Abstract
Heavy metal-resistant plant growth-promoting bacteria may help in reducing the toxic metal accumulation within the plants grown in metal-contaminated soils and thus be effective against biomagnification, enhancing food safety. In the present study, the most potential multi-metal resistant bacterium Pseudomonas aeruginosa JCM 5962 (obtained from arable soil of Uttar Dinajpur) was utilized for Cd biosorption analysis and also to observe the in vivo growth effect on Brassica plant including the estimation of Cd content accumulated within the plant parts of Brassica grown in Cd-contaminated soil. The maximum sorption of Cd ions by the dead bacterial biomass of Pseudomonas aeruginosa JCM 5962 was estimated to be 149.25 mg/g. The adsorption data provided an outstanding fit to the Freundlich isotherm model, indicating multilayer adsorption at heterogeneous surfaces. RL value (0.76, i.e.; <1) implied favourable adsorption. The in vivo experiment revealed the increased length of both roots and shoots in Brassica seedlings grown in Cd-contaminated soil under the effect of bacterial inoculants, which was also statistically significant (p < 0.001). In addition, the studied bacterium was effective in lowering Cd accumulation within the plant parts of Brassica. Approximately 62.76% reduction in Cd content was observed in Brassica seedlings grown in Cd-contaminated soil under the effect of bacterial inoculants than in the non-inoculated control set. This is a pioneering study, which could be further employed to develop strategies for microbe-assisted phytoremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are the major soil contaminants, increasing globally at an unrestrained rate, adversely affecting microbial diversity and agricultural crop productivity. Being non-biodegradable, the persistence and subsequent biomagnification of recalcitrant heavy metals in each trophic level of the ecosystem pose a significant threat to living beings (Srivastava et al. 2017; Rai et al. 2019). Therefore, mitigating metal contaminants from the soil is necessary to reduce potential health risks and maintain environmental sustainability. In this context, soil-inhabitant indigenous bacteria offer a lot of promise. Nowadays, they have gained much more attention as a potential candidate for developing cost-effective green technologies (Pham et al. 2022). Due to the co-existence with metal-rich polluted habitat over a long period of time, soil-dwelling bacteria have established several mechanisms not only to mitigate heavy metals from the contaminated soil but also to enhance plant growth (Jadhav et al. 2010). Various resistance mechanisms, such as biosorption, bioaccumulation, mobilization, precipitation, biotransformation, etc., play a critical role in combating metal toxicity in bacteria (Nies 2003; Ma et al. 2016; Mathivanan et al. 2021). Among all these mechanisms, biosorption by bacterial biomass is the most significant in terms of heavy metal remediation and may be used for the effective removal of toxic heavy metals from aqueous solutions (Pham et al. 2022). Non-specific surface binding or sorption of metal ions onto the bacterial cell wall occurs due to the presence of several potential active sites (Rizvi et al. 2020), which may be further studied through different isotherm models (Mohapatra et al. 2019). All these innate adaptive mechanisms of bacteria assist in altering the metal bioavailability in soils and thus reducing the metal accumulation within the plants (Burd et al. 2000; Vishnupradeep et al. 2022) grown in contaminated fields, resulting in safe food production (Etesami 2018). Such bacteria enhance the nutritional uptake of plants through the secretion of essential phytohormones, enzymes, etc. and solubilizing phosphorous, thus enabling plant growth (Ren et al. 2019). Moreover, they are also involved in alleviating heavy metal-induced oxidative stress by improving the antioxidant activities of the plant. Various soil-inhabitant bacterial genera like Pseudomonas, Bacillus, Rhizobium, Azotobacter, Azospirillum, Burkholderia, Frankia, etc. (Goswami et al. 2016) have been reported to possess such plant growth promotion potentiality. Employing these heavy metal-resistant plant growth-promoting bacterial inoculants has hastened the technology from the laboratory to the cultivable lands.
From the cultivation perspective, Brassica plants are one of the economically significant crop species for oil production globally. This plant has been garnering special attention for phytoremediation due to some essential traits like fast growth rate, profuse biomass production, heavy metal sequestration ability, stress tolerance potentiality etc. (Marchiol et al. 2004). Though the Brassica plant itself possesses the phytoremediation capability (Rizwan et al. 2018), still the metal extraction efficiency of the plant has been reported to be stimulated by the simultaneous inoculation with plant growth-promoting bacteria, revealed in several studies (Adediran et al. 2015; Manoj et al. 2020; Sharma 2021). Hence, the exogenous application of metal-resistant growth-promoting bacteria may overcome the adverse toxic effects of heavy metal on the plant, thus assuring food safety along with the phytomanagement strategy, and may pave the way for effective microbe-assisted phytoremediation also.
This study aimed to analyse the biosorption potential and growth-promoting effect of a heavy metal resistant and plant growth-promoting bacterium Pseudomonas aeruginosa JCM 5962 (isolated from arable soil) on Brassica napus L. The studied bacterium showed effective Cd uptake capability even under cumulative metal (Cd:Zn:Co) stressed condition reported in an earlier study (Saha et al. 2022). Hence, Cd biosorption efficacy of the bacterium was studied including adsorption isotherm modelling to comprehend the Cd uptake ability displayed by the bacterium. Adsorption isotherm models were employed to depict the best-fit model for Cd biosorption of the bacterium. Moreover, the bacterium displayed plant growth promotion potential detected through some in vitro screening tests like phosphate solubilization, zinc solubilization, hydrogen cyanide (HCN) production, indole acetic acid ((IAA) production, ammonia production and siderophore production, formerly (Saha et al. 2022). In continuation with the in vitro assay, an in vivo experiment was performed on Brassica napus to observe the growth-promoting efficacy of the bacterium in Cd-stressed environment including the detection of Cd content accumulated within the plant parts. Therefore, the current study was undertaken to attain the objectives of assessing Cd biosorption by Pseudomonas aeruginosa JCM 5962 through different adsorption isotherm models and evaluating the effect of bacterial inoculation on growth and Cd accumulation by the plant Brassica grown in Cd-contaminated soil.
Materials and methods
Source of the bacterial isolate
The Pseudomonas aeruginosa strain used in this study was obtained from the rhizospheric soil of arable land (25°42'19"N, 88°7'3"E) of Uttar Dinajpur district of West Bengal. Collected soil samples were initially incubated (35°C, 48 h) in metal-supplemented (100 μg/mL metal concentration) Tryptone Soya Broth (TSB) medium separately for each studied heavy metal. Standard serial dilution and subsequent pour plate method was followed to isolate bacterial colonies on culture plates. Through repeated subculturing of single bacterial colony, axenic bacterial culture was obtained and maintained in Tryptone Soya Agar (TSA) slants. A detailed study regarding isolation, characterization, heavy metal resistance and uptake, including whole genome sequence analysis of the studied strain, has been published in another work by the authors (Saha et al. 2022). According to the previous study, this bacterium showed Cd uptake efficiency and plant growth promotion potential (Saha et al. 2022).
Study of Cd biosorption and evaluation through adsorption isotherms
The bacterium was subjected to biosorption study to determine Cd adsorption capacity at an optimized temperature of 35°C and pH 7. Utilizing both Freundlich and Langmuir isotherm models (Al-Ghouti and Da’ana 2020), adsorption isotherms were evaluated in order to understand the mechanism of Cd biosorption by the bacterium. For this study, initially the bacterium was grown in freshly prepared TSB medium (pH 7) at 35°C. After 24 h of incubation, the cell masses were harvested using a mini centrifuge (REMI RM 02 Plus) in 2 mL Eppendorf tubes (RCF = 2000 g for 20 min), followed by a thorough wash in 1X phosphate buffer saline (PBS) solution (pH 7.4). Next to that, the obtained cell masses were dried in a hot air oven at 40–48°C temperature. Finally, the dried cellular biomasses were added separately at a rate of 0.1 g/mL into Cd solutions of different concentrations ranging from 50 to 300 μg/mL. The pH of the Cd solutions was optimized at pH 7 due to the maximum adsorption capacity. After 36 h of contact time at 35°C, the bacterial cell masses were separated from each of the Cd solutions by centrifugation. Through the inductively coupled plasma atomic emission spectroscopic (ICP-AES) analysis, Cd concentrations of each of the solutions were estimated employing an ARCOS, Simultaneous ICP Spectrometer (formulated by SPECTRO Analytical Instruments GmbH, Germany).The experiment was performed in triplicate for each of the studied concentrations of Cd and simultaneously, a control set without the bacterial cell mass was also prepared to avoid the confusion between metal precipitation and biosorption (Chatterjee et al. 2010). The biosorptive metal uptake (qe) by the bacterium was calculated using the following formula:
where, qe is the amount of metal ion biosorbed per gram of dead biomass (mg/g) at equilibrium, Ci denotes initial concentration of metal ions (mg/L) before adsorption, Cf is the final concentration of metal ions (mg/L) after adsorption, V is the volume of metal solutions in the flask (mL), and m is the weight of biosorbent in gram.
In order to evaluate the metal biosorption through adsorption isotherm modelling, Langmuir and Freundlich models were employed to detect the best fit model for Cd biosorption demonstrated by the bacterium. Adsorption isotherm modelling delineates the graphical representation of biosorptive metal uptake per unit weight of biosorbent against the residual metal ion concentration in the medium (Hoyle-Gardner et al. 2021). Out of the two models, Langmuir isotherm model (Langmuir 1918) describes the monolayer sorption which occurs at specific homogeneous or identical sites within the biosorbent, indicating no transmigration of the adsorbate. The linearized equation of the Langmuir model is as follows:
Where, qe represents the metal ion sorbed (mg/g), Ce denotes the equilibrium concentration of metal ion solutions (mg/L), qmax is the maximum amount of metal ion sorbed by biosorbent (mg/g), KL is the Langmuir constant (L/mg). Separation factor (RL) is another essential parameter of Langmuir isotherm spanning the degree of favourability of the adsorption. RL can be depicted by the following formula:
Where, Ci is the initial concentration of metal ions (mg/L) and KL represent the Langmuir constant (L/mg). The RL value may vary depending on the adsorption, such as RL>1 denotes unfavourable adsorption, RL = 1 indicates linear adsorption, while an intermediate value between 0 and 1 (i.e., 0 < RL<1) implies favourable adsorption performed by the adsorbents. The other model, Freundlich adsorption isotherm (Freundlich 1906) narrates a multilayer adsorption that take place at heterogeneous surface. The equation for expressing Freundlich adsorption isotherm is as follows:
Where, KF is the Freundlich constant, and 1/n is an empirical parameter related to the biosorption intensity.
In vivo study on Brassica napus grown in Cd-contaminated soil with bacterial effect
Primarily, the seeds of Brassica napus were surface sterilized by immersion in 70% ethanol for 10 min and subsequently washed three times with sterile distilled water. Then the seeds were soaked in 2% NaClO2 solution for 7–10 min and washed thoroughly with sterile distilled water (Ndeddy Aka and Babalola 2016). After sterilization, the seeds were immersed in bacterial broth cultures of 36 h (treated set) for 30 min, while in the control set, sterile distilled water was used instead of bacterial broth cultures. Bacterial broth cultures containing 108 cfu/mL (cfu = colony forming unit) were prepared by inoculating freshly revived bacteria in sterilized 100 mL Luria Broth (LB) medium followed by incubation at 35°C for 36 h. Before planting in pots, the seeds were allowed to germinate in a Petri dish on wet filter paper at room temperature. Both treated and control sets of seeds were sown at a depth of 2 cm in plastic trays containing 1 kg of autoclaved soil amended with Cd at a rate of 0.1 g/kg of soil. Bacterial suspensions (100 mL per tray) were added to the soil near the root zone during plantation and were applied twice at 10-day intervals. Each tray was watered twice daily and placed in a well-aerated shady area to avoid direct sunlight. The entire experiment consisted of three setups (including one control), each with three replicates. Each setup was carefully observed, and after germination of the seeds, roots lengths and shoots lengths were recorded at seven days intervals.
Statistical analysis
The obtained data regarding root and shoot lengths were then subjected to a one-way ANOVA analysis to detect any significant statistical difference in root length and shoot length between the control and treated sets (Prittesh et al. 2020). One way ANOVA was performed using SigmaPlot 12.0 to detect the significant differences on growth of Brassica at every seven days interval in presence of bacterial inoculants. Standard deviations of the obtained data regarding root and shoot lengths at seven-day intervals were calculated for each treatment group. Statistical significance was defined at the level of p < 0.001. Post-hoc tests were carried out using the most advantageous, stepwise operated Holm-Sidak method for all pairwise multiple comparisons between the treatment groups including the control (Motulsky 2010).
Estimation of Cd content accumulated in plant parts
The Cd accumulation within the plant parts of Brassica, grown in Cd contaminated soil inoculated with bacterial suspension of Pseudomonas aeruginosa JCM 5962 was estimated. After significant growth of the Brassica seedlings (after 45 days), all the plants were carefully removed from the respective trays and washed thoroughly with distilled water to dispel the adhered soil particles (Khanna et al. 2019). After washing, the entire plant body of each experimental setup was wrapped separately in aluminium foil and dried at 70°C for 72 h. For heavy metal analysis, 0.5 g of dried plant samples from the experimental set containing Cd and bacterial inoculants including the control (without bacteria) were taken separately in a 100 mL Erlenmeyer flask containing 24 mL mixture of HCl and HNO3 at 3:1 ratio. Then the mixture was heated on a water bath until the plant parts wholly dissolved in the acid mixture. After digestion, the mixture was allowed to cool and then filtered. The obtained filtrates were used for estimating Cd content using a high-resolution ICP-Mass Spectrometer (Element XR, Thermo Fisher Scientific, Germany).
Results
Cd biosorption study and adsorption isotherm evaluation
This study revealed a relatively higher affinity of bacterial cell surface for binding with Cd ions. The maximum biosorption capacity (qmax) of the bacterium was estimated as 149.25 mg/g. The biosorption data is depicted in Table 1.
Out of the studied two equilibrium models, Freundlich isotherm model was found to be the best fit model to describe the adsorption data. Table 2 depicts the parameters of both the Freundlich and Langmuir isotherm models for Cd biosorption. In Langmuir adsorption isotherm, the obtained RL value was below 1 (RL = 0.76) suggesting favourable adsorption of heavy metal ions onto the bacterial cell surface. Freundlich and Langmuir’s constants were also calculated from the corresponding plots and depicted in Figs. 1 and 2 respectively. The regression coefficients (R2) obtained from the Freundlich (R2 = 0.96), and Langmuir (R2 = 0.93) models for the metal Cd indicated the Freundlich model as the best fit model providing a better correlation with the experimental data.
Effect on the growth ofBrassica napus
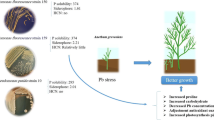
The studied bacterium depicted a significant growth-promoting effect on Brassica grown in Cd-contaminated soil. In each seven-day interval, the root lengths and the shoot lengths of the seedlings growing in each experimental tray were recorded, and the results strongly suggested increased length of both roots and shoots of the seedlings grown in Cd contaminated soil under the effect of Pseudomonas aeruginosa JCM5962 inoculants.
Statistical inference
Statistical analysis corroborated the significant increase in both root and shoot length of Brassica seedlings. The results of one-way ANOVA indicated a significant statistical difference (p < 0.001) in the root length of Brassica seedlings between the treatment groups at 7 days (F = 158.065, p < 0.001), 14 days (F = 331.603, p < 0.001) and 21 days (F = 283.380, p < 0.001) intervals. Similarly, in case of shoot lengths also, the results revealed a significant statistical difference (p < 0.001) at 7 days (F = 123.389, p < 0.001), 14 days (F = 346.241, p < 0.001) and 21 days (F = 324.799, p < 0.001) intervals between the treatment groups. Besides, all pairwise multiple comparison between the treatment groups including control at each seven-day intervals showed a statistically significant difference (p < 0.001) in both root and shoot lengths. The entire statistical data analysis delineated that the differences in the mean values among the treatment groups are greater than would be expected by chance, hence there exist a statistically significant difference at p < 0.001 level. The overall data has been shown in Table 3. The increased root and shoot length of Brassica seedlings grown in Cd-contaminated soil inoculated with bacterial suspension is depicted in Figs. 3 and 4.
Cd content estimation in Brassica
Inductively coupled plasma mass spectrometric (ICP-MS) analysis revealed a reduced amount of Cd content (i.e.; Cd bioreduction) in the plant samples grown in the presence of Pseudomonas aeruginosa JCM5962 compared to that of plants where no bacterial suspension was introduced during growth in Cd-contaminated soil. The reduction in Cd content on the effect of bacterial inoculation has been shown in Fig. 5. Approximately a 62.76% reduction in Cd content was found to occur in the presence of the bacterium.
Discussion
Cd toxicity in agricultural land is a significant concern nowadays (Rashid et al. 2023). In order to get rid of the hazardous impact of Cd toxicity, an eco-friendly, cost-effective strategy employing indigenous metalloresistant bacteria with tremendous Cd uptake potential is of utmost importance. Metal bioaccumulating plant growth promoting bacteria not only possess metal uptake potential but also control the mobilization of toxic metal ions from soil to plant, reducing metal translocation to plant parts using a variety of adaptive mechanisms like biosorption, bioaccumulation, biotransformation, etc. (Pramanik et al. 2021). The studied bacterium Pseudomonas aeruginosa JCM 5962 showed effective Cd biosorption potential in the present work. The maximum adsorption capacity was estimated to be 149.25 mg/g, which is relatively higher than other related strains revealed in another studies by different workers (Ansari and Malik 2007; Li et al. 2014; Liang et al. 2018; Xu et al. 2020). The adsorption data fitted best with Freundlich isotherm model with an R2 value of 0.96. Simultaneously, the RL value of 0.76 (< 1) depicted favourable adsorption of metal ions. Based on the Cd adsorption efficiency of the studied bacterium observed in this study, microbial consortium may be prepared for metal clean-up treatment of contaminated soil or industrial wastewater.
In vivo, pot experiment showed the improved growth of Brassica seedlings in Cd-contaminated soil under the effect of bacterial inoculants. Statistical analysis also demonstrated a significant (p < 0.001) increase in the root and shoot lengths compared to the non-inoculated control. Moreover, reduced Cd accumulation, i.e., Cd bioreduction (approximately 62.76%), was observed in Brassica grown under the bacterial effects. A similar reduction of metal contents within plant parts has also been observed upon the impact of other Pseudomonas species revealed in another recent study (Tripathi et al. 2023). Such enhanced growth with reduced metal accumulation within plants, performed by heavy metal resistant, plant growth promoting native bacteria has been reported in some studies (Jiang et al. 2008; Ren et al. 2019; Wang et al. 2022). The reduced metal accumulation within the plant in the presence of bacterial inoculants may be due to the bacteria-mediated metal extraction through different adaptive resistance mechanisms. Moreover, plant growth promoting (PGP) bacteria improve the phytoremediation efficiency by reducing the availability of heavy metals in soil through their innate mechanisms like biosorption, bioaccumulation, siderophore formation, complexation, etc. All these innate mechanisms of PGP bacteria are very effective in the immobilization of toxic heavy metals and hence reducing metal ion translocation into plant parts grown in contaminated fields. The immobilization and accumulation of heavy metals in such bacteria take place through some sequential steps like metal interaction, metal transport to the cytoplasm, metal reduction, and accumulation (Pande et al. 2022; Pham et al. 2022a). The mechanisms related to the enhanced plant growth under bacterial effects, observed in the present study, might be due to the facilitation in absorbing essential macronutrients through increased bioavailable levels provided by the bacteria. This has also been reported in another different study (Gupta et al. 2023). Besides, siderophore production accomplished by the bacteria play a pivotal role in the easy accessibility of soluble Fe2+ ions for plants (Alves et al. 2022). Additionally, PGP bacteria supply the most important phytohormone IAA that accelerates plant growth. Thus, these indigenous bacteria possess the potential to augment plant growth with enhanced food safety (Liu et al. 2022). The improved heavy metal remediation efficiency of such bacteria might also be accompanied by the secretion of chelating agents, acidification, and redox changes, reported in several studies (Sharma 2021; Gupta et al. 2023).
Conclusion
The present study represents the preliminary work regarding the in vivo plant growth promoting effect of an indigenous Pseudomonas aeruginosa strain. Cd biosorption efficiency coupled with plant growth promoting traits shown by the bacterium resulted in significant growth of Brassica seedlings under Cd stress. The Cd adsorption capacity of the bacterium was estimated as 149.25 mg/g, and the adsorption data fitted best with the Freundlich isotherm model (R2 value 0.96). Due to its tremendous Cd biosorption potential, this bacterium may be employed in creating a microbial consortium for the removal of metal contaminants from polluted environments. Further, detailed analysis needs to be carried out regarding the quantitative estimation of hormones and enzymes responsible for improved plant growth under bacterial effects. Additionally, approximately 62.76% reduction in Cd content has been found in the plant parts grown in Cd-contaminated soil under the effect of bacterial inoculants. Hence, investigation is also required to understand the molecular mechanisms behind such enhanced growth with reduced metal uptake within the plant. Moreover, the biocontrol potential of the bacterium against other plant pathogenic fungi may also be examined. Therefore, in order to reduce the dependence on harmful agrochemicals for crop yield, the application of such heavy metal resistant plant growth-promoting bacteria in arable lands may be helpful as eco-friendly biofertilizers for sustainable agriculture and also for enhanced food security.
Data Availability
All data generated or analysed during this study are included in this article.
Code Availability
Not applicable.
References
Adediran GA, Ngwenya BT, Mosselmans JFW, Heal KV, Harvie BA (2015) Mechanisms behind bacteria induced plant growth promotion and Zn accumulation in Brassica juncea. J Hazard Mater 283:490–499
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383
Alves ARA, Yin Q, Oliveira RS, Silva EF, Novo LAB (2022) Plant growth-promoting bacteria in phytoremediation of metal-polluted soils: current knowledge and future directions. Sci Total Environ 838:156435
Ansari MI, Malik A (2007) Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresour Technol 98(16):3149–3153
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46(3):237–245
Chatterjee SK, Bhattacharjee I, Chandra G (2010) Biosorption of heavy metals from industrial waste water by Geobacillus thermodenitrificans. J Hazard Mater 175(1–3):117–125
Etesami H (2018) Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: mechanisms and future prospects. Ecotoxicol Environ Saf 147:175–191
Freundlich HMF (1906) Over the Adsorption in Solution. J Phys Chem 57:385–471
Goswami D, Thakker JN, Dhandhukia PC (2016) Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food & Agriculture 2(1):1127500
Gupta R, Khan F, Alqahtani FM, Hashem M, Ahmad F (2023) Plant growth–promoting Rhizobacteria (PGPR) assisted bioremediation of Heavy Metal Toxicity. " Applied Biochemistry and Biotechnology
Hoyle-Gardner J, Jones W, Badisa VLD (2021) Lead metal biosorption and isotherms studies by metal-resistant Bacillus strain MRS-2 bacterium. J Basic Microbiol 61(8):697–708
Jadhav JP, Kalyani DC, Telke AA, Phugare SS, Govindwar SP (2010) Evaluation of the efficacy of a bacterial consortium for the removal of color, reduction of heavy metals, and toxicity from textile dye effluent. Bioresour Technol 101(1):165–173
Jiang C-y, Sheng X-f, Qian M, Wang Q-y (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72(2):157–164
Khanna K, Jamwal VL, Gandhi SG, Ohri P, Bhardwaj R (2019) Metal resistant PGPR lowered cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci Rep 9(1):5855
Langmuir I (1918) The adsorption of gases on Plane Surface of Glass, Mica and Platinum. J Am Chem Soc 40(9):1361–1403
Li Y, Wu Y, Wang Q, Wang C, Wang P (2014) Biosorption of copper, manganese, cadmium, and zinc by Pseudomonas putida isolated from contaminated sediments. Desalination Water Treat 52(37–39):7218–7224
Liang Y, Chen J, Mei J, Chang J, Wang Q, Wan G, Yin B (2018) Characterization of Cu and Cd biosorption by Pseudomonas sp. strain DC-B3 isolated from metal mine soil. Int J Environ Sci Technol 16
Liu A, Wang W, Zheng X, Chen X, Fu W, Wang G, Ji J, Jin C, Guan C (2022) Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere 302:134900
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci 7
Manoj SR, Karthik C, Kadirvelu K, Arulselvi PI, Shanmugasundaram T, Bruno B, Rajkumar M (2020) Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J Environ Manage 254:109779
Marchiol L, Assolari S, Sacco P, Zerbi G (2004) Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ Pollut 132(1):21–27
Mathivanan K, Chandirika JU, Vinothkanna A, Yin H, Liu X, Meng D (2021) Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment – a review. Ecotoxicol Environ Saf 226:112863
Mohapatra RK, Parhi PK, Pandey S, Bindhani BK, Thatoi H, Panda CR (2019) Active and passive biosorption of Pb(II)using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: kinetics and isotherm studies. J Environ Manage 247:121–134
Motulsky H (2010) “Power of Holm’s multiple comparison testing compared to others." from https://stats.stackexchange.com/q/109
Ndeddy Aka RJ, Babalola OO (2016) Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int J Phytoremediation 18(2):200–209
Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27(2–3):313–339
Pande V, Pandey SC, Sati D, Bhatt P, Samant M (2022) Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. " Front Microbiol 13:824084
Pham VHT, Kim J, Chang S, Chung W (2022) Bacterial biosorbents, an efficient heavy Metals Green Clean-Up Strategy: prospects, Challenges, and Opportunities. Microorganisms 10. 610. https://doi.org/10.3390/microorganisms10030610
Pramanik K, Mandal S, Banerjee S, Ghosh A, Maiti TK, Mandal NC (2021) Unraveling the heavy metal resistance and biocontrol potential of Pseudomonas sp. K32 strain facilitating rice seedling growth under Cd stress. Chemosphere 274:129819
Prittesh P, Avnika P, Kinjal P, Jinal HN, Sakthivel K, Amaresan N (2020) Amelioration effect of salt-tolerant plant growth-promoting bacteria on growth and physiological properties of rice (Oryza sativa) under salt-stressed conditions. Arch Microbiol 202(9):2419–2428
Rai PK, Lee SS, Zhang M, Tsang YF, Kim K-H (2019) Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ Int 125:365–385
Rashid A, Schutte BJ, Ulery A, Deyholos MK, Sanogo S, Lehnhoff EA, Beck L (2023) Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 13(6):1521
Ren X-M, Guo S-J, Tian W, Chen Y, Han H, Chen E, Li B-L, Li Y-Y, Chen Z-J (2019) Effects of Plant Growth-Promoting Bacteria (PGPB) inoculation on the growth, antioxidant activity, Cu Uptake, and Bacterial Community structure of rape (Brassica napus L.) grown in Cu-Contaminated Agricultural Soil. Frontiers in Microbiology 10
Rizvi A, Ahmed B, Zaidi A, Khan MS (2020) Biosorption of heavy metals by dry biomass of metal tolerant bacterial biosorbents: an efficient metal clean-up strategy. Environ Monit Assess 192(12):801
Rizwan M, Ali S, Zia Ur Rehman M, Rinklebe J, Tsang DCW, Bashir A, Maqbool A, Tack FMG, Ok YS (2018) Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ 631–632:1175–1191
Saha J, Dey S, Pal A (2022) Whole genome sequencing and comparative genomic analyses of Pseudomonas aeruginosa strain isolated from arable soil reveal novel insights into heavy metal resistance and codon biology. Curr Genet 68(3):481–503
Sharma P (2021) Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: an update. Bioresour Technol 328:124835
Srivastava V, Sarkar A, Singh S, Singh P, de Araujo ASF, Singh RP (2017) Agroecological responses of Heavy Metal Pollution with special emphasis on Soil Health and Plant Performances. Front Environ Sci 5(64)
Tripathi M, Kumar S, Makarana G, Goel R (2023) Metal-tolerant Bioinoculant Pseudomonas putida KNP9 mediated enhancement of soybean growth under heavy metal stress suitable for Biofuel production at the metal-contaminated site. Energies 16(11):4508
Vishnupradeep R, Bruno LB, Taj Z, Karthik C, Challabathula D, Tripti A, Kumar H, Freitas, Rajkumar M (2022) Plant growth promoting bacteria improve growth and phytostabilization potential of Zea mays under chromium and drought stress by altering photosynthetic and antioxidant responses. Environ Technol Innov 25:102154
Wang Y, Narayanan M, Shi X, Chen X, Li Z, Natarajan D, Ma Y (2022) Plant growth-promoting bacteria in metal-contaminated soil: current perspectives on remediation mechanisms. Front Microbiol 13:966226
Xu S, Xing Y, Liu S, Hao X, Chen W, Huang Q (2020) Characterization of Cd(2+) biosorption by Pseudomonas sp. strain 375, a novel biosorbent isolated from soil polluted with heavy metals in Southern China. Chemosphere 240:124893
Acknowledgements
The authors would like to acknowledge DST-SAIF of IIT-Bombay for providing sophisticated analytical services (ICP-AES and ICP-MS). The authors also thank Mr. Vivek Roy, Mr. Barnan K. Saha, Ms. Samarpita Adhikary, and Mr. Prajesh Dutta for their support and cooperation.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
JS: Conceptualization, Methodology, Formal analysis and Investigation, Writing - Original Draft preparation, Review & Editing, Data validation. AP: Conceptualization, Supervision, Investigation, Methodology, Writing - Review & Editing, Validation, Visualization, Resources.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors consented to publish the article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saha, J., Pal, A. Cadmium biosorption and plant growth promotion efficacy of a metalloresistant Pseudomonas sp. unveils augmented growth with reduced metal accumulation in Brassica napus L.. Vegetos (2023). https://doi.org/10.1007/s42535-023-00724-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42535-023-00724-z