Abstract
The degradation of two pesticides, carbofuran (CBF) and ioprodine (IPR), was studied by the photolytic decomposition of hydrogen peroxide (UV/H2O2). The influence of two experimental parameters, H2O2 concentration and initial pH, as well as their interactions, was investigated. Optimization was carried out where experimental parameters were determined for the treatment of each pesticide. Both pesticides were totally eliminated by UV/H2O2 system under optimal conditions. However, significant differences were found: CBF degradation was influenced by both parameters and their interactions, while IPR degradation was not statistically affected by initial pH. Interestingly, analysis of degradation pathways showed a major influence of photolysis process and oxidation due to hydrogen peroxide for the CBF degradation, while the synergistic combination between both of them played the most relevant role during IPR degradation. A mixture of both pesticides was also submitted to UV/H2O2 action in which a lower rate was observed for IPR elimination while CBF was not affected. A 90 % of chemical oxygen demand (COD) was removed and 75 % of mineralization was achieved after the treatment of the mixture. Almost 92 % of the toxicity was eliminated making this technique a promising process to treat toxic mixtures of these pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
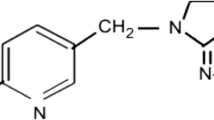
Pesticides are widely used in different forms such as insecticides, herbicides, and fungicides in order to increase agricultural productivity. An example is the carbofuran (CBF), 2,3-dihydro-2,2-dimethyl-7-benzofuranyl methylcarbamate (Fig. 1a), which is widely used in agriculture as an insecticide and it is considered a broad spectrum pesticide mainly used for agricultural crops (Hmimou et al. 2014), ornamental gardens, and plants. CBF is used by spraying upon the plant and the application on the stored crop (Foo 2016; Ruíz-Hidalgo et al. 2016). Among others, there is the iprodione, (IPR) 3-(3,5-dichlorophenyl)-N-isopropyl-2,4-dioxoimidazolidine-1-carboxamide (Fig. 1b), a fungicide of the dicarboximide group. It is mainly used against Botrytis on ornamentals plants such as carnation, chrysanthemum, and rose. It is also used in agricultural crops such as potato, tomato, and onion (Lassalle et al. 2014).
The continuous use of these products has a significant contribution to water contamination (Campos et al. 2015); thus, it raises human health concern, due to their known human toxicity (de Siqueira et al. 2015). Specifically, CBF is known to be highly toxic (LD50 11 mg kg−1 in mice) and inhibitor of acetylcholinesterase, a vital enzyme to the functioning of the central nervous system (Campbell et al. 2004). Also, it has implications as a potential endocrine disruptor (Klotz et al. 1997) and it has a high mobility in soils and a high water solubility (700 mg L−1). Additionally, the presence of this harmful compound in natural waters has been reported (Brkic et al. 2008), which increase the concern about this pollutant. On the other hand, iprodione presents an acute oral toxicity (LD50) >4640 mg kg−1 and an acute dermal toxicity (LD50) >2150 mg kg−1 in rats. It is considered as being mobile in some soils and then will leach to groundwater (Tomlin 1997). In fact, iprodione has been notified to be able to easily diffuse in water; therefore, it can contaminate living organisms of aqueous environments (Wauchope et al. 1992). Additionally, IPR can cause oxidative damage through free radical production. Recently, it has been reported that its presence in natural waters induced oxidative damage in rainbow trout (Camiletti et al. 2016; Radice et al. 2001).
The detrimental impact on the environment caused by these pesticides has turned the attention to the use of feasible treatment technologies for their removal or their transformation into products with no adverse effects on the environment or in human health. Biological treatments are usually the most convenient process to promote natural decontamination (Marco et al. 1997). Unfortunately, many organic pollutants are not biodegradable and so they are known as bio-recalcitrant organic compounds (Trzcinski et al. 2016). This is the case of CBF and IPR which given their relative high toxicity, their degradations could not be reached by biological treatments (de Siqueira et al. 2015). Among the conventional treatments, there are also physical processes, which disadvantage is associated to the pollution phase transfer. Therefore, the need of finding new decontamination processes for these types of compounds increases over the time (Lu et al. 2011a; Zhu et al. 2012).
As alternative technologies of water treatment, advanced oxidation processes (AOPs) have demonstrated to be effective methods for organic pollutants degradation (Chiron et al. 2000; De la Cruz et al. 2013; Brienza et al. 2016; Gupta and Mittal 2016). Particularly, the combination of ultraviolet (UV) light and hydrogen peroxide (H2O2), so-called UV/H2O2 process, has shown high efficiency for the treatment of natural waters and wastewaters contaminated with non-biodegradable and/or toxic organic compounds. The UV/H2O2 process is based in the photolysis of H2O2 to produce hydroxyl radicals (HO•) (Chiron et al. 2000; Zhou et al. 2012), which promotes organic pollutants degradation according to the following reactions:
On the other hand, despite all environmental and human risks of these type of pollutants, up to the date, there are few investigations (Lopez-Alvarez et al. 2011, 2012) were the dangerous pesticides, cabofuran and ioprodine, are intent to be degraded by any AOP but any of those had tested UV/H2O2 process. Moreover, most investigations are based on individual pesticides degradation, but for a real condition, a mixture of the pollutants should be considered. However, under the authors knowledge, there is any report where the effects of a mixture between CBR and IPR have been evaluated. Therefore, the aim of this study is to investigate the efficiency of UV/H2O2 as possible treatment of carbofuran and iprodione, as individual targets, and as a mixture. Operating variables of the UV/H2O2 process, such as pH and hydrogen peroxide dosage, were studied as well as the synergistic and antagonism effects during the pesticides degradation. Degradation routes for each pesticide were also investigated. Finally, the degradation extent in terms of mineralization, chemical oxygen demand, and toxicity removal was also evaluated.
2 Experimental
2.1 Reagents
CBF raw grade (99.10 % of purity), the commercial grade Furadan, with a formulation of 330 g L−1 of CBF and iprodione commercial grade (Rovral), with a formulation of 500 g L−1 of iprodione were supplied by Bayer. IPR raw grade (99.1 % of purity) material was supplied by Bayer Cropscience. Acetonitrile, formic acid, dichloromethane, sodium carbonate, sodium bicarbonate, sodium hydroxide, sulphuric acid, ethyl acetate, and hydrogen peroxide 30 % w/w were supplied by Merck. Potassium hydrogen phthalate was obtained from Carlo Erba. All chemicals were used without any further purification. Milli-Q water was used throughout for the preparation of aqueous solutions. All reagents were the highest available purity and were used as received.
2.2 Experimental Design
In this study, the statistical software Statgraphics Plus 5.1 was used. Using this software, multivariate analysis and factorial design were carried out to evaluate the effects and optimize the two experimental variables: the initial pH (3–9) and the hydrogen peroxide concentration (50–200 mg L−1). Fixed pesticide concentrations were used in order to optimize the process: CBF 55 mg L−1 and IPR 12 mg L−1. Additionally, the polynomial equation associated to the reaction, as well as the response surface and the Pareto diagram, was obtained for both pesticides.
This study was performed with a confidence level of 95 %. The degradation percentage was chosen as the response factor after 60 min of irradiation for CBF and 80 min for IPR, which were selected to accomplish with the best statistical validity and qualification of experiments.
2.3 Photolytic System
After pH adjustment of the CBF solution from pH 5.6 (natural pH) and IPR solution from pH 5.3 (natural pH) to the desired initial pH condition, hydrogen peroxide was added at the appropriate concentration and the resulting mixture was stirred. The tests were carried out under constant stirring in a pilot reactor (manufactured by Atlantic Ultraviolet Corporation) containing 19 L of the pesticides solution. The storage tank with the solution was introduced into recirculation system with the reactor. The tests were performed with a mercury lamp low pressure Atlantic Ultraviolet, model MP36B, surrounded by a stainless steel housing. The lamp tube was 86 cm long and 1.5 cm diameter with a maximum output of 38 W. The UV lamp was a monochromatic wavelength of 254 nm. The system had an inlet and outlet of water and a volumetric capacity of 7 L (see SM 1).
2.4 Analysis
The quantification of the pesticides was performed by liquid chromatography (HPLC) in an Agilent 1100 Series instrument. A Zorbax SB C18 column and a diode array UV detector, set at 220 nm for CBF and 210 nm for IPR, were used. The mobile phase was 40/60 acetonitrile/water for CBF and 40/60 acetonitrile/water with formic acid 0.1 % for IPR. Isocratic mode at 1 mL min−1 for both pesticides was used. For the quantification of the pesticides mixture, IPR conditions were used.
The complete mineralization of organic compounds, i.e., the transformation of organic matter into CO2, H2O and inorganic ions, was done by measuring the total organic carbon (TOC). This was determined in a COT1010 OI-Analytical instrument. During the irradiation time, samples were taken at timed intervals and filtered with filters of 0.45 μm PVDF Millipore Millex®-HV to remove impurities. A solution of potassium phthalate was used as the calibration standard. Chemical oxygen demand (COD) measurements were carried out according to the closed reflux titrimetric method (APHA 2005) in a Nanocolor 500D instrument. The reduction of toxicity was analyzed by using Vibrio Fisheri with a Biofix® Lumi-10 instrument. Chlorine quantification was determined by ionic chromatography Dionex ICS-1000 equipped with an IonPac®AS14A column.
3 Results and Discussion
3.1 Effect of H2O2 Concentration and Initial pH During the Degradation of Carbofuran and Iprodione
Two of the most important chemical parameters in the evaluation of UV/hydrogen peroxide system are the initial pH of the solution and the hydrogen peroxide concentration (Rubio-Clemente et al. 2014). In order to evaluate the influence of these two parameters on the degradation of carbofuran and iprodione by UV/H2O2, a factorial design was implemented using the Statgraphics Plus 5.1 software. Table 1 shows the matrix design obtained by the computer program. The table shows the experimental conditions and the response factor for each experiment, defined as the percentage of degradation after 60 and 80 min of treatment for CBF and IPR, respectively.
Figure 2 shows the response surface methodology (RSM) obtained for the two pesticides (Fig. 2a for CBF and Fig. 2b for IPR). As shown in Fig. 2 and Table 1, the elimination of both pesticides increases by the rise of H2O2 concentration. As stated in Eq. 2, the increment of hydrogen peroxide concentration increases the formation of HO• radicals that consequently improve CBF and IPR degradation. However, for both pesticides, maximum degradation is reached when H2O2 concentration was around 170 mg L−1; and then elimination processes became inhibited at higher doses of hydrogen peroxide. This behavior is associated to H2O2 overdosing that would consume radical species (Eqs. 3–6). A similar situation has been previously reported in other investigations (Guzmán et al. 2016).
Significant differences were observed when the effect of initial pH was evaluated. For a relative low concentration of hydrogen peroxide (50 mg L−1), CBF degradation showed to be improved by the increase of pH. In fact, CBF elimination increased almost 15 % from acidic pH (3) to neutral pH (7) reaching up almost 80 % of elimination and followed by a plateau (Fig. 2a). On the other hand, the pH showed a minor effect for a relative high concentration of hydrogen peroxide (200 mg L−1) with a variation of only less than 5 % and reaching up total elimination of CBF at neutral pH. In contrast, for 50 mg L−1 of added H2O2, IPR degradation showed to be negatively affected by the increment of the solution pH (Fig. 2b). In this case, IPR elimination decreased approximately 20 % from acidic medium (pH 3) to basic medium (pH 9) with a maximum of ∼80 % of elimination at pH 5. Interestingly, the pH effect was contrary for 200 mg L−1 of hydrogen peroxide showing a remarkable increment of IPR elimination while the pH increases from 3 to 7, reaching a maximum of ∼90 % of degradation.
These results could be attributed to the self-decomposition of H2O2 at high pH or to the hydroxyl radical scavenger property of the OH− ions which are highly concentrated at basic pH (Zhou et al. 2012) and compete with the pesticides for the produced hydroxyl radicals. However, both effects are less notable for a relative high concentration of H2O2 (200 mg L−1) where the production of HO• radicals is higher and the scavenger effect or the self H2O2 decomposition can be offset. Furthermore, in the case of CBF degradation, this effect is not significant suggesting that an additional degradation process besides HO• radicals could be involved.
Moreover and based on the interaction plots (SM2), no significant interaction between the variables (pH and H2O2 concentration) was obtained during CBF treatment where the changes in the response were almost parallel. In the case of IPR degradation, in an opposite way, inverse tendencies in the change in response factors were observed, which suggests a significant interaction between the variables for this pollutant. These findings support that different degradation routes are involved in the elimination of the pesticides CBF and IPR.
3.2 Optimization of CBF and IPR UV/H2O2 Degradation
In order to optimize the process, it is necessary to evaluate which variables, and interactions between these variables, have a significant effect on the UV/H2O2 process. The Pareto diagram (Fig. 3a for CBF and Fig. 3b for IPR) represents a valuable tool to evaluate this by showing both magnitude and significance of the effects (variables and interactions) (Rodríguez-Chueca et al. 2012). A positive effect of a variable indicates that the CBF or IPR degradation increases at high values of the respective variable, while negative effect indicates that the degradation increases at low values of the variable. According to Fig. 3a, both variables (H2O2 concentration and pH) can be considered statistically important for the treatment of CBF. Positive effects indicate that CBF degradation increases with the increase of hydrogen peroxide concentration and pH value. Additionally, the Pareto diagram shows a negative effect by the interaction between both variables (AB) on the response factor representing an antagonist effect on the degradation process.
Pareto diagram of the degradation of a CBF (55 mg L−1). b IPR (11 mg L−1) by UV/H2O2. Figure obtained with the Statgraphics Plus 5.1 software according to the design of experiments presented in Table 1
On the other hand, Fig. 3b shows that only the H2O2 concentration (B) has significant effect on IPR degradation. Moreover, interaction (AB) between the two variables (pH and H2O2 concentration) shows a synergistic effect, while AA and BB showed a negative contribution represented at the mathematical description (Eqs. 7 and 8). A relevant fact must be mentioned about the initial pH, where despite the observed effect in Fig. 2b, the Pareto diagram indicates that changes are not statistical significant for the degradation of IPR.
This experimental design methodology also leads to obtaining a mathematical model that directly relates the influential variables and interactions with the response factor (% degradation) thereby the study of the pollutant degradation is facilitated. For this purpose, it becomes relevant to involve the sign of the quadratic value of each variable. Then, for both pesticides, AA as well as BB have negative coefficients (Fig. 3a, b). After multiple regressions using the statistical software, the mathematical models are obtained for CBF (Eq. 7) and IPR (Eq. 8), where Y (%) represents the degradation percentage.
Table 1 shows the comparison between experimental results and those predicted by the models (Eqs. 7 and 8). As shown in Table 1, the proposed models predict the experimental results quite accurately. In fact, less than 1.5 % of error was obtained between experimental and calculated data for CBF, while a maximum of 5.5 % of error was observed for IPR degradation. Additionally, the fraction of the variation response (R 2) was 89.5 % for both models, which demonstrates a sufficient validity between them.
Therefore, according to the response surface diagrams (Fig. 2a, b) and the reduced models (Eqs. 7 and 8), the optimal values for the photocatalytical degradation of the pesticides under the work conditions are initial pH 7.28 and 160 mg L−1 of H2O2 for CBF, while initial pH 6.54 and 142 mg L−1 of H2O2 for IPR.
Both pesticides degradation by UV/H2O2 system showed a common pseudo-first order profile which lead to a kinetic model that describes each process (SM 3). Then, the kinetic models can be compared to the statistic models (Eqs. 7 and 8) by determining the elimination of the pollutants under optimal conditions. Based on the models, 7 % of difference between the statistic model and the kinetic model can be observed for the case of CBF degradation and only 4 % for IPR degradation. This small difference indicates a good correlation between both models describing the degradation processes of the pollutants.
3.3 Characteristics of the Photolytic UV/H2O2 Degradation of CBF and IPR Under Optimal Conditions
3.3.1 Main Degradation Pathways
During the degradation of organic pollutants using the photolytic decomposition of hydrogen peroxide, different processes could be involved according to the target molecules. Given that UV/H2O2 is a photolytic process and several organic pollutants are considered photoactive, degradation due to direct photolysis of the pesticides must be evaluated. Additionally, the use of hydrogen peroxide contributes to the degradation of many organic compounds given that it is a powerful oxidative agent. Figure 4a shows the contribution of both UV radiation and hydrogen peroxide oxidation to the degradation of CBF during UV/H2O2 process. For deeper investigation, experiments using the optimal conditions were carried out (initial pH 7.28 and 160 mg L−1 of H2O2 for CBF (55 mg L−1); initial pH 6.54 and 142 mg L−1 of H2O2 for IPR (12 mg L−1).
a CBF degradation profiles by UV radiation, H2O2 action, and UV/H2O2 system under optimal conditions. 55 mg L−1 of CBF; initial pH 7.28 and 160 mg L−1 of H2O2. b IPR degradation profiles by UV radiation, H2O2 action, and UV/H2O2 system; and chlorine accumulation during UV/H2O2 treatment under optimal conditions. 11 mg L−1 of CBF; initial pH 6.54 and 142 mg L−1 of H2O2
A direct photolysis can be observed reaching 30 % of CBF elimination. However, a plateau is obtained after 100 min of irradiation. On the other hand, the effect of hydrogen peroxide as oxidative agent eliminates 50 % of CBF after 140 min of reaction. These results suggest that CBF could be only partially oxidized by UV light or H2O2, making necessary the application of stronger oxidative techniques, such as UV/H2O2, for its complete removal. In contrast, UV/H2O2 process reaches total elimination of CBF after 140 min of treatment. Then, these findings indicate that photolysis as well as H2O2 oxidation and •OH radicals is involved during CBF degradation by UV/H2O2 process.
On the other hand, the degradation profile of IPR shows a different behavior (Fig. 4b). As observed, UV radiation removes only 17 % of the pollutant after 240 min and hydrogen peroxide removes 22 %. These results indicate that IPR can be considered a more resistant compound than CBF against UV or H2O2 action. Despite that, the combined action of UV radiation and the oxidant (UV/H2O2 system) leads to the total elimination of the pesticide after 160 min of treatment.
The contribution of UV radiation and hydrogen peroxide during the oxidation of both pesticides could result in synergistic or antagonistic effects, which must be determined to a better understanding and optimization of the system. Synergy (S) for both CBF and IPR degradation, by UV/H2O2 system, can be determined using the following equation (Torres et al. 2008):
where % Removed corresponds to the removed percentage of each organic pollutant by each applied system (UV/H2O2, UV, and H2O2). An S value larger than 1 indicates a synergistic effect. Oppositely, S values smaller than 1 indicate an antagonistic effect among the combined processes. In spite after 100 min of treatment more than 90 % for both pesticides is eliminated (Fig. 4a, b), interesting differences in synergy were found. In fact, the calculated synergies were S CBF = 1.3 and S IPR = 7.2. The high synergistic effect found during IPR degradation suggests that this pesticide is much more influenced by HO• radicals, which come from the combined action of UV light and hydrogen peroxide. In contrast, the poor synergistic effect for CBF states that photolysis and hydrogen peroxide action, acting individually, play the main role in the process.
Based in the fact that the main degradation pathway of CBF is through photolysis and hydrogen peroxide action, according to the literature, some possible CBF by-products can be proposed. During the photo-decomposition of CBF, the attack on the C-O bond of the carbamate group is probably the first step of the degradation route. The break of this bond leads to a cation on a basic nitrogen and a phenolic anion that is stabilized by an H- or OH-abstraction to produce 7-phenolcarbofuran or carbamic acid, respectively. The continuous irradiation could produce another by-product identified as 2,3-dihydro-2,2-dimethylbenzofuran-7-ol (Bachman and Patterson 1999). Under our knowledge, no by-products coming from the attack of hydrogen peroxide have been reported. On the other hand, as early indicated, in a lower extend CBF is also degraded by HO•. Therefore, the HO• could attack the C-O bond of the carbamate group to produce 7-phenolcarbofuran and carbamic acid as initial step. Subsequently, hydroxylations have been reported leading to 3-hydroxycarbofuran and 3-hydroxy-7-phenolcarbofuran followed by successive oxidations to produce 3-ketocarbofuran and 3-keto-7-phenolcarbofuran (Fenoll et al. 2013; Lu et al. 2011b; Lopez-Alvarez et al. 2012).
Regarding the IPR degradation, little information is currently available describing its degradation. Given that IPR is a dichloride organic molecule, the evolution of Cl- was also investigated during this pesticide degradation process. Results show a perfect opposite behavior of IPR elimination with chlorine ion accumulation (Fig. 4b, secondary axis). The inverse relationship between the target molecule and Cl- indicates an easily loss of Cl ring substituents, suggesting the HO• attack into the carbon-chloride bond as one of the firsts steps during IPR degradation. The susceptibility of these bonds have been also reported during the photo-decomposition of IPR leading to the substitution of the Cl atoms by OH groups (Lassalle et al. 2014).
3.3.2 Degradation Extend by H2O2 Photolytic Process
It is well known that pollutant removal is not enough evidence to test the efficiency of certain applied treatment. Previous reports have shown that in some cases, AOPs can transform the initial substrate into more dangerous contaminants (Giraldo et al. 2010). That is the case of halogenated organic molecules that could be degraded into hazardous compounds such as mono, di or tri-chloromethane and chlorobenzenes, which are known to be high carcinogenic molecules. Therefore, in order to guarantee the efficiency of the treatment of waters contaminated with CBF or IPR, the evolution of COD, TOC, and the toxicity (1/EC50) of the photo-treated solutions were evaluated (Fig. 5a, b, respectively). For the case of CBF, the toxicity of the initial solution was considerably reduced from 0.09 to almost 0.01 after 390 min of treatment. Additionally, only 16 % of COD and 18 % of TOC remains in the solution at the end of the treatment (390 min). Under this panorama, toxicity could be attributed either to the remained concentration of pesticide or to the presence of toxic degradation by-products. However, the decreasing behavior of both COD and TOC suggest the hazardous compounds could be totally eliminated so as their toxicity with longer process.
In the case of IPR, after 240 min, solution toxicity (1/EC50) was reduced into ∼88 % (Fig. 5b). In it turn, COD measurement showed an elimination of ∼82 % after 240 min, while at the same time, TOC was 76 % removed. Additionally, the elimination tendencies of COD, TOC, and toxicity rapidly achieved a plateau after 160 min of treatment, which is the time where IPR was totally removed from the treated solution. These findings suggest that toxicity is mainly attributed to IPR, but also there some intermediates that are recalcitrant to UV/H2O2 action although they could be considered less dangerous given that toxicity was significantly removed. Furthermore, the TOC removal from the beginning of the experiment suggests that in addition to the IPR dechlorination, others parallel degradation routes, which lead to a fast mineralization of the pesticide, also occur.
The previous discussed differences in terms of synergy during both pesticides degradation turn interesting to investigate this phenomenon for the degradation extents. In order to evaluate this, COD and TOC removal were calculated for each involved process after 60 and 100 min of treatment, respectively (data not shown). Regarding CBF, 14 % of COD and 4 % of TOC was removed by UV irradiation. The sole H2O2 action leads to 8 % of COD and 8 % of TOC removal, while for the combination UV/H2O2 57 % of COD and 26 % of TOC removal was observed. The same experiment was performed with IPR as target molecule. Results show that 15 and 8 % of COD and TOC were removed in the solution using the sole UV irradiation. 12 % of COD and 12 % of TOC were eliminated by hydrogen peroxide action but 72 and 70 % of COD and TOC, respectively, were eliminated by the combination of the processes (UV/H2O2 system). Using Eq. 9, synergistic effect for the degradations extents (S COD and S TOC) can be determined: S COD,CBF = 2.6, S TOC,CBF = 2.1, S COD,IPR = 2.7 and S TOC,IPR = 3.5. These results indicate a synergistic effect on both the COD and TOC removal for both pesticides during UV/H2O2 system attributed to the formation of hydroxyl radicals, which efficiently promote the oxidation of the organic compounds.
3.4 Evaluation of the Photocatalytical Degradation of CBF and IPR in a Mixture
Despite UV/H2O2 system have shown to be an efficient technique to degrade the two tested pesticides, it becomes important to investigate the viability of the process to treat a solution containing a mixture of both pollutants. To evaluate this, a new set of experiments were carried out using an equimolar solution containing 3.3 × 10−5 mol L−1 of each pesticide at pH 7.00 and 150 mg L−1 of H2O2. Figure 6a shows the degradation profiles of the two pesticides individually and both of them at the mixture. Interestingly, CBF showed the same profile in the mixture as individually, which indicates that the presence of IPR in the mixture did not affect the elimination of carbofuran. In the presence and absence of iprodione, carbofuran is practically eliminated after 100 min of treatment. In an opposite way, IPR elimination was significantly inhibited by the presence of CBF. In fact, in the presence of CBF, UV/H2O2 process reached ∼65 % of IPR elimination after 120 min, while 100 % of IPR elimination was achieved at the same time in its absence. As above stated, there is a contribution of each individual process (UV light and H2O2) to promote CBF degradation. Consequently IPR, which is mainly degraded by HO• radicals, do not affect CBF degradation. On the other hand, CBF consume a considerably proportion of both photons and H2O2 and therefore hydroxyl radicals are generated in a lesser extent. As a result, IPR degradation is inhibited by presence of CBF.
On the other hand, Fig. 6b shows the evolution of TOC and COD for the mixture of both pesticides. Results showed a ∼90 % of COD elimination and a ∼75 % of TOC removal. The remaining COD and TOC in solution could be attributed to the amount of IPR that could not be oxidized after 300 min of treatment in the mixture solution and the presence of some by-products. A notable tendency of COD and TOC reduction is observed, which makes possible the application of this technique in a mixture of two pollutants. In order to provide more information about the treated solution, toxicity evolution was determined. As observed in Fig. 6b, almost 92 % of the mixture toxicity was eliminated after only 300 min of treatment, which confirms the high potential of the UV/H2O2 as alternative to the environmental concerns associated to the presence of these pesticides in waters.
4 Conclusions
The evaluation of H2O2 and initial pH effects by experimental design methodology showed that both parameters are statistically significant during CBF degradation. However, for IPR degradation, H2O2 concentration was the only variable statistically significant. The optimal conditions were determined for each pesticide: pH 7.28 and 160 mg L−1 of H2O2 for CBF, while pH 6.54 and 142 mg L−1 of H2O2 for IPR. Different processes were involved during the degradation of the pesticides. CBF degradation was mainly promoted by the additive action of direct photolysis and hydrogen peroxide oxidation. In contrast, the main degradation route of IPR was to the attack of HO• radicals produced by the UV/H2O2 system. Additionally, the inverse relationship between the released chloride ions and IPR along the treatment suggested the attack of HO• radicals into the C-Cl bond as the first step in the degradation process. Both pesticides were totally eliminated under optimal conditions by UV/H2O2 system; where approximately 80 % of COD and TOC were eliminated for CBR and IPR after 6 and 4 h of treatment, respectively. Different behaviors were found when a mixture of the two pesticides was treated. CBF degradation was not significantly affected by the presence of IPR in the mixture solution, while IPR elimination was inhibited. A 90 % of COD was removed during the treatment of an equimolar mixture of the pesticides and 75 % of mineralization was reached after the treatment. The toxicity of the mixture solution was reduced in about 92 %, which highlight the application of this technique as alternative for the treatment of toxic mixtures of the pesticides, transforming them into non-toxic substances.
Abbreviations
- AOPs:
-
Advanced oxidation processes
- CBF:
-
Carbofuran
- COD:
-
Chemical oxygen demand
- EC50:
-
Half maximal effective concentration
- IPR:
-
Iprodione
- LD50:
-
Median lethal dose
- RSM:
-
Response surface methodology
- S:
-
Synergy
- TOC:
-
Total organic carbon
- UV/H2O2:
-
Ultraviolet light radiation and hydrogen peroxide process
References
APHA. (2005). Standard methods for the examination of water and wastewater (Twenty oneth ed.). Washington: American Public Health Association.
Bachman, J., & Patterson, H. (1999). Photodecomposition of the carbamate pesticide carbofuran: kinetic and the influence of dissolved organics matter. Journal of Environmental Science and Technology, 33, 874.
Brienza, M., Mahdi Ahmed, M., Escande, A., Plantard, G., Scrano, L., Chiron, S., et al. (2016). Use of solar advanced oxidation processes for wastewater treatment: follow-up on degradation products, acute toxicity, genotoxicity and estrogenicity. Chemosphere, 148, 473–480. doi:10.1016/j.chemosphere.2016.01.070.
Brkic, D. V., Vitorovic, S. L., Gasic, S. M., & Neskovic, N. K. (2008). Carbofuran in water: subchronic toxicity to rats. Environmental Toxicology and Pharmacology, 25(3), 334–341. doi:10.1016/j.etap.2007.11.002.
Camiletti, B. X., Asensio, C. M., Gadban, L. C., Pecci, M. P. G., Conles, M. Y., & Lucini, E. I. (2016). Essential oils and their combinations with iprodione fungicide as potential antifungal agents against withe rot (Sclerotium cepivorum Berk) in garlic (Allium sativum L.) crops. Industrial Crops and Products, 85, 117–124. doi:10.1016/j.indcrop.2016.02.053.
Campbell, S., David, M. D., Woodward, L. A., & Li, Q. X. (2004). Persistence of carbofuran in marine sand and water. Chemosphere, 54(8), 1155–1161. doi:10.1016/j.chemosphere.2003.09.018.
Campos, M., Perruchon, C., Vasilieiadis, S., Menkissoglu-Spiroudi, U., Karpouzas, D. G., & Diez, M. C. (2015). Isolation and characterization of bacteria from acidic pristine soil environment able to transform iprodione and 3,5-dichloraniline. International Biodeterioration & Biodegradation, 104, 201–211. doi:10.1016/j.ibiod.2015.06.009.
Chiron, S., Fernandez-Alba, A., Rodriguez, A., & Garcia-Calvo, E. (2000). Pesticide chemical oxidation: state-of-the-art. Water Research, 34(2), 366–377. doi:10.1016/S0043-1354(99)00173-6.
De la Cruz, N., Esquius, L., Grandjean, D., Magnet, A., Tungler, A., de Alencastro, L. F., et al. (2013). Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Research, 47(15), 5836–5845. doi:10.1016/j.watres.2013.07.005.
de Siqueira, A., Salvagni, F. A., Yoshida, A. S., Gonçalves-Junior, V., Calefi, A. S., Fukushima, A. R., et al. (2015). Poisoning of cats and dogs by the carbamate pesticides aldicarb and carbofuran. Research in Veterinary Science, 102, 142–149. doi:10.1016/j.rvsc.2015.08.006.
Fenoll, J., Hellín, P., Flores, P., Martínez, C. M., & Navarro, S. (2013). Degradation intermediates and reaction pathway of carbofuran in leaching water using TiO2 and ZnO as photocatalyst under natural sunlight. Journal of Photochemistry and Photobiology A: Chemistry, 251, 33–40. doi:10.1016/j.jphotochem.2012.10.012.
Foo, K. Y. (2016). Value-added utilization of maize cobs waste as an environmental friendly solution for the innovative treatment of carbofuran. Process Safety and Environmental Protection, 100, 295–304. doi:10.1016/j.psep.2016.01.020.
Giraldo, A. L., Peñuela, G. A., Torres-Palma, R. A., Pino, N. J., Palominos, R. A., & Mansilla, H. D. (2010). Degradation of the antibiotic oxolinic acid by photocatalysis with TiO2 in suspension. Water Research, 44(18), 5158–5167. doi:10.1016/j.watres.2010.05.011.
Gupta, M. K., & Mittal, A. K. (2016). Integrated biological and advanced oxidation based treatment of hexamine bearing wastewater: effect of cow-dung as a co-substrate. Journal of Hazardous Materials, 308, 394–401. doi:10.1016/j.jhazmat.2016.01.072.
Guzmán, J., Mosteo, R., Sarasa, J., Alba, J. A., & Ovelleiro, J. L. (2016). Evaluation of solar photo-Fenton and ozone based processes as citrus wastewater pre-treatments. Separation and Purification Technology, 164, 155–162. doi:10.1016/j.seppur.2016.03.025.
Hmimou, A., Maslouhi, A., Tamoh, K., & Candela, L. (2014). Experimental monitoring and numerical study of pesticide (carbofuran) transfer in an agricultural soil at a field site. Comptes Rendus Geoscience, 346(9–10), 255–261. doi:10.1016/j.crte.2014.03.003.
Klotz, D. M., Arnold, S. F., & McLachlan, J. A. (1997). Inhibition of 17 beta-estradiol and progesterone activity in human breast and endometrial cancer cells by carbamate insecticides. Life Sciences, 60(17), 1467–1475. doi:10.1016/S0024-3205(97)00098-2.
Lassalle, Y., Jellouli, H., Ballerini, L., Souissi, Y., Nicol, É., Bourcier, S., et al. (2014). Ultraviolet–vis degradation of iprodione and estimation of the acute toxicity of its photodegradation products. Journal of Chromatography A, 1371, 146–153. doi:10.1016/j.chroma.2014.10.051.
Lopez-Alvarez, B., Torres-Palma, R. A., & Penuela, G. (2011). Solar photocatalitycal treatment of carbofuran at lab and pilot scale: effect of classical parameters, evaluation of the toxicity and analysis of organic by-products. Journal of Hazardous Materials, 191(1–3), 196–203.
Lopez-Alvarez, B., Torres-Palma, R. A., Ferraro, F., & Penuela, G. (2012). Solar photo-Fenton treatment of carbofuran: analysis of mineralization, toxicity, and organic by-products. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 47(13), 2141–2150. doi:10.1080/10934529.2012.696029.
Lu, L.-A., Ma, Y.-S., Kumar, M., & Lin, J.-G. (2011a). Photo-Fenton pretreatment of carbofuran—analyses via experimental design, detoxification and biodegradability enhancement. Separation and Purification Technology, 81(3), 325–331. doi:10.1016/j.seppur.2011.07.040.
Lu, L.-A., Ma, Y.-S., Kumar, M., & Lin, J.-G. (2011b). Photochemical degradation of carbofuran and elucidation of removal mechanism. Chemical Engineering Journal, 166(1), 150–156. doi:10.1016/j.cej.2010.10.045.
Marco, A., Esplugas, S., & Saum, G. (1997). How and why combine chemical and biological processes for wastewater treatment. Water Science and Technology, 35(4), 321–327. doi:10.1016/S0273-1223(97)00041-3.
Radice, S., Ferraris, M., Marabini, L., Grande, S., & Chiesara, E. (2001). Effect of iprodione, a dicarboximide fungicide, on primary cultured rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquatic Toxicology, 54(1–2), 51–58. doi:10.1016/S0166-445X(00)00175-2.
Rodríguez-Chueca, J., Mosteo, R., Ormad, M. P., & Ovelleiro, J. L. (2012). Factorial experimental design applied to Escherichia coli disinfection by Fenton and photo-Fenton processes. Solar Energy, 86(11), 3260–3267. doi:10.1016/j.solener.2012.08.015.
Rubio-Clemente, A., Torres-Palma, R. A., & Peñuela, G. A. (2014). Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: a review. Science of the Total Environment, 478, 201–225. doi:10.1016/j.scitotenv.2013.12.126.
Ruíz-Hidalgo, K., Masís-Mora, M., Barbieri, E., Carazo-Rojas, E., & Rodríguez-Rodríguez, C. E. (2016). Ecotoxicological analysis during the removal of carbofuran in fungal bioaugmented matrices. Chemosphere, 144, 864–871. doi:10.1016/j.chemosphere.2015.09.056.
Tomlin, C. D. S. (1997). The pesticide manual. A world compendium (11th ed.). Farnham, Surrey: British Crop Protection Council.
Torres, R. A., Pétrier, C., Combet, E., Carrier, M., & Pulgarin, C. (2008). Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrasonics Sonochemistry, 15(4), 605–611. doi:10.1016/j.ultsonch.2007.07.003.
Trzcinski, A. P., Ganda, L., Yan Ni, A. S., Kunacheva, C., Qing, Z. D., Lin, L. L., et al. (2016). Identification of recalcitrant compounds in a pilot-scale AB system: an adsorption (A) stage followed by a biological (B) stage to treat municipal wastewater. Bioresource Technology, 206, 121–127. doi:10.1016/j.biortech.2016.01.048.
Wauchope, R. D., Buttler, T. M., Hornsby, A. G., Augustijn-Beckers, P. W. M., & Burt, J. P. (1992). Pesticide properties database for environmental decision making. Reviews of Environmental Contamination and Toxicology, 123, 1–157.
Zhou, C., Gao, N., Deng, Y., Chu, W., Rong, W., & Zhou, S. (2012). Factors affecting ultraviolet irradiation/hydrogen peroxide (UV/H2O2) degradation of mixed N-nitrosamines in water. Journal of Hazardous Materials, 231–232, 43–48. doi:10.1016/j.jhazmat.2012.06.032.
Zhu, J., Xu, M., Meng, X., Shang, K., Fan, H., & Ai, S. (2012). Electro-enzymatic degradation of carbofuran with the graphene oxide–Fe3O4–hemoglobin composite in an electrochemical reactor. Process Biochemistry, 47(12), 2480–2486. doi:10.1016/j.procbio.2012.10.006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopez-Alvarez, B., Villegas-Guzman, P., Peñuela, G.A. et al. Degradation of a Toxic Mixture of the Pesticides Carbofuran and Iprodione by UV/H2O2: Evaluation of Parameters and Implications of the Degradation Pathways on the Synergistic Effects. Water Air Soil Pollut 227, 215 (2016). https://doi.org/10.1007/s11270-016-2903-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2903-2