Abstract
Background
Chloronicotinic insecticide are a class of pesticides that are commonly used as insecticides. Among the frequently used chloronicotinic pesticide, imidacloprid (IM) was developed in 1986. The residual of this insecticide or any pesticides may have serious public health threats.
Methods
Both degradation and mineralization of the imidacloprid (IM) in aqueous solution was studied under various experimental conditions using different advanced oxidation processes namely, ultraviolet C (UVC), UVC + TiO2, and UVC + ZnO. All the experiments were performed using a lab-scale batch photoreactor with a working volume of 100 mL equipped with low-pressure mercury vapor lamp (9 W, 18 cm long, Philips Co.), emitting UV radiation with maximum intensity at 254 nm. The possible intermediates and a reaction pathway for photocatalytic degradation of the IM were also evaluated.
Results
It was observed that under optimal condition for UVC/TiO2 process (C0 = 100 mg/L, pH = 7.5, t = 20 min, TiO2 dose = 100 mg/L), IM was effectively degraded (88.15%) and followed the first order kinetics model. The degradation efficiency increased with increasing of illumination time and is more favorable in alkaline pH compared to acidic pH. Degradation of the IM in photocatalytic process was compared with photolysis showing a significant synergy effect in the case of the photocatalytic degradation process, leading at 20 min illumination time to a 36.7% increase of the IM removal efficiency in comparison to the single UVC. The GC/MS chromatograms before and after treatment confirmed the effectiveness of the UVC/TiO2 process in simplifying the nature of IM and its conversion to more simple and degradable compounds.
Conclusion
The heterogeneous UVC/TiO2 process was found to be an efficient chemical-less method that is appropriate for degradation of IM from aqueous phase.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chloronicotinic insecticide are a class of pesticides that are commonly used as insecticides. Among the frequently used chloronicotinic pesticide, imidacloprid (IM) (neonicotinoid class of insecticide) was developed in 1986 [1]. The chemical structure of the IM is shown in Fig. 1 [2]. This insecticide impacts on motor neurons in insects, causes over-stimulation of the nervous system and finally leads to insect death. The residual of this insecticide or any pesticides may also have serious threat to the aquatic environments [1]. These organic compounds enters into the environment as well as water sources mainly through to the effluents from industries and the agricultural activities. Imidacloprid has been detected in various concentration from 2.09 to 3625 ppb in different water sources [3]. Organophosphate insecticides (OPI) are most frequently detected and major emerging anthropogenic contaminants in drinking water sources in the vicinity of production and application sites, particularly in developing countries [4].
Because of the high water solubility, IM is considered as a high potential contaminant of water bodies [5]. Given the potential hazards of accidental exposure to IM and minimization of these harmful effects, it is necessary to treat IM-containing wastewaters through an appropriate method. A wide range of methods including physical, chemical and biological processes have been studied to remove IM from water and effluents [2, 5,6,7,8]. Among these methods, advanced oxidation processes (AOPS) are preferred. The main mechanism of AOPs processes is the production of highly reactive free radicals such as hydroxyl, capable of decomposing biodegradable materials through non-selectively clinging to organic molecules and converting them into water and carbon dioxide via mineralization [5, 8]. These processes have two main categories: homogeneous and heterogeneous. In the homogeneous processes, the reactions take place in the similar phases, like liquid-liquid, but in the heterogeneous type, the reaction take place in different phases, in which, during one or several stages, the electron pairs are produced. The photolysis process occurs when an organic molecule absorbs ultraviolet radiation. UV rays include wide ranges of wavelengths, including UV-A, UV-B, and UV-C. The photocatalytic oxidation process belongs to heterogeneous category, and has attracted a great interest for decontamination of water and wastes streams [9,10,11]. The photocatalytic processes can be commonly defined as applying a combination of UV irradiation in conjunction with a catalyst agent like Fe2O3, CdS, Sb2S3, ZnO, TiO2, etc. resulting in the production of some oxidative radicals such as hydroxyl radical (•OH), where the latter can oxidize the toxic organic compounds into less toxic products [12,13,14]. In this regard, nano-powders like TiO2 and ZnO have most frequently been used as semiconductor catalysts for degradation of different pollutants in recent years [15]. Titanium dioxide (TiO2) that is resistant to chemical corrosion can be used as a catalyst at ambient temperature and pressure. This catalyst can be irradiated by UV light and leads to producing hydroxyl radicals which attack the refractory organic molecule to degrade it into simple compounds. The main advantages of photocatalytic method are ease of operation, low maintenance, the integration into small places, less-expensive, non-toxic, etc. [12, 15, 16]. The photocatalytic degradation of pesticide takes place on the surface of nano-catalysts by trapping radicals of •OH in the holes of reactive species [17]. The main possible reactions are presented in Eqs. (1)–(5) [5]:
It has been reported that the photocatalytic degradation of organic compounds in the solution is caused through the formation of an electron–hole pair on the catalyst surfaces. The high oxidative potential of the hole (\( {\mathrm{hv}}_{\mathrm{b}}^{+} \)) in the catalyst made it possible the direct oxidation of pesticide to the most simple and oxidized intermediates. A more reactive hydroxyl radicals can also be formed either by the hydrolysis of water or by the reaction of hole with •OH. Electrons in the conduction band (\( {\mathrm{e}}_{\mathrm{cb}}^{-} \)) on the catalyst surface can also reduce molecular oxygen to superoxide anion. This radical, in the presence of organic scavengers, may form organic peroxides or hydrogen peroxide. Moreover, the electrons in the conduction band, are also responsible for the production of such hydroxyl radicals [15].
Accordingly, the objective of this study was to evaluate imidacloprid pesticide degradation and mineralization in aqueous solution using different advanced oxidation processes namely, UVC, UVC/TiO2 and UVC/ZnO. The photocatalytic efficacy of the ZnO and TiO2, regarding the degradation and mineralization of IM were evaluated through the defined experimental conditions.
Materials and methods
Reagents and materials
All the chemicals used in this work were of analytical grade quality and were purchased from Merck Co. Imidacloprid, C9H10O2N5Cl (Fig. 1), 1-[(6-chloro-3-pyridinyl) methyl]-N-nitro-2-imidazolidinimine, technical grade (99% purity) was purchased from PharmaChem Company, Thessaloniki, Greece. TiO2 and ZnO were purchased from Degussa Huells (TiO2 P-25 Degussa, anatase/rutile: 3.6/1, surface area 56 m2/g, nonporous and ZnO with specific surface area and band gap energy of 10 m2/g and 2.92 eV, respectively). The main characteristics of TiO2 and ZnO nanoparticles are shown in Table 1. The solution pH was measured using a lab pH meter (model: EC20, HACH Co., USA) and H2SO4 and NaOH were used to adjust the pH wherever necessary. Double distilled water was used to prepare IM solutions with desired concentrations throughout the work. Aqueous stock solution of IM was prepared every week, protected from light and stored at 25 °C.
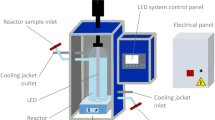
Photoreactor; setup and operational practices
All the experiments for IM degradation were performed using a lab-scale batch photoreactor. A tubular glass photoreactor with a respectively total and working volume of 120 and 100 mL was used to run the experiments. The path length of the reactor was 350 mm. The UVC radiation source for activating TiO2 and ZnO nano-catalysts was a low-pressure mercury vapor lamp (9 W, 18 cm long, Philips Co.) emitting ultraviolet radiation with maximum intensity at 254 nm (flux of 88 μW/cm2 at 1 cm distance from the lamp surface). The UV lamp was mounted in a quartz sleeve having 15 mm diameter.
Figure 2 shows the graphic representation of the experimental setup. For the protecting the lab workers against the UV radiation, the photoreactor apparatus was placed in a wooden chamber. In each test, IM solution with the known concentration was transferred into the reactor. After setting the desired parameter, the lamp was switched on and the suspension was stirred at a constant speed throughout the experiment. The solution in the reaction chamber was sampled at different intervals and analyzed for the target parameters. All experiments were performed in batch mode at ambient temperature (≈25 °C), atmospheric pressure, and at constant stirring of 400 rpm to provide homogenization in the solution. No remarkable increase in liquid temperature was detected in the photoreactor throughout the experiment. The effect of various variables including IM concentration, nano-catalysts dosages, pH and illumination time was assessed on the processes efficiency. In order to demonstrate radical based oxidation of imidacloprid, some compounds that are commonly used as radical scavengers in similar studies including carbonate, sulfate, phosphate, and tert-butanol anions were added to the solution. All the experiments were performed in duplicate and the mean values were reported.
Analysis
To determine the IM concentrations in the influent and effluent samples, HPLC (model LC-2010 AHT, Shimadzu) equipped with UV detector at a wavelength of 270 nm and Phenomenex C-18 column (250 mm × 4.6 mm) was used [19,20,21]. The fate of IM in the photocatalytic process was determined at the optimum experimental conditions using gas chromatography-mass spectrometry (GC-MS) (Agilent Technologies, Tokyo, Japan). A total organic carbon analyzer (Shimadzu Instruments, model TOC-VCSH) was applied for the measurement of dissolved organic carbon (DOC). Scanning electron microscopy (SEM) images were obtained on a JEOL JSM 6700F microscope.
Results and discussion
Scanning electron microscopy (SEM) of nanoparticles
Scanning electron microscopy of ZnO and TiO2 nanoparticles are presented in Fig. 3, where, (a) and (c) revealed the grape-like aggregations of nanoparticles having an appropriate homogeneity [22] for TiO2 and ZnO NPs, respectively. Figure 3b, c illustrate the structure of TiO2 and ZnO NPs aggregations consisting of semi-spherical particles having smoothed surfaces with the average diameter of 30–50 nm for TiO2 and 200–300 nm for ZnO nanoparticles [23]. As a result, the almost one 9th lower diameters of TiO2 NPs comparing with TiO2 NPs confirms 5 times more the TiO2 NPs specific surface area than that of TiO2 NPs.
Effect of initial solution pH on the IM removal
The solubility of neonicotinoides like IM pesticide in aquatic solution depends on multiple factors such as pH, temperature and physical state of the pesticide applied [8]. As seen in Fig. 4, the removal efficiency of IM in both UV/TiO2, and UV/ZnO processes increased with the increase in pH from 3 to 9 with corresponding removal efficiencies of 90.13% and 85.89%, respectively. With the continued increase of pH from 9 to 11, the removal efficiency of IM in UV/TiO2 and UV/ZnO processes was decreased by 6.42% and 7.9%, respectively. However, the lowest removal efficiency was obtained in acidic pH. In contrast, IM removal is more favorable in acidic pH compared to alkaline pH in UVC process. As seen in Fig. 4, at pH = 3 the removal efficiency is 49.28%, but at pH = 11, it is reduced to 26.51%. It should also pointed out that the removal efficiency for all investigated processes is almost constant at pH range from 7 to 9. The pHzpc values for TiO2 and ZnO were equal to 6.5 [24, 25] and 9 [26, 27] and the pKa of IM is 11.12, therefore, IM has a positive charge at pH values less than 11.12. On the other hand, TiO2 and ZnO have a negative charge at the pH values greater than 6.5 and 9, respectively. Accordingly, it can be expected that IM well absorbed on catalyst surfaces in the pH range between 5 and 9, which, in turn, will take part in the reaction with the radicals in the solution and finally decompose [28, 29]. In addition, the optimum pH for ZnO is slightly higher than for TiO2 which is due to their differences in pHzpc. In case of UVC process, the higher IM removal efficacy in the acidic pH arises from pKa and the IM quantum yield.
As at the pH values close to or above 11.12, the ratio of the ionic fraction is increased, but at lower pHs, the molecular fraction ratio of the dissociated IM in the solution will be increased. Therefore, imidacloprid is more active in lower pH or acidic solutions, thus, with a further reduction in pH, the removal efficiency will also be increased [30, 31]. Hong et al. investigated the degradation of imidacloprid with electrolysis. The results of this study showed that pH = 3 was more favorable for IM removal due to the higher efficacy of •OH radicals in acidic conditions [32]. The result of IM removal in UVC process in our study is also consistent with the results of Sedaghat et al. who investigated the imidacloprid degradation by photoelectrofenton and found that acidic conditions are more favorable for IM [33]. Rahmani et al. studied the efficacy of photocatalytic degradation of phenol using UV/TiO2 and found that UV, TiO2 and UV/TiO2 efficiency was in the range of 1.8–19.64%, 2.38–17.8% and 34.62–91.81%, respectively, and the highest removal efficiency was observed at pH = 11, contact time of 9 h, and TiO2 concentration of 0.2 g. Further, phenol removal was also increased with increasing TiO2 dosage, pH, and contact time [34]. Hemmati and coworkers investigated phenolic photocatalytic degradation using UV/TiO2 enriched by trivalent iron and found that ultraviolet radiation alone is less efficient (38.6%) and the highest degradation efficiency (62.4%) was obtained at acidic pH (3). Moreover, the removal efficiency of phenol was decreased with the increase of initial phenol concentration [35]. According to another study, the highest efficiency of photocatalytic oxidation of organophosphorus pesticides using zinc oxide and photocatalytic degradation of metamitron in ZnO water suspensions were at pH = 8–10, where it was shown that at acidic pH, ZnO can react with acids to produce the corresponding salt and dissolution and photodissolution of ZnO considered as the dominant reaction [17, 36].
Abdollahi et al. investigated the removal of benzoquinone 1–4 using ZnO, and showed 100% removal efficiency at pH 9 [37]. Moussavi et al. investigated the photocatalytic degradation of diazinon with ZnO nanoparticles in water and observed that in the presence of catalyst at pH = 7.5, and after 30 min illumination, the removal efficiency of diazinon was 93.3% [4]. Sathiyanarayanan et al. used ZnO photocatalytic process to remove imidacloprid and spirotetramat residues from the water and found highest removal efficiency at the pH 9 [38]. Khani et al. investigated the photocatalytic degradation of azo dye solution by nanosized zero-valent iron-ZnO photocatalyst system and reported that dye removal was the highest in alkaline conditions [15].
Effect of IM initial concentration on the IM removal
The initial concentration of pollutants has a significant impact on the removal efficiency [8]. Based on Fig. 5, when the initial IM concentration increases from 10 to 100 mg/L, mean removal of IM decreased from 100% to 85.15% and from 99.05% to 80.45% for UVC/TiO2 and UVC/ZnO processes, respectively. An increase in initial concentrations of IM reduces the total amount of unoccupied holes (h+) and may decrease the efficacy of formation of OH radicals in the solution [39], which in turn decreased the IM degradation efficiency. Of course, this efficiency reduction is not considerable for the concentrations in the ranges 10 to 100 mg/L, as it is about 15% for UVC/ZnO process and is about 19% for the UVC/TiO2 process. However, further increasing the initial concentration to 150 mg/L, the efficacy of the processes was also decreased by ca. 17% and 21%, respectively. In the case of the UVC process, (as shown in Fig. 5), similar decreasing trend was also observed. For example, the removal efficiency of 65.92% for the initial concentration of 10 mg/L was decreased to only about 11.32% for the initial concentration of 300 mg/L. Therefore, the initial concentration of 100 mg/L was selected as the optimal concentration for the rest of the experiments. Nevertheless, the lower removal efficiency observed in single UVC (e.g. 48.45% for initial concentration of 100 mg/L) compared to other two photocatalytic processes (85.15% for TiO2 at 100 mg/L) was mainly due to that hydroxyl radicals (•OH) was not expected to be formed because of inability of 254 nm irradiation in photolyzing of water [40]. Hemmati and coworkers investigated photocatalytic degradation of phenol using Fe(III)-doped UV/TiO2 and found that the degradation efficiency was decreased with the increasing of phenol concentration [35]. Arbab et al. investigated the photocatalytic degradation of triethyl phosphate using titanium dioxide nano-photocatalyst, and found that at high initial concentrations of triethyl phosphate, the adsorbed molecules of the reactant could occupy the entire catalyst surface, hence reduced the decomposition rate. Besides, the intermediates chemicals produced during the process were competitively adsorbed on the catalyst surface [41] and hindered the further degradation of target pollutant. Khani et al. investigated the photocatalytic degradation of acid orange 7 with ZnO, and found similar results [15].
Effect of illumination time on the IM removal
It can be seen in Fig. 6 that with the increase of reaction time, the removal efficiency of all investigated processes was also increased. As seen, with the increase of illumination time from 0 to 30 min, IM removal efficiency was also increased by 90.37% for UVC/TiO2 process. However, further increase of illumination time to 50 min caused the removal efficiency increase only by ≈ 4%. The main advantage of this process is the relatively lower reaction time compared to other processes, which in turn leads to a reduction in the construction and operating costs. It has also been shown that the removal efficiency was increased a little beyond 30 min reaction time. However, the reaction time over the optimum point can only impose increased energy consumption without considerable increase in the removal efficiency. In the study conducted by Daneshvar et al., integrated UVC/ZnO process was used to degrade diazinon organophosphorus pesticide and showed up to 80% removal in 80 min reaction time [42]. Moza et al. showed that 4 h irradiation of imidacloprid with 290 nm light resulted in 90% degradation efficiency [6]. Moussavi et al. investigated the efficiency of UVC and UVC/ZnO for the oxidation of diazinon insecticide and found the maximum removal of diazinon during 30 min irradiation with UVC in the pH =5 and for UVC/ZnO at pH = 7.5 with the corresponding removal efficiencies of 57.8% and 93.3% [4]. Furthermore, the efficiency of UVC and UVC/TiO2 processes for removing this insecticide was compared, and the results showed that optimum pH for both processes was 5 and for photocatalysis process, the removal efficiency was 2.5 times higher than the UVC process [24].
Effect of catalysts dosages on the on the IM removal
By increasing the dose of TiO2 and ZnO catalysts from 0.005 to 0.1 g/L, the removal efficiency was also increased from 55.14% and 48.41% to 88.91% and 80.15%, respectively (Fig. 7). In the absence of a catalyst (UVC), the removal efficiency is only about 45%. By further increasing of TiO2 and ZnO dosages to 2 g/L, the removal efficiency is reduced by ca. 19% and 15%, respectively. Therefore, the catalyst dosage of 0.1 g/L was selected as the optimal amount for carrying out the experiments. Similar results were also obtained for single absorption process. As seen in Fig. 7, with the increasing of TiO2 and ZnO dose from 0.05 to 2 g/L, the removal efficiencies were also increased from 6.5% and 5.5% to 19.24 and 18.55%, respectively. According to various studies, the rate of decomposition of insecticides were also increased by increasing the concentration of nanoparticles, because with the increase of TiO2 and ZnO, the number of photons were also increased in the solution, resulting in the increase of the amount of adsorbed pollutant molecules on the catalyst surface, thus, a better photocatalytic reaction condition will be expected [17, 43]. Furthermore, by increasing the number of photons, the production of hydroxyl ions (•OH) will also be increased [43] and resulted in higher removal efficiency. However, for the dosages higher than the optimal concentration, the number of pollutant molecules is not proportional to the number of TiO2 nanoparticles. Also at higher concentrations due to the clustering and density of TiO2 particles, the number of available reaction sites at the catalyst surfaces will be decreased [43]. Moreover, excessive catalyst concentrations increase the turbidity of the environment, causing to the reduced light penetration and increasing dispersion, which in turn results in ineffectiveness of the process [41]. Wei et al. [44] reported that an increase in the catalyst dosages provides an increased number of active sites for adsorption and the following degrading reactions; however, the simultaneous increase in the solution opacity inhibits the photon flux penetration, which in turn decreased the degradation efficiency. According to Verma et al., it was found that pollutant removal efficiency was increased with increasing nanoparticle dosages and decreased after optimal dosage for photocatalytic degradation of insecticide Chlorpyrifos with TiO2 nanoparticles under UV irradiation. Sathiyanarayanan et al. who used ZnO photocatalytic process to remove the imidacloprid and Spirotetramat Residues from the water, also found that by increasing the amount of nanoparticle up to 0.07 g/L, the removal efficiency of imidacloprid was also increased [38]. These findings suggest that to ensure efficient absorption of photons and to avoid excess catalyst, the photocatalytic reactor should be operated at optimum catalyst loading to maintain a balance between these two opposing effects.
Effect of the presence of radical scavengers on the photolysis and photocatalytic degradation of imidacloprid
The presence of mineral salts in water can reduce the degradation efficiency of UV oxidation processes. To see the effect of mineral salts in the current study, experiments were conducted with commonly found ions namely carbonate, sulfate, phosphate, and tert-butanol anions. As shown in Fig. 8a, the removal efficiency of photolysis process (no catalyst) is increased by adding these ions and the removal efficiency is about 55% in the absence of these ions, while, the removal efficiency was increased about 18 to 26% by adding these ions, where the most and least effects were noticed in the presence of carbonate and tert-butanol, respectively. In contrast, in case of photocatalytic process, i.e. UVC/TiO2 process, (Fig. 8b), the efficiency of IM removal was decreased by the addition of radical scavengers. For example, the elimination efficiency in the absence of above ions under optimal conditions is about 87.5%, but with the addition of carbonate, phosphate, sulfate and tert-butanol, the removal efficiency decreased to 4.13%, 4.85%, 8.38%, and 16.56%, respectively, where carbonate and tert-butanol have the least and most interfering effects, respectively (Fig. 8b). The results of this study are in agreement with the results of Jafari et al. [24]. In another study conducted by Moussavi et al., it was also observed that the presence of some ions in water, such as carbonate, sulfate and phosphate could reduce the efficiency of the photocatalytic process [4]. In this work, the highest and lowest effects were found with phosphate and bicarbonate, respectively.
Kinetic studies of imidacloprid within photolysis and photocatalytic degradation
Within 270 min of the photocatalytic degradation with ZnO and TiO2, IM successfully removed, reaching 1.2 ± 0.1% of initial concentration of IM. The measurements of the dark absorption of IM showed no change in concentration after same illumination time. Pseudo-first order kinetic expression is commonly used to describe photocatalytic degradation rate of organic compounds, modified as Langmuir–Hinshelwood (L–H) model (Eq. 6) to consider reactions took place at the solid–liquid interface [7]:
Eq. 7 is the linear form of Langmuir–Hinshelwood (L–H) model:
where, ro, Ceq, KLH, and K are the initial degradation rate of the organic substrate, equilibrium bulk-solute concentration, limiting rate constant of reaction at maximum coverage under the given experimental conditions, and the equilibrium constant of adsorption of the organic substrate onto TiO2, respectively. θ in Eq. 7, the surface coverage, is related to the IM concentration (Ceq) and the adsorption equilibrium constant (K).
Figure 9a, b illustrates the non-linear forms of Langmuir–Hinshelwood (L–H) model assigning to photocatalytic and catalytic degradation of IM, respectively, which is obtained by Solver Add-in function in Microsoft Excel [45]. The inset of Fig. 9 depicts the linear forms of L–H model. As shown, the removal kinetics followed the Langmuir-Hinshelwood model. Table 2 presents kLH, and K constants (from L.H linear forms) assigning to photocatalytic and photolysis processes. As mentioned, K represents the equilibrium constant for the degradation of IM in the presence of TiO2, and as represented in Table 2, the value of K attributed to the photocatalytic process is higher than those obtained for the photolysis process. KLH reflects the limiting rate of reaction at maximum coverage (θ) and as it was shown in Table 2, photocatalytic process revealed lower limiting rates than the photolysis in the studied experimental conditions [24, 42]. Figure 9 represents the obvious resemblance between the L.H kinetic model and Langmuir isotherm formal curve (L-shape); at witch, increasing value of degradation rate in the low IM concentrations and reaching to the saturation values in high IM concentrations can be identified. Kitsiou et al., showed the similar finding regarding the photocatalytic degradation of IM in the aqueous solution.
The linear forms of the first and second order kinetic models of IM degradation are presented in the Eqs. 9 and 10 [46], respectively.
Where, C0, and Ct are IM concentrations at times 0 and t (min), respectively. k1 (min−1) and k2 (mg/L.min) are the first and second order reaction coefficients, respectively. Figure 10 shows the graphical illustration of the pseudo-first and pseudo-second order models assigning to different initial concentrations of IM in the photocatalytic process (the data for photolysis process is not shown here). According to the correlation coefficients (R2), at low concentrations, the experimental results reveals better correlations with the pseudo-first model. The corresponding kinetic coefficients for both photolysis and photocatalytic processes are shown in Table 3.
The results suggest that IM is susceptible to degradation but with small differences. Imidacloprid was efficiently degraded within 2 h of the experiment for both nano-catalysts but the highest degradation rate was noticed for TiO2 and the higher removal was also observed in this case. Therefore, in terms of IM removal, the photocatalytic degradation experiments were quite successful. Figure 10b shows the linear form representation of second-order kinetic model to describe the photocatalytic degradation of IM investigated in various concentrations. As represented in Fig. 10b and Table 3, the obtained R2 values confirm high correlation between the pseudo-second model and the experimental results. The R2 values represented in Table 3 confirm that the experimental data have better fit with the pseudo-second order kinetic model both for the low and high IM concentrations. The values of k2 obtained from the photocatalytic study are obviously higher than those obtained for the photolysis study showing the higher reaction rate in terms of photocatalytic IM degradation. These findings are confirmed by those reported by Philippidis et al. [7]. Decreasing the k1 and k2 constants by increasing the IM concentration in the photocatalytic study are represented in Table 3 revealing the UV absorption effect of IM and hindering the efficient activation of TiO2 NPs under UV illumination [24].
The aggregation or dimer formation with increasing IM concentration is a possible explanation for pseudo-second order photocatalytic degradation kinetics (Fig. 11) [46, 47]. Similar findings were also reported in the case of methylene blue and safranin orange photodegradation [46, 48].
Mechanisms involved in the degradation, and mineralization of imidacloprid
In photocatalytic decomposition of IM with TiO2 or ZnO under UV irradiation, electron cavity pairs are formed. Under the UV-irradiated and in the presence of oxygen, the final products of photocatalytic degradation of IM are CO2, Cl− and NO3 [5, 35]. When •OH and O2 radicals are trapped in active holes of cavities, the photocatalytic degradation of IM occurs on the catalyst surface. •OH radicals have sufficient strength to break the bands in insecticidal molecules adsorbed onto the catalyst surface [17]. Figure 12 illustrates three probable intermediate compounds produced during the degradation of IM by the pseudo-first order reactions [47].
UVC/TiO2 process showed the highest degradation and mineralization effects. As shown in Fig. 13a, for all processes at the optimum illumination time of 20 min, the degradation rates (C/C0) for UVC, UVC/TiO2 and UVC/ZnO processes were 0.49, 0.18, and 0.12, respectively. Similar trend was also observed for COD reduction, as after 10 min illumination time for UVC, UVC/TiO2 and UVC/ZnO processes, the IM removal efficiency was obtained ca. 22%, 68% and 64%, respectively. By increasing the reaction time to 20 min, the COD reduction was increased to ≈ 35%, 75%, and 71%, respectively. Finally, after 50 min of reaction, the amount of COD reduction in UVC, UVC/TiO2 and UVC/ZnO processes was 56.4%, 83.8% and 82.3%, respectively. Therefore, it is concluded that UVC/TiO2 process had the highest COD/COD0 reduction rate of 0.25 at the optimum point, while this ratio for UVC and UVC/ZnO was only 0.65 and 0.29. Therefore, based on COD reduction, it can be concluded that the use of titanium dioxide catalyst has the greatest efficiency in removing imidacloprid insecticide. In order to measure the mineralization, the total organic carbon content was also measured at the input and output of the reactor under the optimum conditions, and corresponding removal efficiencies in UVC, UVC/TiO2 and UVC/ZnO processes were obtained 31, 70 and 64%, respectively. The higher mineralization efficiency of UVC/TiO2 process can be attributed to non-selective radical hydroxylation degradation and high oxidation potential (2.80 v). According to Verma et al., it was found that the highest COD reduction was achieved with a concentration of 4 g/L nanoparticles for photocatalytic degradation of chlorpyrifos insecticide with TiO2 nanoparticle [43]. Wu et al. compared the performance of TiO2, SnO2 and ZnO, where the highest removal efficiency was reported for TiO2 [31].
The GC-MS analysis, total organic carbon removal efficiency and final degradation products
The GC/MS chromatogram (not shown here) shows the formation of more stable and simple compounds through ring cleavage of IM aromatic ring structures. The main identified intermediate metabolites included: pyridine and pyrimidine compounds, 6-chloronicotinic acid, and hexamethylene-iminoacetonitrile. Some other peaks were also observed in the library such as silane, thiazole, cyclotetrasiloxane, cyclohexasiloxane, etc. that may be related to column washing. The other compounds including ethylene terephthalate, ethyl et al., etc. may be resulted from sample container [6, 47, 49]. Chloronictinic acid, 1-[(6-chloro-3pyridinyl) methyl]-2-imidazolidinone, and 1-[(6-chloro-3-pyridinyl) methyl]-N-nitroso-2-imidazolidimine were identified as the main degradation products using composite of TiO2 and zeolite H-ZSM-5 in the photocatalytic degradation of IM [50]. Chloronicotinic aldehyde, chloronicotinic acid, 1-(6-chloro-3pyridilmethyl) imidazolidin-2-ona, and two other unknown compunds have been reported as the main degradation by-products in the study conducted by Agüera et al. [51]. 6-chloronicotinamide and 2-pyrrolidinone were determined as the main transformation products in the photocatalysis of IM using TiO2 as the catalyst [52].
It should be noted that the measured concentrations of the identified intermediate metabolites are very low, which also are in agreement with high COD and TOC removal efficiencies, where total organic carbon (TOC) measurements, as an indicator of mineralization [11], at the inlet (61.64 mg/L) and outlet (4.6 mg/L) of the process (under optimum conditions) showed the reasonable photo degradation efficiency of 89.87%, which is high enough compared to similar study. For instance, Sedaghat et al. investigated the imidacloprid degradation by photoelectrofenton process and found only 67% TOC removal efficiency within 3 h [33]. This indicates the effect of the UVC/TiO2 process towards simplifying the nature of IM and its conversion to more simple and degradable compounds.
Comparing the results of studied photolytic and photocatalytic methods for removing pesticides
Table 4 summarizes a variety of methods studied to remove pesticides under various operational conditions. As seen, many studies were conducted to remove pesticides from water or aqueous solutions. For example, Kitsiou et al. [53] studied different photocatalyst methods to remove imidiacloprid from aqueous solutions. They found combined UVA/TiO2 (500 mg/L) + Fe+3 (7 mg/L) + H2O2 (50 mg/L) process as the efficient method (about 80% removal efficiency at 30 min illumination time, pH of 3.2, and initial IM concentration of 20 mg/L) presumably due to the synergistic effect of homogeneous and heterogeneous photocatalytic reaction. Photocatalytic degradation of imidacloprid in aqueous suspension in the presence of TiO2 supported on H-ZSM-5 as a photocatalyst has been studied by Tang et al. [50]. UV lamp with light intensity of 0.5 mW/cm2 at the wavelength of 365 nm (UVA) was used as the main source of irradiation. Although in the mentioned study, higher removal efficiency was obtained compared to our results, higher dosage and illumination time were also required to reach such degradation efficiency. Moussavai et al. also used UVC and UVC/ZnO processes and successfully removed organophosphate pesticide, diazinon as a model organophosphate pesticide. However, higher illumination time was used to attain 93.3% removal efficiency of lower initial concentration of insecticide comparing to our conditions.
Conclusions
The present experimental study aimed at investigating the degradability of imidacloprid via photocatalytic advanced oxidation process under varying working conditions. Applying a low cost and chemically stable catalyst at limited dosage (100 mg/L) and its activation with UVC as a chemical-less agent under relatively low illumination time make this photoreactor as an economically applicable solution for the degradation of organophosphate pesticides and to remove such pollutants from contaminated waters. Photocatalysis of IM is more effective as compared to photolysis process and degradation is favored under alkaline conditions. This is accompanied by oxidation of imidiacloprid to simple and relatively stable transformation by-products that some of these were successfully identified.
References
Chihhao Fan S-JL. Competitive degradation of Imidacloprid in the presence of humic acids by Fenton process at neutral environment. In: 4th international conference on future environment and energy; 2014.
Segura CZC, H’ector D, Mansilla A, Mondaca M. Imidacloprid oxidation by photo-Fenton reaction. J Hazard Mater. 2008;150:679–86.
Zahoor M, Mahramanlioglu MJC. Quarterly be. Adsorption of imidacloprid on powdered activated carbon and magnetic activated. Carbon. 2011;25(1):55–63.
Moussavi G, Hossaini H, Jafari SJ, Farokhi M. Comparing the efficacy of UVC, UVC/ZnO and VUV processes foroxidation of organophosphate pesticides in water. J Photochem Photobiol A Chem. 2014;290:86–93.
Zheng W, Sang-jin L. Photochemistry of insecticide imidacloprid: direct and sensitized in aqueous medium. J Environ Sci. 2004;16(4):539–42.
Moza P, Hustert K, Feicht E, Kettrup A. Photolysis of imidacloprid in aqueous solution. Chemosphere. 1998;36(3):497–502.
Philippidis N, Sotiropoulos S, Efstathiou A, Poulios I. Photoelectrocatalytic degradation of the insecticide imidacloprid using TiO2/Ti electrodes. J Photochem Photobiol A Chem. 2009;204(2–3):129–36.
Yari K, Rahmania A, Asgaria G, Azarian Q, Bhatnagar A, Leili M. Degradation of imidacloprid pesticide in aqueous solution using an eco-friendly electrochemical process. Desalin Water Treat. 2017;86:150–7.
Mahvi AH, Maleki A. Photosonochemical degradation of phenol in water. Desalin Water Treat. 2010;20(1–3):197–202.
Maleki A, Mahvi A, Alimohamadi M, Ghasri A. Advanced oxidation of phenol by ultraviolet irradiation in aqueous system. Pak J Biol Sci. 2006;9(12):2338–41.
Maleki A, Shahmoradi B. Solar degradation of direct blue 71 using surface modified iron doped ZnO hybrid nanomaterials. Water Sci Technol. 2012;65(11):1923–8.
Liang HC, Razaviarani V, Buchanan I. Pesticides and Herbicides, Literature Review. Water Environ Res. 2013;85(10):1601–45.
Moussavi G, Pourakbar M, Aghayani E, Mahdavianpour M, Shekoohyian S. Comparing the efficacy of VUV and UVC/S2O8-advanced oxidation processes for degradation and mineralization of cyanide in wastewater. Chem Eng J. 2016;294:273–80.
Shahmoradi B, Negahdary M, Maleki A. Hydrothermal synthesis of surface-modified, manganese-doped TiO2 nanoparticles for Photodegradation of methylene blue. Environ Eng Sci. 2012;29(11):1032–7.
Khani A, Sohrabi MR. Simultaneous synthesis-immobilization of nano ZnO on perlite for photocatalytic degradation of an azo dye in semi batch packed bed photoreactor. Journal of Chemical Technology. 2012;14(4):69–76.
Bhadiyadra J, Minakshi V. A review on applicability of Photocatalyst titanium dioxide for treatment of greywater. Journal of Engineering Research and Applications. 2015;5(3):102–5.
Dehghani MHFAM. Photocatalytic oxidation of organophosphorus Pesticiides using zinc oxide. Res J ChemEnviron. 2012;16(3):104–9.
Kunal M, Sharma A. Photocatalytic oxidation of pollutant dyes in wastewater by TiO2 and ZnO nano-materials – a mini-review. Nanoscience & Technology for mankind. Indian: The National Academy of Sciences India (NASI); 2014.
Garrett P. Electrochemical degradation of some pesticides in agricultural wastewater by using modified electrode. International Journal of Agr and Env. 2013;61.
Leili M, Pirmoghani A, Samadi MT, Shokoohi R, Roshanaei G, Poormohammadi A. Determination of Pesticides residues in cucumbers grown in greenhouse and the effect of some procedures on their residues. Iran J Public Health. 2016;45(11):1481–90.
Abdel-Gawad SA, Baraka AM, Omran KA, Mokhtar MM. Removal of some pesticides from the simulated waste water by electrocoagulation method using iron electrodes. Int J Electrochem Sci. 2012;7:6654–65.
Tao J, Gong Z, Yao G, Cheng Y, Zhang M, Lv J, et al. Enhanced optical and photocatalytic properties of ag quantum dots-sensitized nanostructured TiO2/ZnO heterojunctions. J Alloys Compd. 2016;688:605–12.
Yang TT, Peng JM, Zheng Y, He X, Hou YD, Wu L, et al. Enhanced photocatalytic ozonation degradation of organic pollutants by ZnO modified TiO2 nanocomposites. Appl Catal B-Environ. 2018;221:223–34.
Jafari SJ, Moussavi G, Hossaini H. Degradation and mineralization of diazinon pesticide in UVC and UVC/TiO2 process. Desalin Water Treat. 2016;57(8):3782–90.
O’Shea K, Pernas E, Saiers J. The influence of mineralization products on the coagulation of TiO2 Photocatalyst. Langmuir. 1999;15(6):2071–6.
O'Shea K, Pernas E, Saiers J. Investigation of adsorption kinetics and isotherms of imidacloprid as a pollutant from aqueous solution by adsorption onto industrial granular activated carbon. Journal of Food Agriculture and Environment. 2007;5(3):425.
Akyol AYH, Bayramoglu M. Photocatalytic decolorization of Remazol red RR in aqueous ZnO suspensions. Appl Catal B Environ. 2004;54(1):19–24.
Cox LKW, Celis R, Hermosin MC, Cornejo J, Yen PY. Sorption of Imidacloprid on soil clay mineral and organic components. Soil Sci Soc Am J. 1998;62(4):911–5.
Mandal A, Singh N. Optimization of atrazine and imidacloprid removal from water using biochars: designing single or multi-staged batch adsorption systems. Int J Hyg Environ Health. 2017;220(3):637–45.
Giri R, Ozaki H, Okada T, Taniguchi S, Takanami R. Factors influencing UV photodecomposition of perfluorooctanoic acid in water. Chem Eng J. 2012;180:197–203.
Wu CLX, Wei D, Fan J, Wang L. Photosonochemical degradation of phenol in water. Water Res. 2001;35(16):3927–33.
Hong S-M, Win Min Z, Mok C, Kwon H-Y, Kim T-K, Kim D-H. Aqueous degradation of Imidacloprid and Fenothiocarb using contact glow discharge electrolysis: degradation behavior and kinetics. Food Sci Biotechnol. 2013;22(6):1773–8.
Sedaghat M, Vahid B, Aber S, Rasoulifard MH, Khataee A, Daneshvar N. Electrochemical and photo-assisted electrochemical treatment of the pesticide imidacloprid in aqueous solution by the Fenton process: effect of operational parameters. Res Chem Intermed. 2016;42(2):855–68.
Rahmani A, Samadi M, Enayati MA. Investigation of photocatalytic degradation of phenol by UV/TiO2 process in aquatic solutions. J Res Health Sci. 2008;8(2):286–92.
Hemmati Borji S, Nasseri S, Nabizadeh R, Mahvi AH, Javadi AH. Photocatalytic degradation of phenol in aqueous solutions by Fe(III)-doped TiO2/UV process. Iran J Health & Environ. 2011;3(4):369–80.
Mijin D, Savic M, Snezana P, Smiljanic A, Glavaski O, Jovanovic M, et al. A study of the photocatalytic degradation of metamitron in ZnO water suspensions. Desalination. 2009;249:286–92.
Abdollahi Y, Abdullah AH, Gaya UI, Ahmadzadeh S, Zakaria A, Shameli K, et al. Photocatalytic degradation of 1,4-benzoquinone in aqueous ZnO dispersions. J Braz Chem Soc. 2012;23(2):236–40.
Sathiyanarayanan S, Ravi PE, Ramesh A. Applications of zinc oxide Nanorods as Photocatalyst for the decontamination of Imidacloprid and Spirotetramat residues in water. The Open Catalysis Journal. 2009;2:24–32.
Patil PN, Bote SD, Gogate PRJUs. Degradation of imidacloprid using combined advanced oxidation processes based on hydrodynamic cavitation. 2014;21(5):1770–7.
Imoberdorf G, Mohseni M. Degradation of natural organic matter in surface water using vacuum-UV irradiation. J Hazard Mater. 2011;186(1):240–6.
Arbab PSM, Fakhraie H. Photocatalytic Degradation of Triethyl phosphate Using Nano TiO2 water & wastewater. 2012;3:103–11.
Daneshvar N, Aber S, Seyed Dorraji M, Khataee A, Rasoulifard M. Photocatalytic degradation of the insecticide Diazinon in the presence of prepared Nanocrystalline ZnO powders under irradiation of UV-C-light. Sep Purif Technol. 2007;58(1):91–8.
Verma A, Poonam DD. Photocatalytic degradability of insecticide Chlorpyrifos over UV irradiated titanium dioxide in aqueous phase. Int J Environ Sci. 2012;3(2):743–55.
Wei L, Shifu C, Wei Z, Sujuan Z. Titanium dioxide mediated photocatalytic degradation of methamidophos in aqueous phase. 2009;164(1):154–60.
Khazaei M, Nasseri S, Ganjali MR, Khoobi M, Nabizadeh R, Mahvi AH. Modeling mercury (II) removal at ultra-low levels from aqueous solution using graphene oxide functionalized with magnetic nanoparticles: optimization, kinetics, and isotherm studies. Desalin Water Treat. 2017;83:144–58.
Rauf MA, Meetani MA, Khaleel A, Ahmed A. Photocatalytic degradation of methylene blue using a mixed catalyst and product analysis by LC/MS. Chem Eng J. 2010;157(2–3):373–8.
Ding T, Jacobs D, Lavine BK. Liquid chromatography-mass spectrometry identification of imidacloprid photolysis products. Microchem J. 2011;99(2):535–41.
El-Kemary M, El-Shamy H. Fluorescence modulation and photodegradation characteristics of safranin O dye in the presence of ZnS nanoparticles. J Photochem Photobiol A Chem. 2009;205(2):151–5.
Bourgin MVF, Debrauwer L, Albet J. Ozonation of imidacloprid in aqueous solutions: reaction monitoring and identification of degradation products. J Hazard Mater. 2011;190(1):60–8.
Tang J, Huang X, Huang X, Xiang L, Wang QJEES. Photocatalytic degradation of imidacloprid in aqueous suspension of TiO2 supported on H-ZSM-5. Environmental Earth Sciences. 2012;66(2):441–5.
Agüera A, Almansa E, Malato S, Maldonado M, I., Fernández-Alba A R. Evaluation of photocatalytic degradation of imidacloprid in industrial water by GC-MS and LC-MS. Analusis 1998;26 (7):245–250.
Malato S, Caceres J, Agüera A, Mezcua M, Hernando D, Vial J, et al. Degradation of Imidacloprid in water by photo-Fenton and TiO2 Photocatalysis at a solar pilot plant: a comparative study. Environmental Science & Technology. 2001;35(21):4359–66.
Kitsiou V, Filippidis N, Mantzavinos D, Poulios I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl Catal B Environ. 2009;86(1):27–35.
Mahalakshmi M, Arabindoo B, Palanichamy M, Murugesan VJ. Photocatalytic degradation of carbofuran using semiconductor oxides. 2007;143(1–2):240–5.
Senthilnathan J, LJCej P. Photocatalytic degradation of lindane under UV and visible light using N-doped TiO2. Chem Eng J. 2010;161(1–2):83–92.
Chen J-Q, Wang D, Zhu M-X, Gao C-J. Photocatalytic degradation of dimethoate using nanosized TiO2 powder. Desalination. 2007;207(1):87–94.
Acknowledgements
The authors are grateful to the Hamadan University of Medical Sciences, Iran for providing financial and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yari, K., Seidmohammadi, A., Khazaei, M. et al. A comparative study for the removal of imidacloprid insecticide from water by chemical-less UVC, UVC/TiO2 and UVC/ZnO processes. J Environ Health Sci Engineer 17, 337–351 (2019). https://doi.org/10.1007/s40201-019-00352-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-019-00352-3