Abstract
Bacteria are regarded as the most effective in the detoxification of heavy metals, being environmental compatible. Metalloresistant bacteria are usually found in nature in highly contaminated environment where they interact with a combination of several toxic metals. For the present research, Arthrobacter oxydans and Arthrobacter globiformis have been isolated from the soil samples of the most polluted regions of Georgia, rich with manganese and iron, and contain co-produced toxic metals such as Cr, V, Zn, Ni, Pb, and Mo. We have studied the effects of the metals with different valence/charge on the metalloresistant Arthrobacter spp., the divalent cation—Zn(II) and the hexavalent anion—Cr(VI). The permanent presence of a nontoxic concentration of zinc alone or zinc together with the subtoxic concentration of chromium at the growth of A. oxydans and A. globiformis as batch culture causes the activation of the zinc primary uptake system transporters from the ZIP family (Zrt1). Chromium does not affect the process. The studied Arthrobacter spp. differ by the character of the activation of the antioxidant defense system. Chromium and zinc concomitant action causes the strongest oxidative stress in the case of A. globiformis that is demonstrated by the increased activity of superoxide dismutase (SOD) and catalase. In the case of A. oxydans, the zinc separate action, and the joint action of zinc and chromium decreases the activity of SOD and catalase. The antioxidant system is active in A. globiformis at the prolonged action of metals (96 h), whereas the cells of A. oxyidans activate the other defense mechanisms to survive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The low concentrations of heavy metals such as zinc, copper, and chromium are essential for growth and development of living organisms, but are toxic at high concentrations. The accumulation of metals in soil and water may cause soil degradation, decline of crop yields and its quality, pollution of surface water and groundwater. These processes could influence the food production and affect human health. It has been demonstrated that the increased level of the heavy metals in the environment complicates the possibility of their removal by the existing contemporary methods (Tang et al. 2014; Tchounwou et al. 2012). Nowadays, the method of microbial remediation could be regarded as an innovative and promising technology. It uses microorganisms for the accumulation and detoxification of the pollutants. Bacteria are more effective in bioremediation, followed by fungi and actinomycetes. Microbial remediation does not destroy the soil environment for plant growth, is not secondary pollution, and does not transfer the pollutants to residuals (Akshata et al. 2014; Ayangbenro and Babalola 2017; Gupta et al. 2016).
The experimental results show that microorganisms differ in their way of heavy metals detoxification. In addition, different strains of the same bacteria species also exhibit different processing capabilities (Tang et al. 2014; Tchounwou et al. 2012). In general, there are two basic heavy metal ion resistance mechanisms: (i) formation of the insoluble toxic metal complexes and (ii) reduced accumulation of toxic metals based on active efflux of the cations. Binding factors and enzymatic transformations such as oxidation, reduction, methylation, and demethylation play a role as defense mechanisms in bacteria as well (Hynninen 2010). The toxicity of heavy metals, apart from their immediate action, is often revealed by the formation of reactive oxygen species (ROS) and oxidative damage (Ercal et al. 2001; Schutzendubel and Polle 2002; Valko et al. 2016).
Arthrobacter is a common genus of soil bacteria, all species of which are Gram-positive, obligate aerobes, metabolically versatile, produce many different enzymes, and grow on a wide range of substrates. The high ability to survive has been attributed to the Arthrobacter’s unique morphological cycle that allows it to remain in a stable, coccoid form at stressful times. The metabolic diversity of Arthrobacter species provides the possibility to biodegrade various types of pollutants in the environment and survive under stressful conditions induced by starvation, ionizing radiation, oxygen radicals, and toxic chemicals (Camargo et al. 2003; Scheublin and Leveau 2013; Westerberg et al. 2000).
Metalloresistant bacteria are usually found in nature in the highly contaminated environment where they interact not only with one particular metal but also with combinations. For the present research, the Arthrobacter species have been isolated from the basalt samples of the most polluted ecological regions of Georgia (Marneuli, Zestafoni, Kazreti). These regions are rich with manganese and iron, and contain co-produced toxic metals such as Cr, V, Zn, Ni, Pb, and Mo (Tsibakhashvili et al. 2011).
The ions with similar valence often reveal the high chemical similarity. For example, Zn(II) chemically is identical to Cd(II) and Pb(II), that the majority of the transcription factors fail to differentiate between them (Brocklehurst et al. 2003; Yoon et al. 1991). The same applies for Cu(I) and Ag(I) (Stoyanov et al. 2001; Stoyanov and Brown 2003). However, it is less known about the joint action of the metals with different valence/charge. We have intended to study coactions of the metals with a different transport system, the transformation processes and the action mechanisms. For this purpose, we have chosen the divalent cation—Zn(II) and the hexavalent anion—Cr(VI). These metals have different physical structures and biochemical features. Chromium transforms from Cr(VI) to Cr(III), producing Cr(IV) and Cr(V) as intermediate products, and zinc is not characterized by transformation.
Zinc possesses a specific transport system that has not been demonstrated for chromium. Chromium and zinc interact with different biomolecules/structural components of biomolecules and activate/inactive different signaling pathways. However, their indirect action causes similarly the increase of the reactive oxygen species and the oxidative damage of cells in the detoxification processes. Consequently, the joint action of these metals may have an influence on the cell antioxidant status and the metalloresistance of bacteria.
The zinc participates in many intracellular processes and is essential for the maintenance of the structural stability of biomacromolecules. Zn(II) serves as a cofactor for more than 300 enzymes (McCall et al. 2000). Zn(II) also plays an important role in gene expression as the structural component of Zn(II)-dependent transcription factors. High concentrations of zinc may suppress the aerobic respiration chain that causes toxicity resulting in the destruction of biological systems (Beard et al. 1995; Hynninen 2010).
Environmental protection from the hexavalent chromium contamination is one of the main problems as the Cr(VI) can spread widely because of its anionic mobility and is a hazard to human health. It is highly toxic, mutagenic, and carcinogenic (Joutey et al. 2015). Chromium does not have any specific transporter. Cr(VI) can cross the cell membrane and penetrate into cellular structures by passive or active transport mechanisms, but mostly via the sulfate transport system (Viti et al. 2014). Cr(VI) does not interact directly with DNA; its genotoxicity is attributed to its intracellular reduction to Cr(III). Cr(III) complexes interact with DNA, that could affect replication and transcription and cause mutagenesis. Additionally, Cr(III) may alter the structure and activity of enzymes by reacting with their carboxyl and thiol groups (Daud et al. 2014; Joutey et al. 2015; Viti et al. 2014). Accompanying products that are generated during intracellular reduction of Cr(VI) to Cr(III) are reactive oxygen species, including singlet oxygen, superoxide, and hydroxyl radicals and hydrogen peroxide, causing various structural damage of biopolymers.
Several intracellular and extracellular mechanisms have been described to account for bacterial resistance to chromate. Extracellular mechanisms include (i) ability to regulate uptake mechanisms such as the sulfate uptake shuttle system that is involved in initial cellular accumulation (Brown et al. 2006); (ii) extracellular capacity to reduce Cr(VI) to Cr(III), which is then removed easily via reactions with functional groups on bacterial cell surfaces (Ngwenya and Chirwa 2011); (iii) capacity to reduce Cr(VI) to Cr(III) in the cell membrane, usually preceded by the adsorption of Cr(VI) to functional groups that are located on the bacterial cell surface (Joutey et al. 2013; Opperman and van Heerden 2008). Intracellular mechanisms include (i) intracellular reduction of Cr(VI) to Cr(III) and (ii) activation of antioxidant ferments (Ackerley et al. 2006; Cervantes and Campos-Garcıa 2007; Joutey et al. 2015).
Regardless of the differences in the mechanisms of zinc and chromium activity, they both produce ROS and activate the antioxidant systems. Consequently, their simultaneous action may change the oxidative status of bacterial cells and their heavy metal resistance. The purpose of this research is to study the separate action of zinc and the simultaneous effect of Zn(II) and Cr(VI) on the cell total protein composition and the status of the antioxidant defense system.
2 Materials and Methods
2.1 Bacterial Culture and Growth Conditions
The identified endolytic bacteria (Arthrobacter oxydans and Arthrobacter globiformis) were isolated from basalts of ecologically most polluted regions of Georgia (Marneuli, Zestafoni, Kazreti) and used to study molecular mechanisms of metal resistance (Tsibakhashvili et al. 2011). In our laboratory, the cells were maintained as a batch culture in a standard medium recommended for Arthrobacter species (Girard and Snell 1983) at a temperature of 21 °C with constant shaking. Culture growth was monitored by measuring optical density at 490 and 590 nm. 50 ppm Zn(II) and 100 ppm Cr(VI) were added (separately or simultaneously) at the late-stationary phase, and the incubation was proceeded for 96 h. Zn(II) was added as ZnCl2, and Cr(VI) was added as K2CrO4. In all cases, bacterial culture growth without or with Cr(VI) and Zn(II) proceeded without medium replenishment. The viability was detected by cell growth on agar plates with a cell suspension dilution.
2.2 Cell Lysate Preparation
Cells were harvested by centrifugation at 10,000 rpm for 10 min (4 °C) and washed twice in 0.15 M NaCl. The cell pellets were treated with 500 μl of lysis buffer B-PER, pH 7.5 (Pierce) per 2 × 109 cells overnight at 4 °C. The lysate was centrifuged at 15,000 rpm for 20 min (4 °C).
2.3 Analytical Methods
Protein concentration was determined by using the BCA (bicinchoninic acid) protein assay reagent from Pierce (Rockford, IL). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (1970), using 10% gels. Molecular weight markers used were as follows: bovine serum albumin (66 kDa), glutamate dehydrogenase (52 kDa), ovalbumin (42 kDa), carbonic anhydrase (30 kDa), and human Cu,ZnSOD (16 kDa). Twenty-five micrograms of the total protein was run per lane. The gels were stained with Coomassie blue R-250.
2.4 SOD in Gel Assay
The technique of SOD assay involves photoreduction of nitro blue tetrazolium (NBT) for the determination of activity of SOD following native polyacrylamide gel electrophoresis. The protein corresponding to SOD can be then visualized as an achromatic zone through the inhibition of NBT (Sigma) reduction via SOD. Achromatic bands were visualized for 50 μg protein equivalent. Crude extract was mixed with the loading buffer in the absence of 2-mercaptoethanol and SDS and loaded into the gel without heating. Following electrophoresis in 12.5% gels for 3 h at 30 mM, the gels were subjected to two-step staining procedure described by Steinman (1985).
2.5 Catalase Activity
Catalase activity in the cell crude extract was determined by measuring the rate of H2O2 (10 mM) decomposition in 50 mM potassium phosphate buffer (pH 7.0), in the presence of the cell crude extract at 240 nm and 25 °C, ε H2O2 = 43.6 M−1 cm−1 (Beers and Sizer 1952).
3 Results and Discussion
3.1 Zinc Influence on the Total Protein Composition of A. oxydans and A. globiformis
The prolonged action of 50 ppm Zn (96 h) generates the impoverishment of the cell total protein composition, especially under 30 kDa (Fig. 1). However, the obtained results have revealed the sharp increase of the clearly visible 51 kDa protein in A. oxydans and the 42 kDa protein in A. globiformis.
The total protein composition of Arthrobacter sp. in response to 50 ppm Zn(II) action. M, marker; C, control cells without Zn(II) action; Zn, Zn(II)-treated cells for 96 h. Zn(II) was added at the stationary phase of bacterial culture growth. The whole protein extract was prepared by cell extraction with bacterial Buffer B-PER™ (in Phosphate Buffer) (Pierce, USA). 35 μg of the total protein was applied to the gel. Green brace marks the group of protein fractions with MW between 52 and 42 kDa. Red arrows mark the protein with MW 42–40 kDa and 50–52 kDa
The stringent maintenance of Zn(II) intracellular/extracellular concentration level is one of the base conditions for the existence of living cells. Since Zn(II) is not capable of passing the cell membrane through diffusion, Zn transporters have been detected in the regulation system (Joutey et al. 2015). Zinc transporters are classified by several different transporter families: ABC (ATP-binding cassette) transporters and RND (resistance nodulation division) transporters, with most notable member CBA (consisting of subunits C, B, and A) proteins, which are found in bacteria, but not in eukaryotes; ZIP—zinc-regulated transporter (ZRT) etc. (Eide 2006; Guerinot 2000; Li et al. 2013).
The primary uptake system for zinc is the Zrt1 transporter of the ZIP family that is required for growth in low zinc conditions. Zrt1 expression levels are upregulated by zinc intracellular concentration at the transcriptional level. Increased level of Zn(II) causes Zrt1 overexpression and increases high affinity uptake of zinc. Zrt1p is the high affinity zinc transporter located on the plasma membrane, which participates in the primary uptake of zinc. ZRT1 expression is highly correlated to the intracellular concentration of zinc (Eng et al. 1998; Gitan et al. 1998; Zhao and Eide 1996, 1997).
According to the literature data, the 42 kDa protein in A. globiformis could correspond to the Zrt1p protein (molecular weight: 41,6 kDa) encoded by ZRT1gene. As for A. oxydans, the increase of the 51 kDa protein could correspond to the Zrt1p protein (molecular weight: 51,2 kDa) (Eng et al. 1998; Gitan et al. 1998; Zhao and Eide 1996, 1997). Therefore, the zinc uptake system is active in the both bacteria isolates grown in the permanent presence of 50 ppm Zn(II) for 96 h.
3.2 The Total Protein Composition of A. oxydans and A. globiformis in Response to Zn(II) and Cr(VI) Joint Action
The simultaneous effect of chromium and zinc on the cell total protein composition at their permanent action for 96 h has been detected (Fig. 2). The Zn (50 ppm) in the presence of Cr (100 ppm) slightly impoverishes the total protein composition of A. oxydans under 30 kDa at 96 h, as it was already observed in case of the separate action of Zn (Fig. 1). In the case of A. globiformis, Zn in the presence of Cr intensifies some proteins, especially with a molecular weight (MW) more than 60 kDa and with low molecular weight with emphasis on the 16 kDa protein (red arrow). The joint action of Zn(II) and Cr(VI) as well as the separate action of zinc, intensifies expression of the 42 kDa and the 51 kDa proteins accordingly in A. globiformis and A. oxydans. Therefore, the active zinc transport system is not affected by chromium in both bacterial isolates.
The total protein composition of Arthrobacter sp. in response to 100 ppm Cr(VI) and 100 ppm Cr(VI) + 50 ppm Zn(II) joint action. M, markers; Cr, Cr(VI)-treated cells for 96 h; Cr + Zn—simultaneously treated cells for 96 h. Metals were added at the stationary phase of bacterial culture growth. The whole protein extract was prepared by cell extraction with bacterial Buffer B-PER™ (in Phosphate Buffer) (Pierce, USA). 35 μg of the total protein was applied to the gel. Red arrow marks the protein with MW 16 kDa
3.3 The Influence of Separate and Joint Action of Zn(II) and Cr(VI) on the SOD Activity
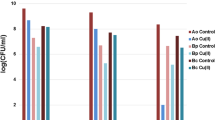
As it follows from the presented data (Fig. 3), both metals inhibit SOD activity in A. oxydans regardless of their separate or simultaneous action. In the case of A. globiformis, Zn (50 ppm) does not influence the SOD activity, Cr at the concentration of 100 ppm inhibits the enzyme activity, but the joint action of metals causes the sharp increase of SOD activity. Therefore, in the case of A. globiformis, separate metals actions on SOD activity strongly differ from their joint action.
The influence of Zn(II) and Cr(VI) separate and joint action on the SOD activity estimated by the photochemical NBT method of SOD detection in 12.5% native gel. 50 ppm Zn(II) and 100 ppm Cr(VI) were added separately or simultaneously at the growth medium at the stationary phase. The bacteria were grown in the presence of metals for 96 h. C, control cells; bacteria were grown without metals in the medium. The whole protein extract was prepared by cell extraction with bacterial Buffer B-PER™ (in Phosphate Buffer) (Pierce, USA). 50 μg of the total protein was applied to the gel. The achromatic zones of SOD positions in a gel are presented in the invert images (panel a). Quantification of the gel bands measuring the peak density (panel b)
3.4 The Influence of Zn(II) and Cr(VI) Separate and Joint Action on the Catalase Activity
The action of the studied metals (Cr, Zn) on the catalase activity differs in the studied Arthrobacter species (Fig. 4). In the case of A. oxydans, Zn significantly affects the catalase activity, decreasing it both in the absence and in the presence of Cr as well. At the same time, Cr alone has no influence on the enzyme activity at the studied concentrations. Therefore, the catalase activity of A. oxydans is sensitive to the action of zinc. In the case of A. globiformis, Cr increases the catalase activity regardless of the Zn presence in the growth medium. The results show that the different mechanisms of antioxidant defense against the Zn and Cr separate and joint action are induced in the studied bacteria.
The influence of Zn(II) and Cr(VI) separate and joint action on the catalase activity. 50 ppm Zn(II) and 100 ppm Cr(VI) were added separately or simultaneously at the growth medium at the stationary phase. The bacteria were grown in the presence of metals for 96 h. The whole protein extract was prepared by cell extraction with bacterial Buffer B-PER™ (in Phosphate Buffer) (Pierce, USA). The data presented are mean values ± SD from three separate sets of experiments
4 Conclusions
The results show that the studied Arthrobacter species (A. oxydans and A. globiformis) growing in the permanent presence of zinc alone or zinc together with chromium could probably activate the zinc primary uptake system transporters from ZIP family (Zrt1) and chromium does not affect the processes that have been demonstrated by the analysis of the total protein composition.
A. oxydans and A. globiformis have different metalloresistance mechanisms. The Cr and Zn joint action produces the strongest oxidative stress on A. globiformis as demonstrated by the activation of the antioxidant defense system (catalase and SOD activities, protein composition). Chromium resistance is influenced by zinc causing the increased activity of SOD and catalase. In the case of A. oxydans, zinc affects the total protein composition, reduces the activity of the antioxidant enzymes that are detected by the decreased activity of SOD and catalase at the zinc separate action, and at the joint action of zinc and chromium. We can conclude that in the case of A. globiformis, the antioxidant defense system still preserves its active state at the prolonged action of zinc and chromium, whereas A. oxydans switches on the other line of defense mechanisms for their metalloresistance.
A search of bacteria with remediation potential is a challenge, as there are some restrictions on the microorganisms’ introduction to the environment. Therefore, it is important to establish the bioremediation potential of native species from a particular location for their further use in the bioremediation/bioaugmentation processes. Arthrobacter spp. isolated from highly polluted regions have been studied and the observed results show their Cr(VI) remediation potential along with resistance to Zn(II) action. In view of this and with Arthrobacter spp. ability to survive at various unfavorable conditions, the bacteria have the potential to be used for the remediation of metals with anionic transport system (Cr(VI), Mn(VII) etc.), as well as potential to sustain metals with cationic transport system (Zn(II), Cu(II), Ag(I) etc.).
References
Ackerley, D. F., Barak, Y., Lynch, S. V., Curtin, J., & Matin, A. (2006). Effect of chromate stress on Escherichia coli K-12. Journal of Bacteriolology, 188, 3371–3381.
Akshata, J. N., Udayashankara, T. H., & Lokesh, K. S. (2014). Review on bioremediation of heavy metals with microbial isolates and amendments on soil residue. International Journal of Science and Research, 3, 118–123.
Ayangbenro, A. S., & Babalola, O. O. (2017). A new strategy for heavy metal polluted environments: a review of microbial biosorbents. International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph14010094.
Beard, S. J., Hughes, M. N., & Poole, R. K. (1995). Inhibition of the cytochrome M-terminated NADH oxidase system in Escherichia coli K-12 by divalent metal cations. FEMS Microbiology Letters, 131(2), 205–210.
Beers, R. F., & Sizer, J. W. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. The Journal of Biological Chemistry, 195, 133–140.
Brocklehurst, K. R., Megit, S. J., & Morby, A. P. (2003). Characterization of CadR from Pseudomonas aeruginosa: a Cd(II)-responsive MerR homologue. Biochemical and Biophysical Research Communications, 308, 234–239.
Brown, S. D., Thompson, M. R., Verberkmoes, N. C., Chourey, K., Shah, M., Zhou, J. Z., Hettich, R. L., & Thompson, D. K. (2006). Molecular dynamics of the Shewanella oneidensis response to chromate stress. Molecular & Cellular Proteomics, 5, 1054–1071.
Camargo, F., Bento, F., Okeke, B., & Frankenberger, W. T. (2003). Hexavalent chromium reduction by an actinomycete, Arthrobacter crystallopoietes ES 32. Biological Trace Element Research, 97, 183–194.
Cervantes, C., & Campos-Garcıa, J. (2007). Reduction and efflux of chromate by bacteria. In D. H. Nies & S. Silver (Eds.), Molecular microbiology of heavy metals (pp. 407–419). New York: Springer.
Daud, M. K., Mei, L., Variath, M. T., Ali, S., Li, C., Rafiq, M. T., & Zhu, S. J. (2014). Chromium (VI) uptake and tolerance potential in cotton cultivars: effect on their root physiology, ultramorphology, and oxidative metabolism. BioMed Research International. https://doi.org/10.1155/2014/975946.
Eide, D. J. (2006). Zinc transporters and the cellular trafficking of zinc. Biochimica et Biophysica Acta (Molecular Cell Research), 1763, 711–722.
Eng, B. H., Guerinot, M. L., Eide, D., & Saier, M. H., Jr. (1998). Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. Journal of Membrane Biology, 166, 1–7.
Ercal, N., Gurer-Orhan, H., & Aykin-Burns, N. (2001). Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Current Topics in Medical Chemistry, 1, 529–539.
Girard, B., & Snell, E. (1983). Biochemical factors. In P. Gerhardt (Ed.), Manual of methods for general bacteriology (pp. 198–276). Moscow: Mir.
Gitan, R. S., Luo, H., Rodgers, J., Broderius, M., & Eide, D. (1998). Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. The Journal of Biological Chemistry, 273, 28617–28624.
Guerinot, M. L. (2000). The ZIP family of metal transporters. Biochimica et Biophysica Acta, 1465, 190–198.
Gupta, A., Joia, J., Sood, A., Sood, R., Sidhu, C., & Kaur, G. (2016). Microbes as potential tool for remediation of heavy metals: a review. Journal of Microbial & Biochemical Technology, 8(4), 364–372.
Hynninen, A. (2010). Zinc, cadmium and lead resistance mechanisms in bacteria and their contribution to biosensing. Dissertation, University of Helsinki.
Joutey, N. T., Bahafid, W., Sayel, H., Ananou, S., & El Ghachtouli, N. (2013). Hexavalent chromium removal by a novel Serratia proteamaculans isolated from the bank of Sebou river (Morocco). Environmental Science and Pollution Research, 21(4), 3060–3072.
Joutey, N. T., Sayel, H., Bahafid, W., & El Ghachtouli, N. (2015). Mechanisms of hexavalent chromium resistance and removal by microorganisms. Reviews of Environmental Contamination and Toxicology, 233, 45–69.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Li, S., Zhou, X., Huang, Y., Zhu, L., Zhang, S., Zhao, Y., Guo, J., Chen, J., & Chen, R. (2013) Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family in maize. BMC Plant Biology. https://doi.org/10.1186/1471-2229-13-114.
McCall, K. A., Huang, C., & Fierke, C. A. (2000). Function and mechanism of zinc metalloenzymes. The Journal of Nutrition. https://doi.org/10.1093/jn/130.5.1437S.
Ngwenya, N., & Chirwa, E. M. N. (2011). Biological removal of cationic fission products from nuclear wastewater. Water Science and Technology, 63, 124–128.
Opperman, D. J., & van Heerden, E. (2008). A membrane-associated protein with Cr(VI)-reducing activity from Thermus scotoductus SA-01. FEMS Microbiology Letters, 280, 210–218.
Scheublin, T. R., & Leveau, J. H. J. (2013). Isolation of Arthrobacter species from the phylloshere and demonstration of their epiphytic fitness. MicrobiologyOpen, 2, 205–213.
Schutzendubel, A., & Polle, A. (2002). Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany, 53(372), 1351–1365.
Steinman, H. M. (1985). Bacteriocuprein superoxide dismutases in pseudomonads. Journal of Bacteriology, 162, 1255–1260.
Stoyanov, J. V., & Brown, N. L. (2003). The Escherichia coli copper-responsive copA promoter is activated by gold. The Journal of Biological Chemistry, 278, 1407–1410.
Stoyanov, J. V., Hobman, J. L., & Brown, N. L. (2001). CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Molecular Microbiology, 39, 502–511.
Tang, M., Chen, J., Sun, Y., Tong, Y., & Liu, Y. (2014). The absorption and scavenging ability of a bacillus in heavy metal contaminated soils (Pb, Zn and Cr). African Journal of Environmental Science and Technology, 8, 476–481.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metals toxicity and the environment. In A. Luch (Ed.), Molecular, clinical and environmental toxicology, volume 3: Environmental toxicology (pp. 133–164). New York: Springer.
Tsibakhashvili, N. Y., Kalabegishvili, T. L., Rcheulishvili, A. N., Ginturi, E. N., Lomidze, L. G., Gvarjaladze, D. N., & Rcheulishvili, O. A. (2011). Effect of Zn(II) on the reduction and accumulation of Cr(VI) by Arthrobacter species. Journal of Industrial Microbiology and Biotechnology, 38(11), 1803–1808.
Valko, M., Jomova, K., Rhodes, C. J., Kuca, K., & Musilek, K. (2016). Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Archives of Toxicology, 90(1), 1–37.
Viti, C., Marchi, E., Decorosi, F., & Giovannetti, L. (2014). Molecular mechanisms of Cr(VI) resistance in bacteria and fungi. FEMS Microbiology Reviews, 38, 633–659.
Westerberg, K., Elvang, A. M., Stackebrandt, E., & Jansson, J. K. (2000). Arthrobacter chlorophenolicus sp. nov., a new species capable of degrading high concentrations of 4-chlorophenol. International Journal of Systematic and Evolutionary Microbiology, 50(6), 2083–2092.
Yoon, K. P., Misra, T. K., & Silver, S. (1991). Regulation of the cadA cadmium resistance determinant of Staphylococcus aureus plasmid pI258. Journal of Bacteriology, 173, 7643–7649.
Zhao, H., & Eide, D. (1996). The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proceedings of the National Academy of Sciences of the United States of America, 93, 2454–2458.
Zhao, H., & Eide, D. J. (1997). Zap1p, a metalloregulatory protein involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae. Molecular and Cellular Biology, 17(9), 5044–5052.
Funding
This work was supported by grants (#2016-39) from the Shota Rustaveli National Science Foundation (SRNSF) and (#6304) from the Science and Technology Center in Ukraine (STCU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asatiani, N., Kartvelishvili, T., Sapojnikova, N. et al. Effect of the Simultaneous Action of Zinc and Chromium on Arthrobacter spp.. Water Air Soil Pollut 229, 395 (2018). https://doi.org/10.1007/s11270-018-4046-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-4046-0