Abstract
Natural habitats are often characterized by the coexistence of Zn and Cr. This study assessed the potential of two Gram-positive, Cr(VI)-reducing, aerobic bacterial strains belonging to Arthrobacter genera, which were isolated from basalt samples taken from the most polluted region of the Republic of Georgia, to remediate Cr(VI) in environments in the presence of Zn(II). Our batch experiments revealed that the addition of Zn(II) to the tested bacterial cells significantly enhanced the accumulation of Cr. According to electron spin resonance (ESR) measurements, the presence of Zn(II) ions did not change the nature of Cr(V) and Cr(III) complexes generated during the microbial reduction of Cr(VI). The efficiency of Cr(VI) reduction also remained unchanged after the addition of 50 mg/l of Zn(II) to the bacterial cells. However, at high concentrations of Zn(II) (higher than 200 mg/l), the transformation of Cr(VI) to Cr(V) and Cr(III) complexes decreases significantly. In addition, it was shown that the accumulation pattern of Zn in the tested bacterial species in the presence of 100 mg/l of Cr(VI) fits the Langmuir–Freundlich model well. The two tested bacterial strains exhibited different characteristics of Zn accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arthrobacter species are common soil bacteria [12]. They have a great potential for detoxification of the environment [13, 20, 24]. Specifically, they can degrade aromatic compounds and humic substances [13, 24]. Arthrobacter species are also resistant to heavy metals [20]. In our recent studies we established that the Gram-positive bacterial strains of Arthrobacter genera isolated from basalt samples which were taken from the most polluted regions of the USA and the Republic of Georgia can reduce and detoxify Cr(VI) with a high efficiency under aerobic conditions [4, 9, 22, 23]. Our electron spin resonance (ESR) experiments revealed that Cr(VI) is reduced to less toxic Cr(III) compounds through the formation of oxo Cr(V) diols at the surface of bacteria [4, 9]. Later, the decomposition of Cr(V) diols to Cr(III) complexes was also investigated [23]. Cr(V) species are mutagenic in bacteria and genotoxic in mammalian cells [11]. Therefore, the formation and the lifetime of Cr(V) intermediates should be evaluated carefully. Thus far, no studies have examined the formation of Cr(V) species in Cr(VI)-reducing bacteria in the presence of other heavy metal ions. Besides, until now only a few laboratory studies have investigated the response of microorganisms to chromium stress in the presence of other metal ions [7, 8, 14].
The purpose of our study was to assess the potential of Arthrobacter species isolated from polluted regions of Georgia to remediate Cr(VI) in the presence of Zn(II). Specifically, our aims were (1) to study the uptake of different concentrations of Cr(VI) by the tested bacteria in the presence of Zn(II), and on the contrary, (2) the uptake of different concentrations of Zn(II) by tested bacteria in the presence of Cr(VI); (3) to investigate dynamics of the formation of Cr(V) and Cr(III) complexes in Arthrobacter–Cr(VI) systems in the presence of Zn(II). These goals are relevant because natural habitats are very often characterized by the coexistence of Zn and Cr, and zinc is an essential metal ion but at higher concentrations it becomes toxic [13].
Materials and methods
Bacterial strains
Two Gram-positive, Cr(VI)-reducing, aerobic bacterial strains belonging to Arthrobacter genera—A. globiformis 151B and Arthrobacter sp. 61B—were used as our model bacteria. These bacteria were isolated from polluted basalts taken from Kazreti (Republic of Georgia) [21].
Bacterial growth conditions and sample preparation
All chemicals used in the experiments were ACS reagent grade, produced by Sigma (St. Louis, MO, USA).
The bacteria were grown aerobically in the following nutrient medium: 10 g of glucose, 10 g of peptone, 1 g of yeast extract, 2 g of caseic acid hydrolysate, 5 g of NaCl, and 1 l of distilled water. Bacterial cells were grown in 500-ml Erlenmeyer flasks as a 100-ml suspension. The medium was inoculated with 0.1 ml of overnight broth and incubated at 21°C being shaken continuously. Cr(VI) as K2CrO4 and Zn(II) as ZnSO4 were added simultaneously to the bacterial cell cultures at the early stationary phase of their growth. Two sets of experiments were performed. In the first one 50 mg/l of Zn(II) was added to the bacterial cells at a given concentration of Cr(VI) within the range of 50–1,000 mg/l. In the second set, 100 mg/l of Cr(VI) was added to the bacterial cells at a given concentration of Zn(II) within the range of 50–1,000 mg/l. After being cultivated for 5 days the cells were harvested by centrifugation (12,000 g, 15 min, 4°C), rinsed twice in a 20 mM phosphate buffer, and subjected to ESR analysis. For atomic absorption spectrometry (AAS) measurements this wet biomass was placed in an adsorption-condensation lyophilizer and dried following the procedure reported in [15].
Methods of measurements
The ESR method was employed to monitor and identify the formation of Cr(V) and Cr(III) complexes in bacterial systems. To estimate both the total chromium and zinc content in bacterial cells, we performed AAS on the cells after the wet ashing of the cells by concentrated nitric acid.
ESR measurements
ESR was the key method employed in this study. ESR measurements were carried out on the RE 1306 radio spectrometer (Russia) with 100-kHz modulation at 9.4 GHz. The experiments were performed at ambient temperature (300 K). The typical settings for spectral acquisition are described elsewhere [9]. In each case, the parameters are given in the figure captions. The ESR signal intensity was measured by the peak-to-peak height of the signal (the width of ESR line does not change and therefore the signal intensity is proportional to the concentration of paramagnetic centers). To measure the value of the g-factor accurately, we took into account the difference in the values of the magnetic field caused by different positions of the samples and by the sensor used for magnetic field measurement. We measured the values of the g-factor of the standards, obtained the small difference, and included them in the measurements of g-factors of the samples. The error in the value of the g-factor was ±0.0003 and ±0.005 for Cr(V) and Cr(III), respectively.
AAS measurements
The total chromium and total zinc contents in bacteria were measured using an atomic absorption spectrometer (Beckman 495, USA) with an acetylene–air flame. The detection was carried out at 357.9 nm (for chromium) and at 213.8 nm (for zinc). The instrumentation detection limit for the Cr measurement was 0.02 μg/ml and for Zn was 0.01 μg/ml.
Results and discussion
We recently showed that under aerobic conditions the accumulation of chromium in A. globiformis 151B and Arthrobacter sp. 61B is dose-dependent and its character changes significantly at higher concentrations of Cr(VI) [22]. Other metal ions can affect the binding efficiency of Cr(VI) through a simple additive effect or by synergetic and antagonistic interactions [6]. To study the effect of coexisting zinc on the uptake of chromium, in the first set of experiments 50 mg/l of Zn(II) was added to the bacterial cells. Note that each set of experiments was replicated at least three times and the data shown in the figures represent averaged values. In Fig. 1, for comparison, the level of Cr accumulation at different Cr(VI) concentrations in the nutrient medium (in the absence of Zn(II)) is also presented which illustrates that for both bacteria the character of Cr accumulation changed significantly when 50 mg/l of Zn(II) was added to the bacterial cells. Specifically, in each case, the level of chromium accumulation was much higher at each given concentration of Cr(VI) and, besides, it became almost constant within the tested range of Cr(VI). The obtained result can be explained as follows: Zn(II) ions can bind to negatively charged groups on the surface of bacterial cells and these metal ions might then assist the contact between CrO4 2− ions and bacterial cells.
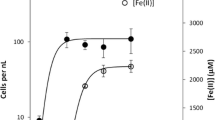
The survival of Arthrobacter species is decreased with the increase of Cr(VI) content in the nutrient medium [1]. Our current experiments revealed that after addition of 50 mg/l of Zn to the nutrient medium, the number of bacterial cells practically did not decrease with the increase of Cr(VI) concentration (Fig. 2a). Zinc plays an important role as an essential trace element in development, growth, and differentiation of all living systems [16]. It is a component of enzymes and is also known to be the stabilizer of membranes and various macromolecules. The second set of experiments, in which the different concentrations of Zn(II) were added to the bacterial growth medium together with 100 mg/l of Cr(VI) at each given concentration of Zn, also points to a certain positive effect of Zn. Figure 2b shows that the biomass of both bacteria increased almost linearly with the increase of Zn content in the nutrient medium. For resistance to physiologically required metals, the survival is optimized by cooperation between the resistance mechanism and the normal cellular metabolism, allowing the cell to accumulate sufficient metal to maintain the metal-dependent activities [2, 3, 17]. According to data presented in Fig. 2b, the concentration of Zn within the range of 50–1,000 mg/l promotes the normal growth of both bacterial species.
a Effect of Cr(VI) concentration on the growth of Arthrobacter sp. 61B (filled circles) and A. globiformis 151B (open squares) in the presence of 50 mg/l of Zn(II); b Effect of Zn(II) concentration on the growth of Arthrobacter sp. 61B (filled circles) and A. globiformis 151B (open squares) in the presence of 100 mg/l of Cr(VI)
Essential metal ions enter the cell through specific and non-specific uptake systems [5]. The first are fast and generally driven by the chemiosmotic gradient across the cytoplasmic membrane of microorganisms. The second uptake systems are inducible. For example, in the yeast Saccharomyces cerevisiae there exist two separate systems for zinc uptake [25]. One system with high substrate affinity is induced in zinc-deficient cells and the second having lower affinity is highly regulated by zinc status. Figure 3 presents the uptake of Zn in A. globiformis 151B and Arthrobacter sp. 61B in the presence of 100 mg/l of Cr(VI) in the nutrient medium. Figure 3 demonstrates that in the tested bacteria the uptake of zinc, similar to chromium uptake, includes two phases—rapid and slow. The rapid phase is connected with the metabolism-independent binding of Zn(II) ions to bacterial surface. The cell wall of Arthrobacter species contains a second bilayer of waxy lipids in addition to the thicker peptidoglycan layer, polysaccharides, menaquinones, teichoic acids, and proteins [18]. Functional groups within these biomolecules provide the amino, carboxylic, sulfydryl, phosphate, and thiol groups that can bind metal ions. The slower rate of zinc accumulation can be explained by the intracellular uptake of Zn(II) ions.
Figure 3 also demonstrates the difference between Zn uptake by A. globiformis 151B and Arthrobacter sp. 61B. Specifically, for Arthrobacter sp. 61B the rate of Zn accumulation slows down earlier than for A. globiformis 151B with the increase of Zn(II) loading.
To quantify Zn uptake by the tested bacteria, the Langmuir–Freundlich (LF) model [19] was used:
Here c is the concentration of metal ions; q max represents the maximum metal accumulation; b is the affinity parameter of the isotherm reflecting the high affinity of the biosorbent for the sorbate; and n is the empirical parameter that varies with the degree of heterogeneity.
It follows from Fig. 3 that the zinc accumulation by cells fits the LF model well. R 2 and the fitting parameters for the LF fit to the accumulation curve of A. globiformis 151B and Arthrobacter sp. 61B are presented in Table 1. According to these data, in the presence of 100 mg/l of Cr(VI), the affinity of zinc accumulation is an order of magnitude higher in Arthrobacter sp. 61B than in A. globiformis 151B. As a result, in the Arthrobacter sp. 61B cells the maximum amount of zinc was reached at lower concentrations of Zn(II) in the nutrient medium. Besides, the A. globiformis–Zn(II) system can be considered as heteregenous (n < 1), contrary to the other bacterial–metal system (here n = 1). It is also found that the maximum amount of Zn accumulated in both bacteria is almost equal but exceeds about 4–5 times that of Cr [22].
In the last set of experiments the effect of Zn on Cr(VI) reduction by A. globiformis 151B was studied. According to ESR measurements, in bacterial systems the Cr(V) ESR line is characterized by a g-factor of 1.980 and a line width of 12 G (corresponding to Cr(V) diols) and the Cr(III) ESR signal is characterized by a g-factor of 2.02 and a line width of 650 G (corresponding to Cr(III) hydroxide) [4, 9]. Thus, the presence of Zn(II) ions did not change the nature of Cr(V) and Cr(III) complexes generated during the reduction of Cr(VI) by this bacterial strain.
Figure 4a presents Cr(V) and Cr(III) ESR signal intensities in A. globiformis 151B at different Cr(VI) concentrations in the presence of 50 mg/l of Zn(II) in the nutrient medium. At each given concentration of Cr(VI), the bacteria were cultivated for 5 days. This figure illustrates the dose-dependent formation of Cr(V) and Cr(III) complexes in A. globiformis 151B. The Cr(V) ESR signal intensity increases with the increase of Cr(VI) concentration in the nutrient medium. Formation of Cr(III) complexes was pronounced at the beginning (within the range of 50–500 mg/l) and then became slower at higher concentrations of Cr(VI). This behavior of Cr(V) and Cr(III) complexes is similar to that of the Arthrobacter–Cr(VI) system without Zn(II) [10], for which it was concluded that the excessive amount of Cr(V) and Cr(III) formed in the interval of 500–700 mg/l probably stressed the bacterial cells and made them lose their ability to transform newly formed Cr(V) complexes into Cr(III).
a Effect of Cr(VI) concentration on the formation of Cr(V) (open circles) and Cr(III) (filled squares) complexes by A. globiformis 151B in the presence of 50 mg/l of Zn(II); b Effect of Zn(II) concentration on the formation of Cr(V) (open circles) and Cr(III) (filled squares) complexes by A. globiformis 151B in the presence of 100 mg/l of Cr(VI)
A completely different picture was obtained when 100 mg/l of Cr(VI) was reduced by A. globiformis 151B in the presence of different concentrations of Zn(II) (Fig. 4b). It follows from this figure that the maximum amount of Cr(V) and Cr(III) complexes was generated at 50 mg/l of Zn(II) in the nutrient medium and then was decreased with the increase of zinc content in the nutrient medium. Besides, it follows from Fig. 4b that the number of Cr(V) diols was already decreased significantly at low concentrations of Zn, while the number of Cr(III) complexes was decreased gradually. For example, at 200 mg/l of Zn(II), the Cr(V) ESR signal intensity was almost halved and the Cr(III) ESR signal intensity decreased only by about 15%. Earlier we established that the reduction of Cr(VI) by Arthrobacter species begins at the surface of bacteria [5]. Therefore, it can be suggested that the high concentrations of Zn made the contact of Cr(VI) with the bacterial surface impossible and, as a result, caused the decrease of transformation of Cr(VI) to Cr(V).
Conclusions
In bacteria isolated from polluted basalts in the Republic of Georgia—A. globiformis 151B and Arthrobacter sp. 61B—we studied: (1) the uptake of different concentrations of Cr(VI) in the presence of 50 mg/l of Zn(II), and (2) the uptake of different concentrations of Zn in the presence of 100 mg/l of Cr(VI). AAS measurements revealed that the addition of Zn(II) to the tested bacterial cells significantly enhanced the accumulation of Cr. Experiments also revealed that the accumulation of Zn is dose-dependent and it follows the Langmuir–Freundlich model well in both bacteria. The comparative analysis of zinc accumulation showed that this process took place differently in the different bacterial strains. However, in both cases the presence of zinc had a positive effect on the growth of bacteria.
According to the ESR data, the presence of Zn(II) ions did not change the nature of Cr(V) and Cr(III) complexes generated during the reduction of Cr(VI) by A.globiformis 151B. Besides, the behavior of Cr(V) and Cr(III) complexes was found to be similar to that of complexes generated in the Arthrobacter–Cr(VI) system without 50 mg/l of Zn(II). However, high concentrations of Zn(II) (more than 200 mg/l) made the contact of Cr(VI) with the bacterial surface impossible and caused the decrease of transformation of Cr(VI) to Cr(V) and then to Cr(III).
References
Asatiani N, Abuladze M, Kartvelishvili T, Bakradze N, Sapojnikova N, Tsibakhashvili N, Tabatadze L, Lejava L, Asanishvili L, Holman HY (2004) Effect of Cr(VI) action on Arthrobacter oxydans. Curr Microbiol 49:321–326
Bruins MR, Kapil S, Oehme F (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207
Choudhury R, Srivastava S (2001) Zinc resistance mechanisms in bacteria. Curr Sci 81:768–775
Codd R, Lay PA, Tsibakhashvili NY, Kalabegishvili TL, Murusidze IG, Holman HY (2006) Chromium(V) complexes generated in Arthrobacter oxydans by simulation analysis of EPR spectra. J Inorg Biochem 100:1827–1833
Gadd GM, Griffiths A (1978) Microorganisms and heavy metal toxicity. Microb Ecol 4:303–317
Gadd GM (1990) Heavy metal accumulation by bacteria and other microorganisms. Experientia 46. Birkhauser, Basel, pp 834–840
Hardoyo KJ, Ohtake H (1991) Effect of heavy metal cations on chromate reduction by Enterobacter cloacae strain H01. J Gen Appl Microbiol 37:519
Ishibashi Y, Cervantes C, Silver S (1990) Chromium reduction in Pseudomonas putida. Appl Environ Microbiol 56:2268–2270
Kalabegishvili TL, Tsibakhashvili NY, Holman HY (2003) Electron spin resonance (ESR) study of chromium(V) formation and decomposition by basalt-inhabiting bacteria. Environ Sci Technol 37:4678–4684
Kalabegishvili TL, Tsibakhashvili NY, Murusidze IG, Pataraya DT, Gurielidze MA, Holman HY (2006) Formation of Cr(V) and Cr(III) in Arthrobacter oxydans exposed to high concentrations of Cr(VI). In: Mendez-Vilas A (ed) Modern multidisciplinary applied microbiology. Wiley-VCH, Weinheim, pp 516–520
Levina A, Codd R, Dillon C, Lay PA (2002) Chromium in biology: toxicology and nutritional aspects. Prog Inorg Chem 51:145–250
Loveland-Curtze J, Sleridan PP, Gutshall KK, Brenchley JE (1999) Biochemical and phylogenetic analysis of psychrophilic isolates belonging to the Arthrobacter subgroup and description of Arhtrobacter psychrolactophilus sp. nov. Arch Microbiol 171:355–363
Marthur SP, Paul EX (1967) Microbial utilization of soil humic acids. Can J Microbiol 13:573–580
McLean J, Berveridge TJ (2001) Chromate reduction by a Pseudomonad isolated from a site contaminated with chromate copper arsenate. Appl Environ Microb 67:1076–1089
Mosulishvili LM, Kirkesali EI, Belokobilsky AI, Khizanishvili AI, Frontasyeva MV, Gundorina SF, Oprea CD (2002) Epithermal neutron activation analysis of blue-green algae Sp. platensis as a matrix for selenium-containing pharmaceuticals. J Radioanal Nucl Chem 252:15–20
Ohnesorge FK, Wilhelm M (1991) Zinc. In: Merian E (ed) Metals and their compounds in the environment. Wiley-VCH, Weinheim, pp 1309–1342
Rouch DA, Lee BT, Morby AP (1995) Understanding cellular responses to toxic agents: a model for mechanism-choice in bacterial metal resistance. J Ind Microbiol Biotechnol 14:132–141
Seltmann G, Host O (2002) The bacterial cell wall. Springer, New York, pp 33–34
Slaveykova VI, Wilkinson KJ (2005) Predicting the bioavailability of metals and metal complexes: critical review of the biotic ligand model. Environ Chem 2:9–24
Suzuki Y, Banfield J (2004) Resistance to accumulation of uranium by bacteria from a uranium-contaminated site. Geomicrobiol J 21:113–121
Tsibakhashvili NY, Mosulishvili LM, Kalabegishvili TL, Pataraya DT, Gurielidze MA, Nadareishvili GS, Holman HY (2002) Chromate-resistant and reducing microorganisms in Georgia basalts: their distribution and characterization. Fresenius Environ Bull 11(9A):562–567
Tsibakhashvili NY, Mosulishvili LM, Kalabegishvili TL, Kirkesali EI, Murusidze IG, Kerkenjia SM, Frontasyeva MV, Holman HY (2008) Biotechnology of Cr(VI) transformation into Cr(III) complexes. J Radioanal Nucl Chem 278:565–569
Tsibakhashvili NY, Kalabegishvili TL, Rcheulishvili AI, Murusidze IG, Kerkenjia SM, Rcheulishvili OA, Holman HY (2009) Decomposition of Cr(V)-diols to Cr(III) complexes by Arthrobacter oxydans. Microb Ecol 57:360–365
Yamada Y, Motoi H, Kinoshita S, Takada N, Okoda H (1975) Oxidative degradation of squalene by Arthrobacter species. Appl Microbiol 29:400–404
Zhao H, Eide DJ (1996) The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci U S A 93:2454–2458
Acknowledgments
This work was funded by Grant # STCU-GNSF 4330/131 from the Ukrainian Science and Technology Centre (STCU) and Georgian National Science Foundation (GNSF). We gratefully acknowledge Prof. D. Pataraya for providing bacterial samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsibakhashvili, N.Y., Kalabegishvili, T.L., Rcheulishvili, A.N. et al. Effect of Zn(II) on the reduction and accumulation of Cr(VI) by Arthrobacter species. J Ind Microbiol Biotechnol 38, 1803–1808 (2011). https://doi.org/10.1007/s10295-011-0967-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-0967-y