Abstract
The concern with the environment and the depletion of natural resources has aroused the interest for the rational use and recycling of water. Therefore, this study evaluated the capacity of the Trametes sp. M3 isolate in the bioremediation of Reactive Blue (RB) 268 dye and its potential for use as an adsorbent in the treatment of textile effluents. In a solid medium containing RB 268, the discoloration rate was 1.00 and the growth rate was 1.4 cm/day. When evaluated in the in vivo biodiscoloration process, 100% of the dye lost its color after 120 h. The oxidative enzyme laccase was found in cultures containing the dye with high activity, indicating that it underwent induction. The chromatogram after cultivation of the fungus showed that there was a change in the structure of RB 268. The mycelium of the culture with the dye was analyzed by FTIR, pointing to an adsorption of RB 268 or its metabolites despite the absence of the color. In the biosorption, the best results were obtained when the mycelium was treated with HCl. The toxicity of the medium decreased after the cultivation of the fungus allowing the survival of the microcrustaceans in the acute toxicity bioassay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The synthetic dyes are used in several types of industries, mainly textiles. Approximately 100,000 types of dyes are available for commercial use, and more than 8 × 105 tons/year of synthetic dyes is produced worldwide (Heinfling et al. 1998). In dyeing processes, 10 to 20% of dyes are lost due to poor attachment to the fibers (Arantes et al. 2006). Despite the economic importance of the textile industry, this activity consumes about 15% of the water used in the industrial sector, generating a large volume of waste with a high organic load and with difficult degradation (Kunz et al. 2002). In addition to color, a variety of chemicals such as surfactants, oils, salts, and greases are present in the textile effluent, contributing to its chemical complexity (Wang et al. 2002). The chemical structure of synthetic dyes is difficult to degrade, posing a danger to man and the environment (Chenaux et al. 2014). The removal of color in industrial effluents is extremely important, considering that color, by preventing the passage of light, decreases photosynthesis and oxygen in the water, essential factors for the survival of aquatic organisms (Chequer et al. 2013).
The discoloration can be carried out through adsorption, filtration, precipitation, chemical degradation, photodegradation, coagulation-flocculation, ion exchange, oxidation, and electrochemical methods, but with low efficiency in color removal. Biological processes are alternatives to conventional treatments and have become interesting due to low cost, high efficiency and, in particular, for not using chemical substances during the process (Li et al. 2009). Bacteria, algae, yeasts, and filamentous fungi have the ability to discolor and degrade a large number of dyes (El-Sheekh et al. 2009; Kaushik and Malik 2009; Saratale et al. 2011). Highlight has been given to white wood rot fungi that produce enzymes capable of degrading the complex structure of lignin (Brugnari et al. 2018). The molecules that have a structure similar to lignin such as some pesticides, polycyclic aromatic hydrocarbons, synthetic polymers, and synthetic dyes, among others, can also be degraded by the action of enzymes due to their nonspecific action (Asgher et al. 2008).
The role of the oxidative enzymes from white rot fungi has been extensively studied (Araújo et al. 2017; Contato et al. 2020), and the catalytic action promotes not only discoloration but also biotransformation and mineralization. The great diversity of dye structures invites to the discovery of microorganism species capable of bioremediate these pollutants, and several studies demonstrate the importance of enzymatic degradation in bioremediation processes. Besides that, biosorption has been considered an advantageous alternative since activated carbon can be a good adsorbent of dyes from different classes, but its high acquisition and regeneration cost discourages its use (Hubbe et al. 2012). In the last two decades, the biosorption of pollutants from aquatic environments by the biomass of fungi, bacteria, and algae has been considered an adequate method of effluent bioremediation (Russoa et al. 2010).
The synthetic dye Reactive Blue (RB) 268, also known as Cibacron Brilliant Blue FN-G, is widely used in jeans laundries, whose effluent after treatment maintains a large amount of color (Sun et al. 2020). The dyes most used on cotton garments are the reactants whose main characteristic is the formation of covalent bonds with the cellulose fiber. Of all types of dyes, the reactive ones are the most permanent, since the covalent bond with the cellulose or with the protein molecule allows the dye to be part of the fiber, providing greater color stability and washability of the garment (Ríoz-Gómez et al. 2018). Therefore, reactive dyes for cotton are the most used in the textile industry (Phan et al. 2020).
The concern with the environment and the depletion of natural resources has aroused interest for the rational use and recycling of water, requiring proper treatment of effluents. Therefore, the present study had as an innovative nature the evaluation of the bioremediation capacity of Trametes sp. M3 isolate against the Reactive Blue 268 dye, as well as its potential for use as an adsorbent in the treatment of textile effluents.
2 Materials and Methods
2.1 Synthetic Dye and Chemicals
Reactive Blue (RB) 268 dye was obtained from AGS Química (Barueri, Sao Paulo, Brazil) as trade name Briiliant Blue Reafix BFN-G. RBBR (Remazol Brillant Blue R) and Poly R 478 were obtained from the same place. Dye solution was prepared by dissolving 0.01 g in 100 mL of distilled water and filtered by 0.2 μm membrane (Millipore, Bedford, MA, USA) after use.
Methanol HPLC grade (Sigma-Aldrich, St. Louis, MO, USA) was used in chromatographic analysis, and all other chemicals used throughout this study were analytical grade.
2.2 Fungus
The white rot fungus M3 was collected from decaying wood and isolated at the Laboratory of Biochemistry of Microorganisms of the State University of Maringá (UEM, Maringá, Brazil). This isolate was identified as Trametes sp. by molecular methods at the Division of Microbial Resources of the Chemical, Biological and Agricultural Pluridisciplinary Research Center (CPQBA) at University of Campinas (UNICAMP, Campinas, Brazil). It can be found at the fungi library of Laboratório de Bioquímica de Microrganismos (LBM) from State University of Maringá (UEM, Maringá, Brazil). Its sequence is deposited in GenBank, and its access code is MT538335. The phylogeny and identification were already described by Araújo et al. (2017). The strain was cultivated on potato dextrose agar (PDA) Petri dishes for up to 2 weeks at 28 °C. When the Petri dish was fully covered, mycelial plugs measuring 15 mm in diameter were used as inoculum for liquid cultures.
2.3 Pellet Preparation and Culture Conditions
Five mycelial plugs (15 mm of diameter) from Trametes sp. M3 were obtained of Petri dishes with PDA fully covered with mycelium and transferred to 250 mL Erlenmeyer flasks containing 50 mL of potato-dextrose broth. The cultures were incubated at 28 °C and 120 rpm for 7 days. The mycelium pellets were harvested aseptically, washed with distilled water, and used in discoloration and biosorption experiments.
The cultures were carried on 50 mL of Vogel medium (Vogel 1956), glucose (1%), and RB 268 (100 mg/L). Homogenized and standardized (460 mg) pellets from shaken cultures were used to evaluate the discoloration and enzymatic activity. The flasks were incubated at 28 °C in a rotatory shaker at 120 rpm for 5 days. Samples were removed at regular periods and centrifuged for 10 min at 10.000 g at 4 °C. The supernatants were used to evaluate lignin-modifying enzymes, dye concentration and RB 268 metabolites. The mycelia were washed with distilled water and dried to a constant weight at 50 °C. The fungus was also inoculated in medium liquid without dye. Abiotic controls with and without RB 268 were carried. Results were expressed as the mean of three different cultures.
2.4 Enzyme Assays
Laccase activity (EC 1.10.3.2) was measured with 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid; ABTS) in 50 mM sodium acetate buffer (pH 4.5). Oxidation of ABTS was determined by the increase in absorbance at 420 nm (ε = 36 mM−1 cm−1) (Brugnari et al. 2018). Manganese peroxidase activity (MnP, EC 1.11.1.13) was assayed spectrophotometrically by following the oxidation of 1 mM MnSO4 in 50 mM sodium malonate, pH 4.5, in the presence of 0.1 mM H2O2. Manganic ions, Mn3+, form a complex with malonate, which absorbs at 270 nm (ε = 11.59 mM−1 cm−1) (Wariishi et al. 1992). The lignin peroxidase activity (EC 1.11.1.14) was determined by spectrophotometric measurement at 310 nm of the H2O2-dependent veratraldehyde formation (ε = 9.3 mM−1 cm−1) from veratryl alcohol (Tien and Kirk 1983). The aryl-alcohol oxidase (AAO) (EC 1.1.3.7) was estimated as oxidation of veratryl alcohol at 5 mM to veratraldehyde (A310 = 9300 M−1 cm−1) in phosphate buffer 100 mM (pH 6.0) (Muñoz et al. 1997). The released reducing sugars were quantified by the 3,5-dinitrosalicylic acid reagent (Miller 1959). Enzyme activities were expressed as international enzymatic units (U, μmol min−1) per L (U/L).
2.5 Analysis of Reactive Blue 268 by Ultraviolet-Visible Spectra
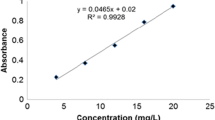
The dye concentration on samples was measured using a spectrophotometer UV/VIS Shimadzu UV-1601 PC (Shimadzu Corp., Kyoto, Kyoto, Japan). The discoloration rate was determined at 635 nm, and percentage of discoloration of the dye was calculated as
where Ci and Cf represent the initial and residual concentrations of dye, respectively. The average values of replicates were used in calculations.
2.6 High-Performance Liquid Chromatography Analysis
The HPLC analysis of filtered samples (0.2 μm) (Millipore, Bedford, MA, USA) was performed on a Dionex Ultimate 3000 HPLC system (Dionex, Idstein, Germany) equipped with UltiMate 3000 Pump, UltiMate 3000 Autosampler Column Compartment, UltiMate 3000 Photodiode Array Detector and Chromeleon software. A reversed phase Acclaim® 120 column, C18 5 m 120 Å (4.6 mm × 250 mm), was used in the experiments. The column was maintained at 40 °C throughout the analysis ,and the detection was at 260 nm. The mobile phase used was a mixture of methanol-water (50:50, v/v) with a flow rate of 1 mL min.−1. The injection volume was 10 μL.
2.7 Characterization of Mycelium by Fourier Transform Infrared Spectroscopy
FTIR spectra of dried mycelia harvested from culture media after 24 and 120 h were analyzed on a Shimadzu IRAffinity-1 FTIR spectrophotometer in the 4000–400 cm−1 region using potassium bromide (KBr) pellets.
2.8 Bioassay of Toxicity
Artemia salina eggs (100 mg) were hatched in a recipient with artificial sea water that was prepared with 10 g of commercial salt and 500 mL of distilled water. The eggs were incubated at 28 °C in the dark with low aeration. After 48 h the content of the recipient was transferred to a separation funnel and the superior side was illuminated. The phototropic nauplii were collected, and ten larvae were transferred to each well of 24-well cell culture plate using a Pasteur pipette. An aliquot of samples after discoloration by the fungus (2 mL) was added to each well. The same volume of dye and artificial sea water were used as positive control and as negative control respectively. After 24 h, the dead nauplii were counted and the survival rates determined. The data were plotted, and the LC50, the dose in which 50% of nauplii died, was calculated by graphical interpolation.
2.9 Biosorption Assay
After a 5-day incubation period at 28 °C on an orbital shaker (120 rpm), the mycelial pellets were harvested by filtering in a sieve and thoroughly washed with distilled water to collect the live biomass and a part of the biomass was heat-treated by autoclaving at 121 °C for 15 min. After autoclaving the pellets were submerged in acid (HCl 0.1 M), basic (NaOH 0.1 M), and saline solutions (NaCl 0.1 M) for 1 h and then washed successively. Approximately 73 mg of dry weight was placed in Erlenmeyer flasks (125 mL) containing 100 mg of RB 268 where they stayed under shaking for 24 h, at 120 rpm and 28 °C. After, the samples were centrifuged to analyze.
The supernatant was evaluated spectrophotometrically at 635 nm. The amount of adsorbed dye into mycelium was calculated according to the equation [10]:
where q is the sorption capacity (mg g−1); C0 and Cf are concentrations (mg) of RB 268 in the initial solution and after biosorption, respectively; V is volume of the aqueous phase (L); and M is the amount of biomass (g).
2.10 Scanning Electron Microscopy Analysis
The changes in the cell wall of the pellets after the heat treatments, acid solutions, and saline were observed by scanning electron microscopy (SEM) analysis (Shimadzu SS-550 Superscan) (Shimadzu Corp, Kyoto, Kyoto, Japan). The samples were fixed in a 2.5% glutaraldehyde solution and 0.1 M cacodylate buffer for 72 h at 4 °C. Thereafter, the samples were washed twice in 0.1 M cacodylate buffer. Dehydration was done in increasing gradient of ethanol. After the samples passed to step critical and treated with gold using a sputter Shimadzu IC-50 as described by Yang et al. (2008) with modifications.
2.11 Statistical Analysis
The data were analyzed by Student’s t test and one-way ANOVA with Tukey’s multiple comparison test (P < 0.05) using the GraphPad Prism Program (GraphPad Software, San Diego, CA, USA).
3 Results
3.1 Bioremediation of the Dyes
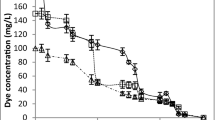
The ability of Trametes sp. M3 to discolor the synthetic dyes was evaluated using RBBR, Poly R 478, and RB 268 in solid medium. The results obtained are shown in Table 1 and Fig. 1. The medium containing RB 268 provided greater growth as well as a greater discoloration coefficient. The evaluation in liquid medium was followed between 12 and 120 h after inoculation of the pellets. The Trametes sp. M3 demonstrated the ability to discolor 94.4% of the RB 268 dye from the culture medium in 48 h (Fig. 2, tube 4). During this period, dye adsorption to biomass occurred and the complete discoloration of the mycelium occurred in 120 h (Fig. 2, tube 5). The activity of the laccase enzyme was detected in the two cultures evaluated: cultures with dye and control cultures (without RB 268). The highest enzyme activity was observed in the cultures with dye after 72 h, reaching 515.67 U/L in 5 days. The color percentage found at this time was only 1.6% after 72 h. Manganese peroxidase, lignin peroxidase and aryl alcohol oxidase were not detected under the studied conditions.
3.2 HPLC Analysis of Discolored Samples
Chromatographic analyses of the samples after 120 h of cultivation are shown in Fig. 3. The chromatogram shows a change in the profile of the samples of the cultures when compared with the abiotic control containing the dye and with the dye solution. Peaks with the following retention times, 2.560, 2.873, 2.960, 4.127, 5.697, and 7.467, were detected after treatment with the fungus.
3.3 Fourier Transform Infrared
The biomass from the fungus cultures with and without dye at 24 and 120 h was analyzed by FTIR. The spectra are shown in Fig. 4. Although very similar in relation to the peaks found, peak number 1317 appears only in 24 h in cultures containing the dye and the peak around 920, presents in cultures with dye, and does not appear in the biomass spectrum of cultures without dye. In the region between 500 and 1000 there is a difference in the profile found in the biomass of cultures without dye when compared with the biomass of cultures with dye.
3.4 Toxicity Bioassay
The toxicity of the samples was verified in Artemia salina larvae. Nauplii were dipped for 24 h in dye samples treated with the fungus and in different concentrations. The same procedure was carried out with dye solution and with the culture medium without the dye. The LD50 values obtained were 19.25 ± 0.23 mg/L of the dye solution treated with the fungus, 15.9 ± 1.37 mg/L of the solution without the dye (abiotic culture medium) and 15.01 ± 1.67 mg/L of the solution with culture medium only. There was a significant difference (P < 0.05) between the LD50 of the treated dye and the other evaluated solutions, showing that the fungal growth decreased the toxicity of the medium.
3.5 Biosorption
The adsorption capacity of active and inactive biomass and active and inactive mycelium was subjected to three treatments: acid, alkaline, and saline. Table 2 shows the results of the dye biosorption study. Greater amount of dye was adsorbed by both active and inactive biomass and when they were treated with HCl, with a significant difference between untreated active biomass and the treated with HCl (P < 0.05) and the inactive biomass in treatment and the treated (P < 0.001). There was no significant difference when the active biomass was treated with NaCl but the inactive biomass treated with the salt adsorbed 78.66% when compared with 18.83 of the inactive biomass. The treatment of active and inactive biomass with NaOH resulted in low adsorption.
The analyses performed by scanning electron microscopy show that the acid solution caused the formation of cavities in the mycelium (Fig. 5B), A parede celular hifal e as biomoléculas associadas (mais os ácidos orgânicos dissolvidos liberados) formam uma estrutura/organização tridimensional complexa que pode apresentar diferentes níveis de acessibilidade ao ambiente aquático perturbado. In the mycelium treated with the basic solution, the hyphae were grouped (Fig. 5D). The hyphae immersed in the saline medium formed cavities, but of smaller sizes (Fig. 5C) and hyphae without chemical treatment formed aggregates (Fig. 5A).
4 Discussion
The colored effluents when discharged untreated into aquatic bodies can be harmful to human and animal health and cause damage to the environment. Within the several types of treatments proposed, the biological one, promoted by the action of microorganisms, can occur through the action of its metabolites or by the adsorption phenomenon. The fungus Trametes sp. M3 was able to grow in a solid medium containing the RB 268 dye, and the halo of discoloration was superior to its growth showing the potential of this fungus for use in colored effluent bioremediation processes. Some dyes can inhibit the growth of fungi, which limits their use for the treatment processes of contaminated environments.
The dyes are classified according to their chromophore group, and RB 268 is a triphenodioxazine, according to this classification (Table 1). High percentages of discoloration occurred in the liquid cultures of Trametes sp. M3, and these values are higher than those found in the literature for some basidiomycetes. Scientific articles show that discoloration is dependent on factors such as type of dye, pH, temperature, fungus, and cultivation time. The white rot fungus Phanerochaete chrysosporium discolored 87% of artificial effluent with acid dyes after 7 days and Pleurotus ostreatus, 66% after 14 days (Faraco et al. 2009). In other studies, Pleurotus sajor-caju and Pleurotus florida discolored 80% and 75%, respectively; the Black astrazone dye after 24 h (Yesilada et al. 2003), P. chrysosporium 50.6 and 27.8% the Congo red dye and Poly R-478, respectively; and Pycnoporus cinnabarinus discolored 50.6% and 55.7% for the same dyes within 8 days (Diwaniyan et al. 2010).
Among the microorganisms used in the treatment of effluents, the fungi of white wood rot have been highlighted for their efficient mechanisms involved in the degradation of xenobiotics such as, for example, the cytochrome P-450 monooxygenase and ligninolytic enzymes involved in the degradation of aromatic hydrocarbons (Coelho-Moreira et al. 2013). The presence of laccase in Trametes sp. M3 appears to be related to the discoloration process of the dye solution. Some white rot fungi produce all the ligninolytic enzymes used in the degradation of lignin and dyes, while others produce only one or two enzymes (Hatakka 1994) and laccase is generally the main enzyme present in the discoloration processes (Saito et al. 2003).
The dye absorption spectrum and HPLC analysis show that after cultivation, there was, in addition to the alteration of the chromophore group (assessed by the absence of color), a structural change that was noticeable due to the formation of several peaks (Fig. 3) between the retention time interval of the evaluated dye. According to Chen et al. (2003) the adsorption and degradation processes can be analyzed through the disappearance or reduction of the absorption peak represented by the dye and the appearance of new peaks. Other authors such as Shedbalkar et al. (2008) and Ramya et al. (2007) showed by HPLC analysis the appearance of new absorption peaks regarding the metabolites of degradation of dyes studied in their works.
The properties of the cell wall surface are crucial for biosorption processes. The fungal cell wall is composed of polysaccharides (chitin and chitosan), proteins, lipids, and melanin with several functional groups (such as amino, carboxyl, thiol, and phosphate groups) that are able to bind to the dye molecules (Yesilada et al. 2003). The FTIR analysis of the mycelium obtained in culture without dye showed the common peaks in fungal preparations (Arica and Bayramoğlu 2007; Kalyani et al. 2009). In low-frequency regions between 900 cm−1 and 675 cm−1, more important bands are found involved in the structure of aromatic compounds (Maciel et al. 2013) and peaks around 535 cm−1 can indicate C-C=O curvature of carboxylic acid (Das et al. 2012). Although with the naked eye the mycelium has lost its color in 120 h, FTIR analysis show that the dye and its metabolites may have become adsorbed to the mycelium (Fig. 4) since a peak at 920 cm−1, indicating =C-H of aromatic groups, appears in the FTIR of the mycelium grown in medium containing dye after 120 h of treatment. This peak was not identified in the control.
The adsorption results (Fig. 5; Table 2) showed that the treatments improve the ability of mycelium to adsorb polluting materials. The results with untreated active biomass suggest the participation of fungus enzymes in the dye discoloration processes, since the percentage of discoloration of the solution was high and the amount of dye adsorbed was low, which reinforces the hypothesis that the laccase enzyme participated in the process of discoloration of the dye in liquid media during cultivation. Intracellular enzymes and other degradation systems may have altered the structure of the dye adsorbed on mycelial cells, possibly degrading it or altering its chromophore group. Some authors have already reported the reduction of recalcitrant compounds by oxidative enzymes present in cytochrome P450 (Subramanian and Yadav 2009), such as in the biotransformation of malachite green by the fungus Cunninghamella elegans (Cha et al. 2001). When the mycelium is disturbed with a base, it reacts against proton-releasing processes (like a range of acids and structures in different levels of organization, membrane proteins, oligopeptides, and others). The hifal cell wall and the associated biomolecules (plus the released organic acids dissolved) form a complex tridimensional structure that is able to show different levels of accessibility to the disturbed aquatic environment (Almeida et al. 2016).
Due to their nature, dyes are highly detectable with the naked eye, being visible in some cases even at concentrations as low as 1 ppm. Spectrophotometric analysis allow an efficient evaluation of color removal and can be used to control the dilution of dye in river waters; however, they do not detect whether toxic compounds have been degraded, generating major problems such as accumulation and bioavailability (Guaratini and Zanoni 2000). The color change in a dye does not mean that there has been a change in its structure to the extent that its toxicity has been eliminated. Toxicity tests help to evaluate the efficiency of environmental pollutant treatments. The acute toxicity bioassays involve organisms sensitive to small amounts of toxic material. The short-lived provide a quick estimate of the lethal toxic effects of pollutants on aquatic organisms. Its main objective is to determine the lethal doses (LD50) in reduced exposure time, which generally varies between 24 to 96 h. The LD50 is defined as the concentration of the toxic agent that causes 50% mortality in the population of organisms subjected to the test. The tests of this nature provide basic information becoming an indicator of environmental clean-up. The LD50 of the artificial effluent treated with Trametes sp. M3 was higher, indicating that the fungal treatment resulted in a decrease in the toxicity of the medium, although there was no significant difference between the controls.
5 Conclusion
The analysis confirmed that the isolate Trametes sp. M3 is able to degrade the Reactive Blue 268 dye. The discoloration is strongly related to the presence of the laccase secreted by the fungus in the medium containing the dye and by the adsorption mechanism observed in the artificial dye solution. Compounds were generated from the biodegradation of the dye confirmed by new peaks found through HPLC analysis. FTIR identified the chemical groups adsorbed by the mycelium, confirming the structure of the dye used in the jeans laundry. Bioassays have shown that the metabolites formed after treatment with the fungus are less toxic than the original solutions. Our data show that our studied fungus has great potential to be used as an alternative in the treatment of textile effluents.
References
Almeida, V. R., Szpoganicz, B., Chou, L., Baert, K., Hubin, A., & Bonneville, S. (2016). Equilibrium and out-of-equilibrium investigation of proton exchange and CuII and ZnII complexation on fungal mycelium (Trametes hirsuta). Journal of the Brazilian Chemical Society, 27.
Arantes, V., Baldocchi, C., & Milagres, A. M. F. (2006). Degradation and decolorization of a biodegradable-resistent polymeric dye by chelator-mediated Fenton reactions. Chemosphere, 63, 1764–1772.
Araújo, C. A. V., Maciel, G. M., Rodrigues, E. A., Silva, L. L., Oliveira, R. F., Brugnari, T., Peralta, R. M., & Souza, C. G. M. (2017). Simultaneous removal of the antimicrobial activity and toxicity of sulfamethoxazole and trimethoprim by white rot fungi. Water, Air, & Soil Pollution, 228, 341.
Arica, M. Y., & Bayramoğlu, G. (2007). Biosorption of Reactive Red-120 dye from aqueous solution by native and modified fungus biomass preparations of Lentinus sajor-caju. Journal of Hazardous Materials, 149, 499–507.
Asgher, M., Bhatti, H., Ashradf, M., & Legge, R. (2008). Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation, 19, 771–783.
Brugnari, T., Pereira, M. G., Bubna, G. A., Freitas, E. N., Contato, A. G., Corrêa, R. C. G., Castoldi, R., Souza, C. G. M., Polizeli, M. L. T. M., Bracht, A., & Peralta, R. M. (2018). A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Science of the Total Environment, 634, 1346–1351.
Cha, C. J., Doerge, D. R., & Cerniglia, C. E. (2001). Biotransformation of malachite green by the fungus Cunninghamella elegans. Applied and Environmental Microbiology, 67, 4358–4360.
Chen, K. C., Wu, J. Y., Liou, D. J., & Hwang, S. C. J. (2003). Decolorization of the textile dyes by newly isolated bacterial strains. Journal of Biotechnology, 101, 57–68.
Chenaux, P. R., Lalji, N., & Lefebvre, D. D. (2014). Trametes meyenii possesses elevated dye degradation abilities under normal nutritional conditions compared to other white rot fungi. AMB Express, 4, 74.
Chequer, F.M.D., Oliveira, G.A.R., Ferraz, E.R.A., Cardoso, J.C., Zanoni, M.V.B., & Oliveira, D.P. (2013). Textile dyes: dyeing process and environmental impact, eco-friendly textile dyeing and finishing, Dr. Melih Gunay (ed), InTech.
Coelho-Moreira, J. S., Bracht, A., Souza, A. C. S., Oliveira, R. F., Sá-Nakanishi, A. B., Souza, C. G. M., & Peralta, R. M. (2013). Degradation of diuron by Phanerochaete chrysosporium: role of ligninolytic enzymes and cytochrome P450. BioMed Research International, 2013, 1–9.
Contato, A. G., Inácio, F. D., Brugnari, T., Araújo, C. A. V., Maciel, G. M., Haminiuk, C. W. I., Peralta, R. M., & Souza, C. G. M. (2020). Solid-state fermentation with orange waste: optimization of laccase production from Pleurotus pulmonarius CCB-20 and decolorization of synthetic dyes. Acta Scientiarum. Biological Sciences, 42, e52699.
Das, D., Basak, G., Lakshmi, V., & Das, N. (2012). Kinetics and equilibrium studies on removal of zinc(II) by untreated and anionic surfactant treated dead biomass of yeast: Batch and column mode. Biochemical Engineering Journal, 64, 30–47.
Diwaniyan, S., Kharb, D., Raghukumar, C., & Kuhad, R. C. (2010). Decolorization of synthetic dyes and textile effluents by basidiomycetous fungi. Water, Air, & Soil Pollution, 210, 409–419.
El-Sheekh, M. M., Gharieb, M. M., & Abou-El-Souod, G. W. (2009). Biodegradation of dyes by some green algae and cyanobacteria. International Biodeterioration & Biodegradation, 63, 699–704.
Faraco, V., Pezzella, C., Miele, A., Giardina, P., & Sannia, G. (2009). Bio-remediation of colored industrial wastewaters by the white-rot-fungi Phanerochaete chrysosporium and Pleurotus ostreatus and their enzymes. Biodegradation, 20, 209–220.
Guaratini, C. C. I., & Zanoni, M. V. B. (2000). Corantes têxteis. Quimica Nova, 23, 71–78.
Hatakka, A. (1994). Lignin-modifying enzymes from selected white-rot-fungi: production and role in lignin degradation. FEMS Microbiology Reviews, 13, 125–135.
Heinfling, A., Martínez, J., & Marínez, A. T. (1998). Transformation of industrial dyes by manganese-independent reaction. Applied and Environmental Microbiology, 64, 2788–2793.
Hubbe, M. A., Beck, K. R., O’Neal, W. G., & Sharma, Y. C. (2012). Cellulosic substrates for removal of pollutants from aqueous systems: a review. BioResources, 7, 2592–2687.
Kalyani, D. C., Telke, A. A., Dhanve, R. S., & Jadhav, J. P. (2009). Ecofriendly biodegradation and detoxification of Reactive Red 2 textile dye by newly isolated Pseudomonas sp. SUK1. Journal of Hazardous Materials, 163, 735–742.
Kaushik, P., & Malik, A. (2009). Fungal dye decolourization: recent advances and future potential. Environment International, 35, 127–141.
Kunz, A., Peralta-Zamora, P., Moraes, S. G., & Duran, N. (2002). Novas tendências no tratamento de efluentes têxteis. Química Nova, 25, 78–82.
Li, L., Dai, W., Yu, P., Zhao, J., & Qu, Y. (2009). Decolorization of synthetic dyes by crude laccase from Rigidoporus lignosus W1. Journal of Chemical Technology & Biotechnology, 84, 399–404.
Maciel, G. M., Souza, C. G. M., Araujo, C. A. V., Bona, E., Haminiuk, C. H. I., Castoldi, R., Bracht, A., & Peralta, R. M. (2013). Biosorption of herbicide picloram from aqueous solutions by live and heat-treated biomasses of Ganoderma lucidum (Curtis) P. Karst and Trametes sp. Chemical Engineering Journal, 215-216, 331–338.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Muñoz, C., Guillén, F., Martínez, A. T., & Martínez, M. J. (1997). Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Current Microbiology, 34, 1–5.
Phan, K., Van Den Broeck, E., Van Speybroeck, V., De Clerck, K., Raes, K., & De Meester, S. (2020). The potential of anthocyanins from blueberries as a natural dye for cotton: a combined experimental and theoretical study. Dyes and Pigments, 176, 108180.
Ramya, M., Anusha, B., Kalavathy, S., & Devilaksmi, S. (2007). Biodecolorization and biodegradation of Reactive Blue by Aspergillus sp. African Journal of Biotechnology, 6, 1441–1445.
Ríoz-Gómez, J., Ferrer-Monteagudo, B., López-Lorente, A. I., Lucena, R., Luque, R., & Cárdenas, S. (2018). Efficient combined sorption/photobleaching of dyes promoted by cellulose/titania-based nanocomposite films. Journal of Cleaner Production, 194, 167–173.
Russoa, M. E., Di Natalea, F., Prigioneb, V., Tiginib, V., Marzocchellaa, A., & Vareseb, G. C. (2010). Adsorption of acid dyes on fungal biomass: Equilibrium and kinetics characterization. Chemical Engineering Journal, 162, 537–535.
Saito, T., Hong, P., Kato, K., Okazaki, M., Inagaki, H., & Maeda, S. (2003). Purification and characterization of an extracellular laccase of a fungus (family Chaetomiaceae) isolated from soil. Enzyme and Microbial Technology, 33, 520–526.
Saratale, R. G., Saratale, G. D., Chang, J. S., & Govindwar, S. P. (2011). Bacterial decolorization and degradation of azo dyes: a review. Journal of the Taiwan Institute of Chemical Engineers, 1, 138–157.
Shedbalkar, U., Dhanve, R., & Jadhav, J. (2008). Biodegardation of triphenylmethane dye cotton blue by Penicillium ochrochloron MTCC 517. Journal of Hazardous Materials, 157, 472–479.
Subramanian, V., & Yadav, J. S. (2009). Role of P450 monooxygenases in the degradation of the endocrine-disrupting chemical nonylphenol by the white rot fungus Phanerochaete chrysosporium. Applied and Environmental Microbiology, 75, 5570–5580.
Sun, Q., Jiang, L., Li, M., & Sun, J. (2020). Assessment on thermal hazards of reactive chemicals in industry: state of the art and perspectives. Progress in Energy and Combustion Science, 78, 100832.
Tien, M., & Kirk, T. K. (1983). Lignin degrading enzyme from Phanerochaete chrysosporium: purification, characterization and catalytic properties of unique H2O2-requiring oxygenase. Proceedings of the National Academy of Sciences of the United States of America, 81, 2280–2284.
Vogel, H. J. (1956). A convenient growth medium for Neurospora crassa. Microbial Genetics Bulletin, 13, 42–47.
Wang, C., Yediler, A., Lienert, D., Wang, Z., & Kettrup, A. (2002). Toxicity evaluation of reactive dyestuffs auxiliaries and selected effluents in textile finishing industry to luminescent bacteria Vibrio fischeri. Chemosphere, 46, 339–344.
Wariishi, H., Valli, K., & Gold, M. H. (1992). Manganese (II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Journal of Biological Chemistry, 267, 23688–23695.
Yang, Q., Zhan, H., Wang, S., Fu, S., & Li, K. (2008). Modification of eucalyptus CTMP fibres with white-rot fungus Trametes hirsute—effects on fibre morphology and paper physical strengths. Bioresource Technology, 99, 8118–8124.
Yesilada, O., Asma, D., & Cing, S. (2003). Decolorization of textile dyes by fungal pellets. Process Biochemistry, 38, 933–938.
Funding
The authors received financial support from the Conselho Nacional de Desenvolvimento Científico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Araújo, C.A.V., Contato, A.G., Aranha, G.M. et al. Biodiscoloration, Detoxification and Biosorption of Reactive Blue 268 by Trametes sp. M3: a Strategy for the Treatment of Textile Effluents. Water Air Soil Pollut 231, 349 (2020). https://doi.org/10.1007/s11270-020-04723-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04723-7