Abstract
Nanoparticles (NPs) are becoming more widely produced, used, and released into the aquatic environment. In aquatic ecosystems, these NPs affect different populations of photosynthesizing organisms, such as cyanobacteria. This study aimed to evaluate the effects of titanium dioxide (TiO2) NPs (48 mg l−1) combined with low (0.04 mM) and high (9 mM) concentrations of urea and nitrate on Microcystis aeruginosa. Microcystins (MCs) production and release were monitored in the cyanobacterium. The results showed that high urea concentration (9 mM) combined with TiO2 NPs inhibited growth, pigment, and malondialdehyde (MDA) content by 82%, 63%, and 47%, respectively. The treatment also increased the reactive oxygen species (ROS) and glutathione S-transferase (GST) activity by 40.7% and 67.7%, respectively. Similarly, low nitrate (0.04 mM) combined with TiO2 NPs inhibited growth by 40.3% and GST activity by 36.3% but stimulated pigment production and ROS concentration in M. aeruginosa. These responses suggest that high urea combined with TiO2.NPs and high nitrate combined with TiO2 NPs induced oxidative stress in cyanobacteria. The peroxidase (POD) activity of M. aeruginosa decreased by 17.7% with increasing urea concentrations. Our findings suggest that TiO2 NPs combined with changing nutrient (urea and nitrate) concentrations may adversely affect cyanobacterial development and antioxidant defense enzymes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to advances in nanotechnology, the concentration of nanoparticles (NPs) released into water bodies during their manufacture, transport, use, and disposal cycles has been steadily increasing (Ju-Nam & Lead 2008). One of the most popular manufactured nanoparticles is nanosized titanium dioxide (TiO2 NPs), which is increasingly used in numerous consumer items. The detrimental impact of nanoparticles on aquatic ecosystems has recently garnered much attention (Piotrowska-Niczyporuk et al. 2012). According to estimates, titanium dioxide nanoparticle concentration in effluents from wastewater treatment plants could reach 3 mg/L, which is anticipated to soon rise in the natural ecosystem (Keller et al. 2010; Liang et al. 2019; Xiao et al. 2019; Zhang et al. 2020). To ensure the safety of nanotechnologies and manufacture materials that have minimal adverse effects on aquatic ecosystems, it is essential to carefully analyze the environmental and ecological impacts of extensive nanoparticle use. The worldwide environmental impact of significant NP applications must be considered extensively (Song et al. 2012).
Titanium dioxide nanoparticles have been extensively studied for their inhibitory effects on algae, zooplankton, fish, and higher plants (Chen et al. 2012; Ilona 2008; Wiench et al. 2009). When suspended particles, such as titanium dioxide nanoparticles, come into contact with cyanobacteria, they can alter their photosynthesis and antioxidant defense mechanisms. In some studies, TiO2 NPs exposure decreased the growth and nitrogen fixation of Anabaena variabilis, whereas other studies found no significant association between growth rate and TiO2 NPs dose in cyanobacterial cultures (Cherchi & Gu 2010; Kulacki & Cardinale 2012). Similarly, Chlamydomonas reinhardtii and Ochromonas danica exposed to a mixture of Ag nanoparticles (10 nm) and sorbed carboxylated PS nanoparticles (20 nm) suffered an additive decline in their physiological parameters (Huang et al. 2019). However, combining glyphosate and nPS-NH2 had antagonistic interactive effects on M. aeruginosa growth inhibition because they resulted in comparatively lower effects than their effects (Zhang et al. 2018a, b).
There have also been some documented benefits of TiO2 NPs in contrast to their negative consequences. For example, Zheng et al. (2005) found that TiO2 NPs increase the germination, growth, dry weight, pigment production, and metabolism of plants. Nanoparticles affect nutritional dynamics, nutrient (nitrogen and phosphorus) uptake, and metabolism (Chaudhary & Singh 2020; Chen et al. 2012). Nitrogen (N) and phosphorus (P) are the most vital nutrients in waterways, but aquatic environments also deal with environmental contaminants, such as TiO2 NPs (Liang et al. 2019). In a field investigation, Das et al. (2014) found that the interaction of phosphate fluctuations with silver nanoparticles resulted in a 70–90% reduction in phytoplankton biovolumes. The carbohydrate levels of phytoplankton community were dramatically affected by changing nitrogen levels in the presence of n-TiO2, as opposed to the overall lipid and protein content (Dauda et al. 2020). Microalgal growth, a significant source of nutrition for several small invertebrates and fish, depends on macronutrients (Chaffin & Bridgeman 2014). Cyanobacterial biomass is controlled by nitrate and urea concentrations and availability (Hampel et al. 2019; Li et al. 2016). While nitrogen forms and concentrations are critical in determining phytoplankton growth, aquatic ecosystems face the reality of the presence and increasing concentrations of emerging environmental contaminants like TiO2 NPs.
Aquatic nitrogen mainly exists in the form of nitrate (NO3−), ammonium (NH4+), and urea (Donald et al. 2011). Aquatic ecosystems receive increased quantities of bioavailable nitrogen (Elser et al. 2009) and TiO2 NPs (Tripathi et al. 2016). In light of the likelihood that nanoparticles interact with nutrients that regulate the development of cyanobacteria, it is critical to study the possible interactive effect of nitrogen forms combined with potential pollutants such as TiO2 NPs (Mahler et al. 2012; Dauda et al. 2017). Studies on the effect of TiO2 NPs on M. aeruginosa under various nitrogen forms and concentrations are scarce. Cyanobacteria under varying nitrogen forms and concentrations are more likely to interact with suspended materials such as TiO2 NPs, affecting their photosynthesis and antioxidant protection mechanisms. Therefore, this study aimed to examine the effects of TiO2 NPs under varying nitrogen forms on M. aeruginosa growth, oxidative stress response, and microcystin production. The findings of this research will enhance our knowledge of the impact of TiO2 NPs on aquatic ecosystems. Our results provide insight into the consequences of nitrogen forms, their concentrations, and TiO2 NPs exposure on M. aeruginosa.
Materials and methods
Sample collection and maintenance
The cyanobacterium M. aeruginosa (EAWAG 198) was acquired from the Gobler Laboratory of the State University of New York, USA. M. aeruginosa was kept in BG11 medium (pH 7.4) under carefully regulated laboratory settings, including light intensity of 40 µmol m−2 s−1, photoperiod of 16:8 h light: dark, and temperature of 23 ± 1 °C. Growth medium was autoclaved (121 °C for 30 min) to ensure sterility. The cyanobacteria were acclimated by a series of culture transfers during the exponential growth phase to acquire accurate physiological results. Pre-cultures were all axenic and samples for the studies were grown under nutrient-sufficient conditions. Cultures were in the exponential growth phase when the experimental vessel was filtered and inoculated to ensure enough biomass and the proper physiological state for effective observation.
Experimental design
Nitrate was provided as sodium nitrate (NaNO3) and urea as carbamide (CH4N2O). Sigma-Aldrich (Sigma-Aldrich, USA) provided the titanium dioxide anatase nano-powder (CAS number: 637254), having a particle size of 25 nm. The BG 11 medium was used to suspend the TiO2 powder to prepare a stock solution. We exposed exponentially growing cultures to low nitrate/urea, high nitrate/urea, low nitrate/urea + TiO2 NPs, and high nitrate or urea + TiO2 NPs in 250 mL of modified BG 11. At the same time, experimental control cultures were grown in unmodified BG 11 medium without TiO2 NPs. Each experimental condition was tested in triplicate. All cultures were started at a cell density of 5.0 × 105 cells mL−1. The study lasted seven days, and samples were taken on the first, third, fifth, and seventh days to measure growth, microcystin, biochemical, and antioxidant responses.
Our limited and high concentrations of both urea and nitrate were 0.04 mM and 9 mM, respectively. These concentrations were based on the levels reported in oligotrophic and eutrophic aquatic environments (Reynolds 2006). The actual urea and nitrate concentrations in the medium were determined using the Magnesium Oxide-Devarda alloy method, where the first distillation in 2% boric acid was with Mg oxide to estimate the ammonia content, and the second distillation in fresh 2% boric acid was performed with Devarda alloy to estimate the nitrate content (Bremner & Keeney 1965). The TiO2 NPs level used in this study was 48 mg L−1, which showed substantial activity in phytoplankton, according to our previous studies (Dauda et al. 2017, 2020). The increasing discharge of NPs into aquatic ecosystems may present scenarios where organisms are exposed to concentrations approaching the milligram range (Arabia 2010). The Agilent Technologies Calibration mix 1 for AA and ICP OES was used for the preparation of titanium standards. An Agilent 4200 microwave plasma-atomic emission spectroscopy (Agilent Technologies, CA-USA) was used to measure the amount of TiO2 NPs in the cultures.
Collection of data
Measurements of cell density, growth, and pigment content
Microcystis aeruginosa cells were counted using an improved bright-lined Neubauer hemocytometer under a light microscope. Daily growth observations were also performed using a UV–VIS spectrophotometer to determine optical density at 750 nm. Specific growth rate (μ) was determined according to Liang et al. (2013).
For chlorophyll-a quantification, 10 mL of M. aeruginosa culture was centrifuged at 4000 rpm for 10 min. The resulting pellets were extracted with 3 mL acetone at − 20 °C for 24 h in the dark. The absorbance of the extracts was measured using a UV–VIS spectrophotometer, and the total chlorophyll-a concentrations were calculated using the equation of Ritchie (2006).
Quantification of total proteins and oxidative stress parameters
Cultures were extracted to quantify total proteins and oxidative stress parameters. A 40 mL culture aliquot collected from the cultures was centrifuged at 4000 rpm for 10 min, and the resulting pellets were stored at − 20 °C. The pellets were homogenized in a vortex for 10 s before being mixed with 2.5 mL of 0.1 M phosphate buffer [pH 6.5, containing 1% (w/v) polyvinylpyrrolidone (PVP)]. The resulting homogenate was centrifuged at 4000 rpm for 10 min, and the supernatant was stored at − 20 °C until analysis.
Bovine serum albumin (BSA) was used as a standard to quantify the total intracellular protein from the extracted supernatant, according to the Bradford (1976) technique. The supernatant (500 µL) was mixed with Bradford reagent (2.5 mL) containing 0.01% Coomassie blue, 4.7% methanol, and 8.5% phosphoric acid. After incubation at room temperature for 5 min, the absorbance was measured at 595 nm. The concentration of BSA used as a standard ranged from 10 to 400 μg mL−1 in phosphate buffer solution (pH 7.2).
The Jana & Choudhuri (1982) approach was used to extract and quantify the intracellular hydrogen peroxide (H2O2). 400 µL H2O2 extract was combined with 133 µL 0.1% titanium chloride in 20% H2SO4. After one minute, the absorbance of the red–orange mixture at 410 nm was measured spectrophotometrically. Using the extinction coefficient of 0.28 L mmol−1 cm−1, the amount of H2O2 in µmol/mg was calculated.
Malondialdehyde (MDA) levels in the cells of M. aeruginosa were measured to determine lipid peroxidation according to the method of Heath & Packer (1968). One milliliter of the extract was added to 2 mL 10% trichloroacetic acid and 0.5% thiobarbituric acid. The mixture was maintained at 95 °C in a water bath for 15 min before being cooled quickly in an ice bath. A spectrophotometer was used to read the absorbance of the mixture at 532 and 600 nm, and an extinction coefficient of 155 mM−1 cm−1 was used to compute the MDA content.
Glutathione S-transferase (GST) activity was assessed following the method of Habig et al. (1974). One hundred microliters of enzyme extract was combined with 2 mL of the reaction mixture (3.6 mM reduced glutathione and 1 mM 1-chloro-2,4-dinitrobenzene in 0.1 M potassium phosphate buffer, pH 6.5) to start the reaction. The GST activity showed a linear relationship with the change in absorbance at 340 nm. The method of Reddy et al. (1996) for measuring peroxidase activity was used. After combining 3 mL of pyrogallol solution (0.05 M in 0.1 M phosphate buffer, pH 6.5) and 0.5 mL of 1% H2O2, 0.1 mL of enzyme extract was introduced into the mixture in a cuvette. The activity of the enzyme, measured in nkat per mg protein, was directly related to the change in spectrophotometric absorbance at 430 nm per minute.
Quantification of microcystins
Microcystins were extracted from the cell pellet obtained after centrifuging 20 mL culture aliquots of M. aeruginosa for 10 min at 4000 rpm. The pellet was vortexed for 10 s in 3 mL of 80% methanol before storing at − 20 °C. (Turner et al. 2018). Microcystin levels were quantified with the Abraxis Microcystins-ADDA ELISA 96 well plate kit (Eurofins Abraxis Inc., Warminster, PA, USA), with absorbance read at 450 nm.
Statistical analyses
A two-way analysis of variance (ANOVA) was used to measure significant differences among the treatments. Where significant differences occurred, Tukey's HSD test separated the means at a 5% significance level. The homogeneity of variance and normality of the experimental data were determined using Levene’s homogeneity of variance and Shapiro–Wilk tests, respectively. Principal component analysis (PCA) was used to ascertain whether the response parameters and treatments were related.
Results
Cell density, chlorophyll content, total protein, and specific growth rate of M. aeruginosa
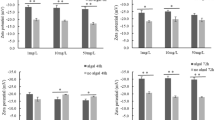
The cell density of M. aeruginosa on day 7 (Fig. 1a) was significantly (p < 0.05) reduced by all treatments (low and high nitrogen and urea and their combinations with TiO2 NPs), with high urea combined with TiO2 NPs producing the lowest cell density. Chlorophyll a content of M. aeruginosa (Fig. 1b) declined to a lesser degree than cell density to the treatments, with only high urea and high urea combined with TiO2 NPs producing significantly (p < 0.05) reduced chlorophyll a. The growth rate of M. aeruginosa (Fig. 1c) was significantly (p < 0.05) reduced by urea (both low and high) and all urea and nitrate combined with TiO2 NPs treatments, and the total protein content of M. aeruginosa was significantly (P < 0.05) increased by the low urea combined with TiO2 exposure, whereas high nitrate and its combination with TiO2 NPs resulted in the lowest total protein content (Fig. 1d).
Cell density (cells mL−1) (a), chlorophyll-a (µg mL−1) (b), total protein (pg cell−1) at day 7 (c), and specific growth rate (day−1) at day 7 (d) of Microcystis aeruginosa EAWAG 198 exposed to low and high urea and nitrate concentrations, and their combinations with titanium dioxide nanoparticles. Error bars indicate the standard deviation for a sample size of 3. Bars with different letters are significantly different (p < 0.05)
Response of Microcystis aeruginosa EAWAG 198 cells to oxidative stress
The intracellular H2O2 content of M. aeruginosa (Fig. 2a) increased significantly (p < 0.05) in the urea treatments (both low and high urea) and in all nitrate and urea combinations with TiO2 NPs. However, a significant (p < 0.05) decrease in H2O2 was recorded in nitrate-only (low and high) treatments. The peroxidase activity of M. aeruginosa increased significantly (p < 0.05) after high urea treatment (Fig. 2b). To a greater degree, the combination of TiO2 NPs with high and low nutrient (urea and nitrate) concentrations stimulated POD activity (p < 0.05). Because of lipid peroxidation, the increase in malondialdehyde (MDA) content can be used to evaluate the extent of membrane lipid damage. High urea significantly (p < 0.05) increased the MDA levels of M. aeruginosa (Fig. 2c), whereas the combination of high nitrate, and low and high urea with TiO2 NPs produced the lowest MDA levels. High urea combined with TiO2 NPs caused the greatest increase in GST activity in M. aeruginosa, whereas nitrate (low and high) combined with TiO2 NPs produced the lowest GST activity (Fig. 2d).
Changes in intracellular H2O2 (pM cell−1) (a), malondialdehyde content (pM cell−1) (b), peroxidase activity (nKat 10–6 cells) (c), and glutathione-S-transferase activity (nKat 10–6 cells) (d) of Microcystis aeruginosa EAWAG 198 after 7 days of exposure to low and high urea and nitrate concentrations, and their combinations with titanium dioxide nanoparticles. Error bars show the standard deviation for a sample size of 3. Bars with different letters are significantly different (p < 0.05)
Microcystin production by Microcystis aeruginosa
Microcystin concentrations significantly (p < 0.05) increased when M. aeruginosa was subjected to both low and high amounts of nitrate in the culture medium (Fig. 3). The highest significant (p < 0.05) stimulation of microcystins was recorded for low nitrate combined with TiO2 NPs and high urea combined with TiO2 NPs exposure (Sadiq et al. 2011).
Microcystins concentration (fg cell−1) of Microcystis aeruginosa EAWAG 198 after 7 days of exposure to low and high urea and nitrate and their combinations with titanium dioxide nanoparticles. Error bars show the standard deviation for a sample size of 3. Bars with different letters are significantly different (p < 0.05) different
Relationship between response parameters of the investigated organisms and treatment conditions: PCA
The first two principal components represented 61.6% of the total variation in the data generated from experiments involving M. aeruginosa (Fig. 4). The highest growth and biomass (cell density and chlorophyll-a content) of M. aeruginosa were recorded in the control treatment. However, M. aeruginosa growth and biomass were negatively correlated with peroxidase activity and H2O2 content.
Principal component analysis biplot of biomass, growth, antioxidant response, and microcystin content of Microcystis aeruginosa as a function of low and high nitrate and urea levels and their combination with TiO2 NPs. The variables on opposing axes are negatively related, while those on the same orthogonal axis are positively correlated
Discussion
Growth and pigment content of Microcystis aeruginosa
In this study, the cell density, chlorophyll a, and growth rate of M. aeruginosa responded differently to the two concentrations (low and high) of both nitrate and urea and their combinations with TiO2 NPs. Cell density was most sensitive to the treatments, indicating that cyanobacterial cell division was the first to be impaired. The difference in the growth response of M. aeruginosa to nitrate and urea shows that this cyanobacterium preferentially uses nitrate and not urea as a nitrogen source. In this study, we found that high urea (9 mM) inhibited the chlorophyll-a content and growth of M. aeruginosa, whereas high nitrate (9 mM) level maintained these response variables within the control range. Thus, depending on the nutrient and TiO2 NPs scenario, cyanobacteria are likely to change their intracellular carbon and nitrogen equilibria (Cherchi et al. 2021). Phytoplankton species, such as Microcystis, have preferences for the form of nitrogen and the concentration thresholds needed for optimum growth (Glibert et al. 2016).
Our findings extend the understanding of the impact of urea on M. aeruginosa growth, showing that while low concentrations maintain high growth rates, high concentrations of urea do not. Thus, the extra nitrogen building blocks and low energy investment (Finlay et al. 2010), when urea is used as a nitrogen source, become compromised at substantially high concentrations, resulting in M. aeruginosa growth reduction. Another explanation for the influence of high urea on cyanobacteria may be related to the cost decision by the cell to either produce microcystins or invest in growth. Therefore, the carbon and nitrogen produced by urea hydrolysis are probably used for physiological processes other than growth and photosynthesis, such as toxin synthesis (Erratt et al. 2018; Harke et al. 2016). However, when uptake and cellular incorporation are impaired, high concentrations of ammonium cation in the medium further create unfavorable growth conditions for cyanobacteria and microalgae (Collos and Harrison 2014; Dai et al. 2012; Glibert et al. 2016). Previous studies by Wu et al. (2015) and Dai et al. (2016) revealed that high urea levels hindered the growth and development of M. aeruginosa, whereas lower concentrations stimulated growth. On the other hand, our findings support the existing knowledge that Microcystis spp. can withstand high nitrogen levels, explaining the non-significant differences between the control and high nitrate conditions. It is also essential to note that the control also contained nitrate as the nitrogen source, albeit at a lower concentration than the high nitrate treatment. These concentrations fall within the levels reported for natural waters that experience Microcystis blooms (Brêda-Alves et al. 2021).
A considerable decrease in cell density, growth, chlorophyll a, and total protein was recorded under high urea concentration and in combination with TiO2 NPs. The reduction in the growth of M. aeruginosa as a result of the addition of TiO2 NPs could have been a result of the TiO2 NPs coating phytoplankton cells and self-shading, which inhibited cell division (Aruoja et al. 2009; Hartmann et al. 2010; Clément et al. 2013). Similarly, Dauda et al. (2017) found that TiO2 NPs suppressed the growth of Chlorella vulgaris in a nutrient-rich environment. Treatment with TiO2 NPs decreased the growth rate and photosystem II activity, while boosting the LPO of Scenedesmus obliquus, Anabaena sp., and Chattonella subsalsa (Li et al. 2020; Tang et al. 2013). These changes indicate the induction of oxidative stress. The high urea concentration in the culture medium could also have aggregated with TiO2 NPs, causing damage to the cell membrane by increasing its permeability and disrupting its structural integrity (Ma et al. 2014; Perreault et al. 2012). Such changes alter the structural integrity of organelles, consequently affecting their function. Our findings also demonstrated a decline in the chlorophyll-a levels of M. aeruginosa when exposed to high urea and TiO2 NPs, which correlated with the additive inhibitory effect recorded on the growth of the cyanobacterium. Following our findings, (Deng et al. 2017) also discovered that 40 mg L−1 TiO2 NPs inhibited photosynthetic activity in Phaedactylum tricornutum in a nutrient-replete culture. Exposure to TiO2 NPs has been associated with reduced chlorophyll content, which leads to a reduction in electron transport through PSII, and an overall decrease in photosynthetic activity in phytoplankton (Wu et al. 2019). High nitrogen generally interacts with different pollutants to cause antagonistic effects, as our findings showed that high nitrate and urea combined with TiO2 NPs had an interactive effect in reducing the cell density of M. aeruginosa.
Oxidative stress, POD, and GST activity of Microcystis aeruginosa
During photosynthesis, chloroplasts are more likely to produce more reactive oxygen species ROS, which leads to high cellular ROS levels. Phytoplankton stress leads to ROS overproduction, which can disrupt normal metabolism by oxidizing lipids, nucleic acids, and proteins. Our study showed that high urea and nitrate induced high intracellular H2O2 content, lipid peroxidation, and antioxidant enzyme activity (POD and GST) in M. aeruginosa. Fluctuations in POD and GST activities defend cells against the harmful effects of ROS and lipid peroxidation (Pejić et al. 2009). The combination of TiO2 NPs with different nitrate and urea concentrations increased the levels of H2O2, but to varying degrees depending on the nutrient concentration. A smaller increase in H2O2 was produced in the TiO2 NPs treatment combined with high nitrate than in that combined with high urea, which means that high nitrate provided a better growth condition. This hypothesis is further supported by the change in POD activity, which is not surprising as H2O2 is among the major substrates of the enzyme. Despite the differences in POD and GST activities to suppress the toxic effects of ROS (Pejić et al. 2009; Xia et al. 2014) induced by the investigated conditions, membrane damage via peroxidation was significantly increased by high urea treatment.
Despite being dependent on the form and concentration of nitrogen, the changes in oxidative stress recorded in M. aeruginosa when subjected to TiO2 NPs under different nutrient conditions confirmed the TiO2 NPs’ stress-inducing mechanism in cyanobacteria (Melegari et al. 2013; Planchon et al. 2013). The augmented GST activity observed under high urea plus TiO2 NPs revealed that the enzyme detoxified the TiO2 NPs (Li et al. 2015; Xu et al. 2021). GST repairs oxidized macromolecules in damaged cellular organelles. Hence, the highest GST activity was observed under the treatment (high urea combined with nanoparticles) that caused the most elevated stress and growth inhibition.
Microcystin production
Microcystis spp. toxin concentrations can be regulated in part by nutrients (Chia et al. 2018). Microcystins biosynthesis is associated with nitrogen availability (Harke et al. 2016; Harke & Gobler 2013). In the current study, the stimulation of microcystin production by M. aeruginosa when treated with low and high nitrate concentrations was most likely caused by toxin production in these circumstances. It is generally hypothesized that microcystins are produced in proportion to their growth. Nevertheless, M. aeruginosa cultured under low/high nitrate and high urea conditions in this study had a decreased growth rate and increased microcystin concentration. Accordingly, our results showed that nitrogen forms and concentrations affected microcystin production, but did not show that microcystin production was controlled by growth. This outcome is in agreement with previous findings (Chen et al. 2019; Peng et al. 2018; Wagner et al. 2021). We discovered that a combination of low nitrate and high urea with titanium dioxide increased microcystin formation. This stimulation could be because microcystin is synthesized as a defense mechanism to protect/adapt the cells to stress (Chen et al. 2010; Wang et al. 2017). In several studies, increased microcystin production has also been linked to the suppression of protein membrane formation and cell permeability (Zhang et al. 2018a, b). Microcystin synthesis is promoted by high inorganic nitrate (Hu 2006; Pimentel et al. 2014) and low urea concentrations, but it is decreased by high urea concentration (Hu 2006; Pimentel et al. 2014; X. Wu et al. 2015). However, some studies show that amino acids and urea can make M. aeruginosa produce more microcystins.
Conclusion
The results of this study reveal variable responses of M. aeruginosa to urea and nitrate levels, together with their combinations with TiO2 NPs, depending on the treatment conditions. Cyanobacteria were more sensitive to changes in urea conditions with and without TiO2 NPs. Specifically, growth and chlorophyll a synthesis inhibition were recorded, and oxidative stress was generally induced when M. aeruginosa was exposed to high urea and high urea combined with TiO2 NPs conditions. Variations in pigment content lead to changes in light absorption and photosynthesis. These findings indicate that the increasing presence of urea and nanometals in the environment could influence aquatic organisms and decrease the population structure and primary productivity of M. aeruginosa in the aquatic environment. Therefore, the effect of TiO2 NPs on aquatic organisms in water bodies may depend on the form and concentration of nitrogen. Adding TiO2 NPs to high urea disrupted normal metabolism by oxidizing lipids, resulting in lipid peroxidation by 43% compared to the control. Microcystin production was also stimulated by high urea and low nitrate combined with TiO2 NPs by 81% and 97%, respectively, to combat the adverse effects of the induced stress.
Data availability
The corresponding author will provide the datasets produced and analyzed during the current work upon request.
References
Arabia S (2010) Differential responses of epiphytic and planktonic toxic cyanobacteria to allelopathic substances of the submerged macrophyte Stratiotes aloides. Int Rev Hydrobiol. https://doi.org/10.1002/iroh.200911219
Aruoja V, Dubourguier HC, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407(4):1461–1468. https://doi.org/10.1016/j.scitotenv.2008.10.053
Brêda-Alves F, de Oliveira Fernandes V, Cordeiro-Araújo MK, Chia MA (2021) The combined effect of clethodim (herbicide) and nitrogen variation on allelopathic interactions between Microcystis aeruginosa and Raphidiopsis raciborskii. Environ Sci Pollut Res 28(9):11528–11539. https://doi.org/10.1007/s11356-020-11367-x
Bremner JM, Keeney DR (1965) Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal Chimica Acta 32:485–495. https://doi.org/10.1016/S0003-2670(00)88973-4
Chaffin JD, Bridgeman TB (2014) Organic and inorganic nitrogen utilization by nitrogen-stressed cyanobacteria during bloom conditions. J Appl Phycol 26(1):299–309. https://doi.org/10.1007/s10811-013-0118-0
Chaudhary I, Singh V (2020) Titanium dioxide nanoparticles and its impact on growth, biomass and yield of agricultural crops under environmental stress: a review. Res J Nanosci Nanotechnol 10(1):1–8. https://doi.org/10.3923/rjnn.2020.1.8
Chen W, Liu HL, Zhang Q, Dai S (2010) Effect of nitrite on growth and Microcystins production of 30 Microcystis aeruginosa PCC7806. J Appl Phycol 23:665–671
Chen H, Chen J, Guo Y, Wen Y, Liu J, Liu W (2012) Evaluation of the role of the glutathione redox cycle in Cu ( II ) toxicity to green algae by a chiral perturbation approach. Aquat Toxicol 120–121:19–26. https://doi.org/10.1016/j.aquatox.2012.04.011
Chen Q, Wang M, Zhang J, Shi W, Mynett AE, Yan H, Hu L (2019) Physiological effects of nitrate, ammonium, and urea on the growth and microcystins contamination of Microcystis aeruginosa: implication for nitrogen mitigation. Water Res 163:114890. https://doi.org/10.1016/j.watres.2019.114890
Cherchi C, Gu AZ (2010) Impact of titanium dioxide nanomaterials on nitrogen fixation rate and intracellular nitrogen storage in Anabaena variabilis. Environ Sci Technol 44(21):8302–8307. https://doi.org/10.1021/es101658p
Cherchi C, Lin Y, Gu AZ (2021) Nano-titanium dioxide exposure impacts nitrogen metabolism pathways in cyanobacteria. Environ Eng Sci 38(6):469–480. https://doi.org/10.1089/ees.2020.0150
Chia MA, Jankowiak JG, Kramer BJ, Goleski JA, Huang IS, Zimba PV, do Carmo Bittencourt-Oliveira M, Gobler CJ (2018) Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae 74:67–77. https://doi.org/10.1016/j.hal.2018.03.002
Clément L, Hurel C, Marmier N (2013) Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants—effects of size and crystalline structure. Chemosphere 90(3):1083–1090. https://doi.org/10.1016/j.chemosphere.2012.09.013
Collos Y, Harrison PJ (2014) Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar Pollut Bull 80(1–2):8–23. https://doi.org/10.1016/j.marpolbul.2014.01.006
Dai GZ, Shang JL, Qiu BS (2012) Ammonia may play an important role in the succession of cyanobacterial blooms and the distribution of common algal species in shallow freshwater lakes. Glob Change Biol 18(5):1571–1581. https://doi.org/10.1111/j.1365-2486.2012.02638.x
Dai R, Wang P, Jia P, Zhang Y, Chu X, Wang Y (2016) A review of factors affecting microcystins production by algae in aquatic environments. World J Microbiol Biotechnol 32:51. https://doi.org/10.1007/s11274-015-2003-2
Das P, Metcalfe CD, Xenopoulos MA (2014) Interactive effects of silver nanoparticles and phosphorus on phytoplankton growth in natural waters. Environ Sci Technol 48(8):4573–4580
Dauda S, Chia MA, Bako SP (2017) Toxicity of titanium dioxide nanoparticles to Chlorella vulgaris beyerinck (beijerinck) 1890 (trebouxiophyceae, chlorophyta) under changing nitrogen conditions. Aquat Toxicol 187:108–114. https://doi.org/10.1016/j.aquatox.2017.03.020
Dauda S, Gabriel AM, Idris OF, Chia MA (2020) Combined nanoTiO2 and nitrogen effects on phytoplankton: a mesocosm approach. J Appl Phycol 32(5):3123–3132. https://doi.org/10.1007/s10811-020-02187-0
Deng XY, Cheng J, Hu XL, Wang L, Li D, Gao K (2017) Biological effects of TiO2 and CeO2 nanoparticles on the growth, photosynthetic activity, and cellular components of a marine diatom Phaeodactylum tricornutum. Sci Total Environ 575:87–96. https://doi.org/10.1016/j.scitotenv.2016.10.003
Donald DB, Bogard MJ, Finlay K, Leavitt PR (2011) Comparative effects of urea, ammonium, and nitrate on phytoplankton abundance, community composition, and toxicity in hypereutrophic freshwaters. Limnol Oceanogr 56(3):2161–2175. https://doi.org/10.4319/lo.2011.56.6.2161
Elser JJ, Andersen T, Baron JS, Bergström AK, Jansson M, Kyle M, Nydick KR, Steger L, Hessen DO (2009) Shifts in lake N: P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326(5954):835–837. https://doi.org/10.1126/science.1176199
Erratt KJ, Creed IF, Trick CG (2018) Comparative effects of ammonium, nitrate and urea on growth and photosynthetic efficiency of three bloom-forming cyanobacteria. Freshw Biol 63(7):626–638. https://doi.org/10.1111/fwb.13099
Finlay K, Patoine A, Donald DB, Bogard MJ, Leavitt PR (2010) Experimental evidence that pollution with urea can degrade water quality in phosphorus-rich lakes of the Northern Great Plains. Limnol Oceanogr 55(3):1213–1230. https://doi.org/10.4319/lo.2010.55.3.1213
Glibert PM, Wilkerson FP, Dugdale RC, Raven JA, Dupont CL, Leavitt PR, Parker AE, Burkholder JM, Kana TM (2016) Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol Oceanogr 61(1):165–197. https://doi.org/10.1002/lno.10203
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hampel JJ, McCarthy MJ, Neudeck M, Bullerjahn GS, McKay RML, Newell SE (2019) Ammonium recycling supports toxic planktothrix blooms in sandusky bay, lake erie: evidence from stable isotope and metatranscriptome data. Harmful Algae 81:42–52. https://doi.org/10.1016/j.hal.2018.11.011
Harke MJ, Gobler CJ (2013) Global transcriptional responses of the toxic cyanobacterium, Microcystis aeruginosa, to nitrogen stress, phosphorus stress, and growth on organic matter. PLoS ONE. https://doi.org/10.1371/journal.pone.0069834
Harke MJ, Davis TW, Watson SB, Gobler CJ (2016) Nutrient-controlled niche differentiation of western lake erie cyanobacterial populations revealed via metatranscriptomic surveys. Environ Sci Technol 50(2):604–615. https://doi.org/10.1021/acs.est.5b03931
Hartmann NB, Von der Kammer F, Hofmann T, Baalousha M, Ottofuelling S, Baun A (2010) Algal testing of titanium dioxide nanoparticles—testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 269(2–3):190–197. https://doi.org/10.1016/j.tox.2009.08.008
Heath L, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch biochem Biophys 125(1):189–198
Hu H (2006) Nitrate and phosphate supplementation to increase toxin production by the marine dinoflagellate Alexandrium tamarense. Mar Pollut Bull 52:756–760. https://doi.org/10.1016/j.marpolbul.2005.11.005
Huang B, Wei ZB, Yang LY, Pan K, Miao AJ (2019) Combined toxicity of silver nanoparticles with hematite or plastic nanoparticles toward two freshwater algae. Environ Sci Technol 53(7):3871–3879. https://doi.org/10.1021/acs.est.8b07001
Illona V (2008) Aquatic ecotoxicity tests of some nanomaterials. Environ Toxicol Chem 27(9):1942–1947
Jana S, Choudhuri MA (1982) Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat Botany 12:345–354
Ju-Nam Y, Lead JR (2008) Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2008.06.042
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Robert M, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44(6):1962–1967
Kulacki KJ, Cardinale BJ (2012) Effects of nano-titanium dioxide on freshwater algal population dynamics. PloSOne. https://doi.org/10.1371/journal.pone.0047130
Li F, Liang Z, Zheng X, Zhao W, Wu M, Wang Z (2015) Toxicity of nano-TiO 2 on algae and the site of reactive oxygen species production. Aquat Toxicol 158:1–13. https://doi.org/10.1016/j.aquatox.2014.10.014
Li J, Zhang J, Huang W, Kong F, Li Y, Xi M, Zheng Z (2016) Comparative bioavailability of ammonium, nitrate, nitrite and urea to typically harmful cyanobacterium microcystis aeruginosa. Mar Pollut Bull 110(1):93–98. https://doi.org/10.1016/j.marpolbul.2016.06.077
Li R, Li T, Zhou Q (2020) Impact of titanium dioxide (TiO 2) modification on its application to pollution treatment—a review. Catalysts 10(7):804
Liang F, Wen X, Geng Y, Ouyang Z, Luo L, Li Y (2013) Growth rate and biomass productivity of Chlorella as affected by culture depth and cell density in an open circular photobioreactor. J Microbiol Biotechnol 23(4):539–544. https://doi.org/10.4014/jmb.1209.09047
Liang X, Cui S, Li H, Abdelhady A, Wang H (2019) Removal effect on stormwater runoff pollution of porous concrete treated with nanometer titanium dioxide. Transp Res Part D 73:34–45. https://doi.org/10.1016/j.trd.2019.06.001
Ma S, Zhou K, Yang K, Lin D (2014) Hetero-agglomeration of oxide nanoparticles with algal cells: effects of particle type, ionic strength and pH. Environ Sci Technol 49(2):932–939. https://doi.org/10.1021/es504730k
Mahler GJ, Esch MB, Tako E, Southard TL, Archer SD, Glahn RP, Shuler ML (2012) Oral exposure to polystyrene nanoparticles affects iron absorption. Nat Nanotechnol 7:264–271. https://doi.org/10.1038/nnano.2012.3
Melegari SP, Perreault F, Costa RHR, Popovic R, Matias WG (2013) Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat Toxicol 142:431–440. https://doi.org/10.1016/j.aquatox.2013.09.015
Pejić S, Todorović A, Stojiljković V, Kasapović J, Pajović SB (2009) Antioxidant enzymes and lipid peroxidation in endometrium of patients with polyps, myoma, hyperplasia and adenocarcinoma. Reprod Biol Endocrinol 7:1–9. https://doi.org/10.1186/1477-7827-7-149
Peng G, Martin RM, Dearth SP, Sun X, Boyer GL, Campagna SR, Lin S, Wilhelm SW (2018) Seasonally relevant cool temperatures interact with N chemistry to increase microcystins produced in lab cultures of microcystis aeruginosa NIES-843. Environ Sci Technol 52(7):4127–4136. https://doi.org/10.1021/acs.est.7b06532
Perreault F, Bogdan N, Morin M, Claverie J, Popovic R (2012) Interaction of gold nanoglycodendrimers with algal cells (Chlamydomonas reinhardtii) and their effect on physiological processes. Nanotoxicology 6(2):109–120. https://doi.org/10.3109/17435390.2011.562325
Pimentel JSM, Giani A, Pimentel JSM, Giani A (2014) Microcystin production and regulation under nutrient stress conditions in toxic Microcystis Strains. Appl Environ Microbiol 80(18):5836–5843. https://doi.org/10.1128/AEM.01009-14
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Z̈yłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65. https://doi.org/10.1016/j.plaphy.2011.11.009
Planchon M, Jittawuttipoka T, Cassier-chauvat C, Guyot F, Gelabert A, Benedetti MF, Chauvat F, Spalla O (2013) Exopolysaccharides protect Synechocystis against the deleterious effects of Titanium dioxide nanoparticles in natural and artificial waters. J Colloid Interface Sci 405:35–43. https://doi.org/10.1016/j.jcis.2013.05.061
Reddy JK, Suga T, Mannaerts GP, Lazarow PB, Subramani S (eds) (1996) Peroxisomes: biology and role in toxicology and disease. Annals of the New York academy of sciences, vol 804. New York Academy of Sciences, New York, pp 1–795
Reynolds CS (2006) Ecology of phytoplankton. Cambridge University Press, Cambridge
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41. https://doi.org/10.1007/s11120-006-9065-9
Sadiq IM, Dalai S, Chandrasekaran N, Mukherjee A (2011) Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp. Ecotoxicol Environ Safety 74(5):1180–1187. https://doi.org/10.1016/j.ecoenv.2011.03.006
Song G, Gao Y, Wu H, Hou W, Zhang C, Ma H (2012) Physiological effect of anatase TiO2 nanoparticles on Lemna minor. Environ Toxicol Chem 31(9):2147–2152. https://doi.org/10.1002/etc.1933
Tang Y, Li S, Qiao J, Wang H, Li L (2013) Synergistic effects of nano-sized titanium dioxide and zinc on the photosynthetic capacity and survival of Anabaena sp. Int J Mol Sci. https://doi.org/10.3390/ijms140714395
Tripathi DK, Gaur S, Singh S, Singh S, Pandey R, Singh VP, Sharma NC, Prasad SM, Dubey NK, Chauhan DK (2016) An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2016.07.030
Turner AD, Dhanji-rapkova M, Neill AO, Coates L, Lewis A, Lewis K (2018) Analysis of microcystins in cyanobacterial blooms from freshwater bodies in England. Toxins 10(1):39. https://doi.org/10.3390/toxins10010039
Wagner ND, Quach E, Buscho S, Ricciardelli A, Kannan A, Naung SW, Phillip G, Sheppard B, Ferguson L, Allen A, Sharon C, Duke JR, Taylor RB, Austin BJ, Stovall JK, Haggard BE, Chambliss CK, Brooks BW, Scott JT (2021) Nitrogen form, concentration, and micronutrient availability affect microcystin production in cyanobacterial blooms. Harmful Algae 103:102002. https://doi.org/10.1016/j.hal.2021.102002
Wang Z, Chen Q, Hu L, Wang M (2017) Combined effects of binary antibiotic mixture on growth, microcystin production, and extracellular release of Microcystis aeruginosa: application of response surface methodology. Environ Sci Pollut Res 25:736–748
Wiench K, Wohlleben W, Hisgen V, Radke K, Salinas E, Zok S, Landsiedel R (2009) Chemosphere acute and chronic effects of nano- and non-nano-scale TiO 2 and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere 76(10):1356–1365. https://doi.org/10.1016/j.chemosphere.2009.06.025
Wu X, Yan Y, Wang P, Ni L, Gao J, Dai R (2015) Bioresource Technology Effect of urea on growth and Microcystins production of Microcystis aeruginosa. Biores Technol 181:72–77. https://doi.org/10.1016/j.biortech.2015.01.035
Wu D, Yang S, Du W, Yin Y, Zhang J, Guo H (2019) Effects of titanium dioxide nanoparticles on Microcystis aeruginosa and Microcystins production and release. J Hazard Mater 377:1–7. https://doi.org/10.1016/j.jhazmat.2019.05.013
Xia B, Chen B, Sun X, Qu K, Ma F, Du M (2014) Science of the Total Environment Interaction of TiO 2 nanoparticles with the marine microalga nitzschia closterium: growth inhibition, oxidative stress and internalization. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2014.11.066
Xiao B, Zhang Y, Wang X, Chen M, Sun B (2019) Occurrence and trophic transfer of nanoparticulate Ag and Ti in the natural aquatic food web of Taihu Lake China. Environ Sci Nano. https://doi.org/10.1039/c9en00797k
Xu K, Li Z, Juneau P, Xiao F, Lian Y, Zhang W, Shu L, Jiang H, Zhang K, Wang C, Wang S, Yan Q, He Z (2021) Toxic and protective mechanisms of cyanobacterium Synechocystis sp. in response to titanium dioxide nanoparticles. Environl Pollut 274:116508. https://doi.org/10.1016/j.envpol.2021.116508
Zhang M, Wang X, Tao J, Li S, Hao S, Zhu X, Hong Y (2018a) PAHs would alter cyanobacterial blooms by affecting the microcystin production and physiological characteristics of Microcystis aeruginosa. Ecotoxicol Environ Saf 157:134–142. https://doi.org/10.1016/j.ecoenv.2018.03.052
Zhang Q, Qu Q, Lu T, Ke M, Zhu Y, Zhang M, Zhang Z, Du B, Pan X, Sun L, Qian H (2018b) The combined toxicity effect of nanoplastics and glyphosate on microcystis aeruginosa growth. Environ Pollut 243:1106–1112. https://doi.org/10.1016/j.envpol.2018.09.073
Zhang J, Jiang L, Wu D, Yin Y, Guo H (2020) Effects of environmental factors on the growth and microcystin production of microcystis aeruginosa under TiO2 nanoparticles stress. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.139443
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Funding
No specific grant was provided for this research by funding organizations in the public, private, or nonprofit sectors.
Author information
Authors and Affiliations
Contributions
SSA collected data and wrote the main manuscript text, and MAC and SD performed data analysis and prepared all the Figs. 1, 2, 3, 4. WNY, MAC, WSJ, and JH supervised the project and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no known competing financial interests or personal ties that could be perceived as having influenced the work described in this study.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samuel, S.A., Chia, M.A., Yusufu, W.N. et al. Nitrogen forms and concentration influence the impact of titanium dioxide nanoparticles on the biomass and antioxidant enzyme activities of Microcystis aeruginosa. Arch Microbiol 205, 177 (2023). https://doi.org/10.1007/s00203-023-03500-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03500-4