2) the need to include microbial food web complexity into soil components of plant–soil interaction models when determining microbial mortality. These lines of reality will be explored as important determinants on ecosystem dynamics. Microbe growth efficiency for N affected most of the variables in the model, but the efficiency for C only affected certain variables. N deposition and leasing also primarily affected annual C and N cycling in the forest. These results also suggest that with the default parameters, this model forest system is generally limited by N, rather than by C. This is in contrast to the DAYCENT model (Frey et al. 2013), which predicts high sensitivity of SOC accumulation to microbial C growth efficiency. This discrepancy might imply that further study of coupled C and N dynamics and their affects to microbe-mediated soil processes is needed; this may also be a potential limitation of the model because of its simplicity.
Bridging from conceptual model to process-based ecosystem model and implications for empirical studies
Our modeling study aimed to bridge the gap among conceptual models of phenological shift (e.g., Kramer 1994; Kramer et al. 2000; Nakazawa and Doi 2012) and plant–soil feedback (Miki and Kondoh 2002; Ushio et al. 2013; Miki et al. 2010; Miki 2012; Ke et al. 2015) and process-based forest models [e.g., CENTURY (Parton et al. 2009) and ecosystem models (Grant et al. 2001)]. Phenological shift models have demonstrated the importance of a phenological shift as a determinant of inter-specific interactions such as trophic interactions between phytoplankton and zooplankton (Winder and Schindler 2004) and plant–pollinator interactions (Doi et al. 2008). Meanwhile, plant–soil feedback models have demonstrated the importance of plant–microbial interactions as a determinant of plant growth, reproduction, and nutrient cycling. Our LGS-PSF model represents the first attempt to incorporate these two features into a single framework with relatively realistic but simple parameterization of C and N standing stock and fluxes in a forest. We here summarized the pros and cons of our model. In addition, the structure of the LGS-PSF model is more complex than typical conceptual models in mathematical ecology, which prevents the mathematical analysis on model behavior but it is necessary to formulate C–N coupling to gain a better understanding of the dynamic interactions between plants and microbial decomposers. Furthermore, it is much simpler than typical process-based forest models in the field of ecosystem modeling. In particular, (1) the effects of increasing atmospheric CO2 and temperature on the photosynthesis rate (GPP) and C:N of plants and (2) the roles of soil water availability in plant and microbial activities are not considered. This simplicity prevents a direct comparison with other process-based models as well as observed data and results in quantitative uncertainty of its behavior, but enables us to elucidate possible outcomes of the interactions between LGS and PSF in a very clear way. For better comparison with previous process-based forest models (Sitch et al. 2003; Matala et al., 2005) one should consider common processes, e.g. dynamic allocation of C and nutrients into aboveground and belowground parts of plants and temperature dependence of GPP. In addition, seasonality of N deposition is also an important factor for providing a good description of the seasonal dependence of plant–microbial interactions.
Both phenological shift in plants and interactions with microbes (i.e., microbe-mediated PSF) have been largely ignored in existing models that predict productivity and element cycling in forest ecosystems in response to increasing levels of CO2 and air temperature. However, Peng et al. (2009) recently suggested that global modeling should consider the microbe and phenological shifts that occur under climate change. Our model demonstrates how the roles of microbe and phenological shift can be considered in a global model to predict future C and nutrients fluxes from temperate forests. Our model and simulations focused on temperate forests, because the prominence of deciduous tree species causes extensions of the GS to primarily occur in these forest ecosystems. Furthermore, Pan et al. (2011) recently determined that approximately 30 % of the global forest C sink occurred in temperate forests (0.72 Pg C per year). Thus, the inclusion of an extension of the GS in a global C-cycling model would substantially improve model predictions. By considering both the extension of the GS and microbe-mediated PSF effects on plant productivity and element cycling in a forest, global models of forest production and C cycling can better predict the effects of rising temperatures than the models lacking these drivers.
Our estimated NPP changes (+9 %) under a typical temperature change scenario (+3 °C), but excluding a shift in phenology and microbial activity (Fig. 7), are comparable to other model predictions (−7 to +24 %, Ollinger et al. 2008; Peng et al. 2009; Kirschbaum et al. 2012). Interestingly, the inclusion of microbial population dynamics results in higher predicted NPP (around +30 %; Fig. 7). Similarly, the changes in SOC in our model (3–28 % reduction) are comparable to other model predictions (−9 to +3 %, Ollinger et al. 2008; Kirschbaum et al. 2012), but also highly depend on the presence/absence of the PSF effects (Fig. 7). This also implies that a better understanding of microbial components in theoretical studies and experiments will revise and improve our understanding of the forest response to warming.
In conclusion, we performed simple theoretical modeling to estimate the effects of leaf phenology on C and N dynamics in temporal forests under future climate change. To predict forest productivity and functions under future climate-change conditions, both the shift in the LGS in plants and microbe-mediated PSF should be considered. Further experimental and observational studies are needed to fill current knowledge gaps related to climate change and forest ecosystem dynamics. In particular, the following factors should be considered. First, some simulation studies have indicated that enhanced levels of CO2 driven by climate change always result in an increase in NPP (Ollinger et al. 2008; Peng et al. 2009; Kirschbaum et al. 2012); however, the interactive effects among CO2 fertilization, plants, and microbes should also be considered. Second, water and N availability for forest ecosystems will be affected by climate change caused by expected changes in precipitation and N deposition (IPCC 2007). Because our simulations show the importance of N limitation as well as competition between plants and soil microbes for the N in response to temperature increase, the change in N supply may be also important in determining the relationship between plants and microbes. Additionally, microbial communities may suffer from water deficiency caused by asymmetric competition with plants; therefore, future experimental and observational studies as well as simulation models should estimate the interactive effects of both water and N availability for plants and microbes. Finally, decomposer (microbe and fungi) diversity may buffer competition between plants and microbes (Miki and Kondoh 2002; Miki et al. 2010; Miki 2012; Ushio et al. 2013). Our simulations indicated the importance of competition for N between plants and microbes, so the buffering effect of decomposer diversity would change the degree of competition among these communities.
References
Allison SD, Wallenstein MD, Bradford MA (2010) Soil-carbon response to warming dependent on microbial physiology. Nature Geo 3:336–340
Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK (2005) A temporal approach to linking aboveground and belowground ecology. Trend Ecol Evol 20:634–641
Bever JD (1994) Feedback between plants and their soil communities in an old field community. Ecology 75:1965–1977
Christensen JH et al. (2007) Regional climate projections. In: Solomon S. et al. (eds.) Climate change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, UK and New York, pp 847–940
Chuine I (2010) Why does phenology drive species distribution? Phil Trans Royal Soc B 365:3149–3160
Chuine I, Beaubien EG (2001) Phenology is a major determinant of tree species range. Ecol Lett 4:500–510
Churkina G, Schimel D, Braswell BH, Xiao XM (2005) Spatial analysis of growing season length control over net ecosystem exchange. Global Change Biol 11:1777–1787
Cramer W et al (2001) Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Global Change Biol 7:357–373
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Doi H (2012) Response of the Morus bombycis growing season to temperature and its latitudinal pattern in Japan. Int J Biometeorol 56:895–902
Doi H, Katano I (2008) Phenological timing of leaf budburst with climate change in Japan. Agric For Meteorol 148:512–516
Doi H, Gordo O, Katano I (2008) Heterogeneous intra-annual climatic changes drive different phenological responses at two trophic levels. Clim Res 36:181–190
Doi H, Takahashi M, Katano I (2010) Genetic diversity increases regional variations in phenological responses to climate change. Global Change Biol 17:373–379
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant–soil system. Annu Rev Env Resour 30:75–115
Fitter AH, Fitter RSR (2002) Rapid changes in flowering time in British plants. Science 296:1689–1691
Forsythe WC, Rykiel EJ Jr, Stahl RS, We H, Schoolfield RM (1995) A model comparison for daylength as a function of latitude and day of year. Ecol Model 80:87–95
Franklin O (2007) Optimal nitrogen allocation controls tree responses to elevated CO2. New Phytol 174:811–822
Frey SD, Lee J, Melillo JM, Six J (2013) The temperature response of soil microbial efficiency and its feedback to climate. Nature Clim Change 3:395–398
Gordo O, Sanz JJ (2005) Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia 146:484–495
Gordo O, Sanz JJ (2009a) Long term temporal changes of plant phenology in the western Mediterranean. Global Change Biol 15:1930–1948
Gordo O, Sanz JJ (2009b) Long term temporal changes of plant phenology in the western Mediterranean. Global Change Biol 15:1930–1948
Grant RF, Jarvis PG, Massheder JM, Hale SE, Moncrieff JB, Rayment M, Scott SL, Berry JA (2001) Controls on carbon and energy exchange by a black spruce—moss ecosystem: testing the mathematical model Ecosys with data from the BOREAS experiment. Glob Biogeochem Cycles 15:129–147
Hu JIA, Moore DJP, Burns SP, Monson RK (2010) Longer growing seasons lead to less carbon sequestration by a subalpine forest. Global Change Biol 16:771–783
IPCC (2007) Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Climate change 2007: impacts, adaptations, and vulnerability. Cambridge University Press, New York
Ke PJ, Miki T, Ding TS (2015) The soil microbial community predicts the importance of plant traits in plant–soil feedback. New Photol 206:329–341
Kirschbaum MUF, Watt MS, Tait A, Ausseil AGE (2012) Future wood productivity of Pinus radiata in New Zealand under expected climatic changes. Global Change Biol 18:1342–1356
Kramer K (1994) A modelling analysis of the effects of climatic warming on the probability of spring frost damage to tree species in The Netherlands and Germany. Plant Cell Environ 17:367–378
Kramer K, Leinonen I, Loustau D (2000) The importance of phenology for the evaluation of impact of climate change on growth of boreal, temperate and Mediterranean forests ecosystems: an overview. Int J Biometeorol 44:67–75
Kulmatiski A, Beard KH, Stevens JR, Cobbold SM (2008) Plant–soil feedbacks: a meta-analytical review. Ecol Lett 11:980–992
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669
Manzoni S, Porporato A (2007) Theoretical analysis of nonlinearities and feedbacks in soil carbon and nitrogen cycles. Soil Biol Biochem 39:1542–1556
Manzoni S, Porporato A (2009) Soil carbon and nitrogen models: mathematical structure and complexity across scales. Soil Biol Biochem 41:1355–1379
Matala J, Ojansuu R, Peltola H, Sievanen R, Kellomaki S (2005) Introducing effects of temperature and CO2 elevation on tree growth into a statistical growth and yield model. Ecol Model 181:173–190
Matsumoto K, Ohta T, Irasawa M, Nakamura T (2003) Climate change and extension of the Ginkgo biloba L. growing season in Japan. Global Change Biol 9:1634–1642
Medlyn BE, Duursma RA, Zeppel MJB (2011) Forest productivity under climate change: a checklist for evaluating model studies. WIREs Clim Change 2:332–355
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659
Menzel A et al (2006) European phenological response to climate change matches the warming pattern. Global Change Biol 12:1969–1976
Miki T (2012) Microbe-mediated plant–soil feedback and its roles in a changing world. Ecol Res 27:509–520
Miki T, Kondoh M (2002) Feedbacks between nutrient cycling and vegetation predict plant species coexistence and invasion. Ecol Lett 5:624–633
Miki T, Ushio M, Fukui S, Kondoh M (2010) Functional diversity of microbial decomposers facilitates plant coexistence in a plant–microbe–soil feedback model. Proc Natl Acad Sci USA 107:14251–14256
Miller-Rushing AJ, Katsuki T, Primack RB, Ishii Y, Lee SD, Higuchi H (2007) Impact of global warming on a group of related species and their hybrids: cherry tree flowering at Mt. Takao, Japan. Am J Bot 94:1470–1478
Ollinger SV, Goodale CL, Hayhoe K, Jenkins JP (2008) Potential effects of climate change and rising CO2 on ecosystem processes in northeastern U.S. forests. Mitig Adapt Strateg Glob Change 13:467–485
Pan Y et al (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993
Parton WJ, Schimel DS, Cole CV, Ojima DS (2009) Analysis of factors controlling soil organic-matter levels in Great-Plains grasslands. Soil Sci Soc Am J 51:1173–1179
Peng C et al (2009) Quantifying the response of forest carbon balance to future climate change in Northeastern China: model validation and prediction. Global Planet Change 66:179–194
Piao S, Friedlingstein P, Ciais P, Viovy N, Demarty J (2007) Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Global Biogeochem Cy 21:GB3018
Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83:553–559
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106
Saxe H, Cannell MGR, Johnsen B, Ryan MG, Vourlitis G (2001) Tree and forest functioning in response to global warming. New Phytol 149:369–399
Sitch S et al (2003) Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Global Change Biol 9:161–185
Ushio M, Miki T, Balser TC (2013) A coexisting fungal-bacterial community stabilizes soil decomposition activity in a microcosm experiment. PLoS One 8:e80320
Van der Putten WH, Van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune communities. Nature 362:53–56
White MA, Running SW, Thornton PE (1999) The impact of growing-season length variability on carbon assimilation and evapotranspiration over 88 years in the eastern US deciduous forest. Int J Biometeorol 42:139–145
Winder M, Schindler DE (2004) Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85:2100–2106
Xu XL et al (2011) Spatio-temporal patterns of plant-microbial competition for inorganic nitrogen in an alpine meadow. J Ecol 99:563–571
Zheng D, Hunt ER Jr, Running SW (1993) A daily soil temperature model based on air temperature and precipitation for continental applications. Clim Res 2:183–191
Acknowledgments
National Taiwan University (Grant No. 10R70604-3) and the Ministry of Science and Technology (Grant No. NSC101-2621-B-002-004-MY3) supported this research for T. M.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Miki, T., Doi, H. Leaf phenological shifts and plant–microbe–soil interactions can determine forest productivity and nutrient cycling under climate change in an ecosystem model. Ecol Res 31, 263–274 (2016). https://doi.org/10.1007/s11284-016-1333-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-016-1333-3